Abstract
Background antibiotic use (i.e., administration of antibiotics not directly related to Chlamydia trachomatis or Neisseria gonorrhoeae infections) has been associated with a lower prevalence of genital C. trachomatis infection in a clinical setting. Associations with specific antibiotic types or with N. gonorrhoeae are lacking. Here, we assessed the prevalence of antibiotic use, the different classes and agents used, and their association with a subsequent sexually transmitted infection (STI) clinic C. trachomatis and N. gonorrhoeae test result. At our STI clinic, we systematically registered whether antibiotics were used in the past month (in 29% of the cases, the specific antibiotic agent was named). Patients were screened for urogenital C. trachomatis and N. gonorrhoeae; a third of them were also screened for anorectal and oropharyngeal C. trachomatis and N. gonorrhoeae. The proportion of antibiotics used and their association with C. trachomatis and N. gonorrhoeae prevalence were assessed for heterosexual men, men who have sex with men (MSM), and women. During 14,775 clinic consultations, antibiotic use was reported by 12.2% (95% confidence interval [CI], 11.7% to 12.7%), i.e., 14.8% of women, 8.6% of heterosexual men, and 11.6% of MSM. The most reported antibiotics were penicillins, tetracyclines, and macrolides, respectively. The prevalence was 11.0% (95% CI, 10.3% to 11.3%) for C. trachomatis and 1.9% (95% CI, 1.7% to 2.1%) for N. gonorrhoeae. Only tetracycline use was associated with a lower C. trachomatis prevalence (3%). Overall antibiotic use was associated with lower anorectal C. trachomatis prevalence in MSM only (odds ratio, 0.4; 95% CI, 0.2 to 0.8). STI clinic visitors commonly report recent antibiotic use. Even in a country with low antibiotic consumption, tetracycline use impacted C. trachomatis prevalence, while there was a notable absence of association with azithromycin.
INTRODUCTION
It is a continuous challenge to control the spread of sexually transmitted infections (STIs). Many cases are hidden to care and remain untested and untreated, for example, cases of Chlamydia trachomatis and Neisseria gonorrhoeae (1, 2). Furthermore, control of N. gonorrhoeae is hampered by growing antimicrobial resistance (3–5). C. trachomatis and N. gonorrhoeae are among the most common bacterial STIs. If not adequately treated, they may result in serious complications such as epididymitis in men and pelvic inflammatory disease, infertility, and ectopic pregnancy in women (6). C. trachomatis infection can be treated by several classes of antibiotics; macrolides (i.e., azithromycin) and tetracyclines (i.e., doxycycline) are the recommended options. Alternatively, fluoroquinolones and quinolones (i.e., ofloxacin) can be prescribed (7, 8, 9). N. gonorrhoeae can be treated with ceftriaxone or with ciprofloxacin when ceftriaxone is contraindicated and strains show no ciprofloxacin resistance (7, 8, 9). Treatment of N. gonorrhoeae is complicated by increasing rates of resistance to quinolones, tetracyclines, and penicillins and decreasing susceptibility to cephalosporins (3–5).
Australian research has postulated that C. trachomatis may be incidentally treated in countries with a relatively high background antibiotic consumption (10, 11), as the overall annual number of antibiotic prescriptions generally outweighs the frequency of C. trachomatis testing. The volume of outpatient systemic antibiotic use increased in most European countries between 1997 and 2003, while consumption remained stable between 2007 and 2011 at a median consumption of 19.5 defined daily doses (DDD) per 1,000 inhabitants per day (12, 13). An ecological analysis of studies from 12 European countries demonstrated an inverse correlation between tetracycline and macrolide use in the year 2002 and genital C. trachomatis prevalence in all countries except the Netherlands (12). Tetracyclines and macrolides belong to the most commonly prescribed group of systemic antibacterial antibiotics after penicillins (13).
The Netherlands has historically had the lowest prescription rate of all European countries, although an increase has been noted (reaching 11.4 DDD per 1,000 inhabitants per day in 2011) (13). The C. trachomatis test rate in the Netherlands has been estimated to be 3 per 1,000 persons in the community (14). While background antibiotic use may impact the transmission of STIs on a population level, it may also impact clinical practices since it affects the outcome of an STI diagnostic test and may interact with subsequent treatment. Studies among men and young women receiving antenatal, general practitioner, or sexual health care in Australia demonstrated that recent antibiotic use was associated with a lower prevalence and incidence of genital C. trachomatis (1, 15, 16, 17). However, the specific antibiotic agents were not studied.
Such reports are lacking for N. gonorrhoeae, yet a similar phenomenon may be observed. The consumption of antibiotics may lead to increasing rates of resistance in the population, so overall antibiotic use may impact the clinical practice of STI testing. The number of incidentally treated N. gonorrhoeae infections is probably minimal, as N. gonorrhoeae is more symptomatic and is therefore more likely to be identified and treated promptly.
In this study, conducted in a country with low per capita antibiotic consumption (i.e., the Netherlands), we assessed the proportion of systemic antibiotic use before C. trachomatis and N. gonorrhoeae genital and extragenital (anorectal and oropharyngeal) screening in women and men visiting an STI clinic. Further, we tested the association between overall systemic antibiotic use and the specific agent and the result of subsequent C. trachomatis and N. gonorrhoeae tests.
MATERIALS AND METHODS
Procedures and study population.
The outpatient STI clinic of the South Limburg Public Health Service offers free-of-charge examination and treatment for STIs. The clinic has four fixed testing sites in South Limburg (population, 630,000). The study population includes surveillance data from all patients 18 years and older who visited our STI clinic between August 2010 and October 2013 (n = 14,945). At every new consultation, patients were tested urogenitally for C. trachomatis and N. gonorrhoeae on first-void urine (men) or self-swab (women), and some of the patients (38%; n = 5,691) were also tested anorectally by self-swab (men and women) and/or oropharyngeally by nurse-taken swab (men and women). Testing was done by commercially available nucleic acid amplification tests (NAAT) (strand displacement amplification [SDA] [ProbeTec ET system; Becton Dickinson, MD, USA] or PCR [Cobas Amplicor or Cobas 4800; Roche, CA, USA); positive N. gonorrhoeae tests were confirmed by an in-house PCR.
In accordance with national guidelines, patients who tested positive were asked to return for treatment with azithromycin or doxycycline (in the case of C. trachomatis) or with ceftriaxone (in the case of N. gonorrhoeae). From August 2010, we systematically registered (by self-report) whether patients had used antibiotics in the month preceding the screening test. An additional open question was asked about the type of regimen used; patients filled in their prescribed course and/or their indication for use.
Variables and statistical analyses.
We assessed the prevalence of systemic antibiotic use and its association with C. trachomatis or N. gonorrhoeae diagnoses. To reduce confounding by indication (thereby excluding people who were recently treated for an STI), we removed certain consultations from the data: those that occurred within 45 days after a previous STI clinic consultation (n = 100) or consultations in which a client reported a C. trachomatis or N. gonorrhoeae diagnosis in the past month (n = 70). This resulted in 14,775 consultations in our analyses. Of all the people who answered “yes” for antibiotic use (n = 1,994), 132 reported using medication other than systemic antibacterial treatment (e.g., painkillers, nonsystemic [e.g., topical] antibiotics, antifungals, inhalation medication, or antihistamines), and an additional 61 reported no systemic antibiotic agent but an indication that was not likely for systemic antibiotic use (i.e., fungal infection, herpes, eye infection, parasitic worms, hay fever, or impetigo). In our analyses, we considered the patients in these 193 consultations not to have used systemic antibiotics.
Of the 1,801 remaining consultations in which antibiotic use was reported, named agents were given in 541 and no agents were named in the remaining 1,260. We constructed several variables on antibiotic use (yes/no) by agent based on the reported antibiotic agent that was recommended for use against C. trachomatis or N. gonorrhoeae: doxycycline, azithromycin, ofloxacin, erythromycin, amoxicillin, ceftriaxone, and ciprofloxacin. Other variables were constructed on the combined classes of reported agents: tetracyclines, macrolides, fluoroquinolones and quinolones, penicillins, cephalosporins, nitrofurantoin/fosfomycin/trimethoprim, and other (mainly metronidazole).
First, we used chi-square analyses to compare characteristics of the study population, including antibiotic use, between women, heterosexual men, and men who have sex with men (MSM). Second, we used chi-square tests to make associations between overall antibiotic use and age, gender, sexual orientation, and HIV positivity in the total group of 14,775 consultations. Third, we used univariate and multivariable logistic regression analyses, accounting for repeated measures, to assess overall antibiotic use and antibiotic use by agent, gender, sexual orientation, HIV status, and age (and their interaction) as determinants for C. trachomatis and for N. gonorrhoeae positivity. In analyses evaluating antibiotic use by specific agent, we excluded consultations in which antibiotics were reported but no specific agent was named (n = 1,260, resulting in n = 13,515 used in analyses). Overall presence of C. trachomatis and N. gonorrhoeae (at any site) was used as the outcome, but we also performed analyses stratified by anatomic site. We noted differences between anatomic sites and considered a P value of <0.05 to be statistically significant. Analyses were performed using the SPSS package version 20 (IBM Inc., Somers, New York, USA).
Ethical approval.
The medical ethical committee of Maastricht University approved the study (no. 11-4-108).
RESULTS
Antibiotic use before STI testing.
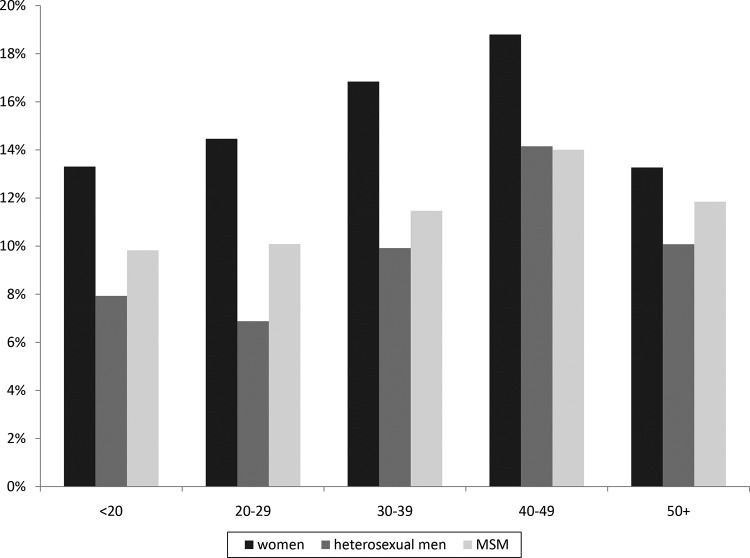
During 14,775 STI clinic testing consultations, 12.2% (n = 1,801) (95% confidence interval [CI], 11.7% to 12.7%) of clinic patients reported recent antibiotic use. Women had higher rates of antibiotic use than heterosexual men or MSM, and antibiotic use increased with age (all P < 0.001) (Fig. 1). Antibiotic use was also higher for those who were HIV positive (18.6%, versus 12.1% for HIV-negative patients) (P = 0.004). Antibiotics were used for various reasons (e.g., for urinary tract infections [UTI] and respiratory tract infections [RTI]), yet in the majority of cases (63%), data about antibiotic usage were absent.
FIG 1.
Proportion of antibiotic use in the past month by patients who visited an STI clinic for Chlamydia trachomatis and Neisseria gonorrhoeae screening, divided by sexual orientation and age, for a total of 14,775 STI clinic consultations.
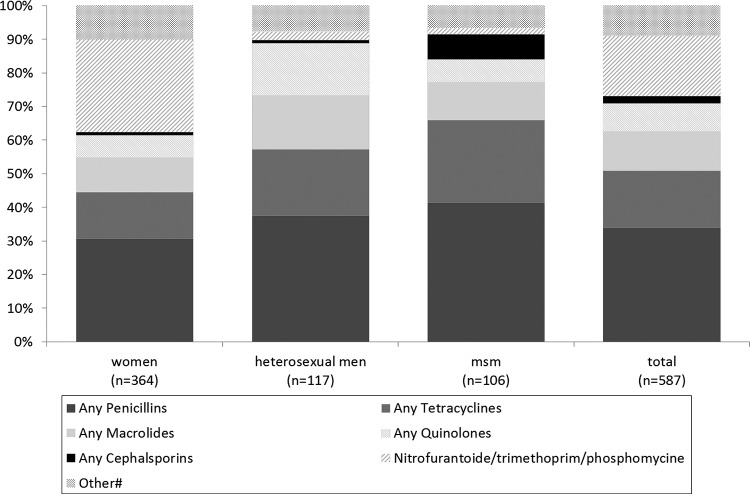
The specific agent(s) was named in 541 (30.0%) of the consultations where patients reported antibiotic use. The reported frequencies of use are displayed in Table 1 and the shares of use in Fig. 2. Of the named agents, 26.2% (n = 142) consisted of any of the following: doxycycline, azithromycin, ofloxacin, or erythromycin. Another 6.3% (n = 34) consisted of ceftriaxone or ciprofloxacin. In 46 consultations, patients reported using a combination of two different agents. The antibiotics used varied by gender and sexual orientation (Table 1). Penicillins were most frequently reported, followed by nitrofurantoin/trimethoprim/fosfomycin (commonly used for UTI) in women and tetracyclines and macrolides in both men and women (Fig. 2).
TABLE 1.
Characteristics of the study population and self-reported antibiotic use in the past month, by sexual orientation, from a total of 14,775 STI clinic consultationsa
| Characteristic | Women (n = 7,419) | Heterosexual men (n = 5,007) | MSM (n = 2,349) | Pb | Total (n = 14,775) |
|---|---|---|---|---|---|
| Median age, yr (interquartile range) | 23 (20–30) | 25 (22–36) | 37 (25–48) | ** | 24 (21–37) |
| C. trachomatis diagnosis | |||||
| Any sitec | 10.6 (788) | 11.8 (593) | 9.2 (216) | ** | 10.8 (1,597) |
| Genitald | 10.2 (757) | 11.7 (588) | 3.2 (76) | ** | 9.6 (1,421) |
| Anorectald | 6.5 (136) | 0.9 (7) | 7.3 (162) | ** | 6.0 (305) |
| Oropharyngeald | 1.4 (22) | 0.2 (2) | 0.7 (16) | ** | 0.8 (40) |
| N. gonorrhoeae diagnosis | |||||
| Any sitec | 1.1 (82) | 1.0 (50) | 6.4 (150) | ** | 1.9 (282) |
| Genitald | 0.6 (45) | 0.6 (30) | 1.4 (32) | ** | 0.7 (107) |
| Anorectald | 0.7 (15) | 0.4 (3) | 4.0 (88) | ** | 2.1 (106) |
| Oropharyngeald | 2.7 (43) | 2.2 (19) | 3.4 (79) | 3.0 (141) | |
| HIV positivee | 0.2 (13) | 0.1 (7) | 9.2 (216) | 1.6 (236) | |
| Overall antibiotic use (past month) | 14.8 (1,099) | 8.5 (428) | 11.7 (274) | ** | 12.2 (1,801) |
| Antibiotic use per named indication | |||||
| Urinary tract infections | 3.1 (229) | 0.2 (12) | 0.3 (6) | ** | 1.7 (247) |
| Respiratory tract infections | 1.4 (105) | 1.1 (57) | 1.5 (35) | ** | 1.3 (197) |
| Other (non-STI) indications | 1.4 (102) | 1.4 (71) | 1.5 (36) | 1.4 (209) | |
| Unknown indications | 8.9 (663) | 5.8 (288) | 8.4 (197) | ** | 7.8 (1,148) |
| Any antibiotic agent reported | 4.5 (331) | 2.2 (109) | 4.3 (101) | ** | 3.7 (541) |
| Antibiotic use per named agent/classf | |||||
| Doxycycline | 0.6 (39) | 0.4 (20) | 0.8 (18) | 0.5 (77) | |
| Azithromycin | 0.4 (28) | 0.4 (17) | 0.6 (12) | 0.4 (57) | |
| Ofloxacin | 0.1 (4) | 0.1 (4) | 0 (0) | 0.1 (8) | |
| Amoxicillin | 1.1 (76) | 0.7 (31) | 1.5 (33) | ** | 1.0 (140) |
| Erythromycin | 0.1 (4) | 0 (0) | 0 (0) | 0 (4) | |
| Ceftriaxone | 0 (1) | 0 (1) | 0.2 (5) | ** | 0.1 (7) |
| Ciprofloxacin | 0.2 (12) | 0.2 (9) | 0.3 (6) | 0.2 (27) | |
| Any tetracyclines | 0.8 (50) | 0.5 (23) | 1.2 (26) | ** | 0.7 (99) |
| Any macrolides | 0.6 (38) | 0.4 (19) | 0.6 (12) | 0.5 (69) | |
| Any quinolones | 0.4 (24) | 0.4 (18) | 0.3 (7) | 0.3 (49) | |
| Any cephalosporins | 0 (3) | 0 (1) | 0.4 (8) | ** | 0.1 (12) |
| Any penicillins | 1.7 (112) | 0.9 (44) | 2.0 (44) | ** | 1.5 (200) |
| Nitrofurantoin/trimethoprim/fosfomycin | 1.5 (100) | 0.1 (3) | 0.1 (2) | ** | 0.8 (105) |
| Otherg | 0.6 (37) | 0.2 (9) | 0.3 (7) | * | 0.4 (53) |
Data are reported as percentages (numbers of subjects) unless otherwise indicated.
*, P < 0.05; **, P < 0.01 (by chi-square test on the difference between women, heterosexual men, and MSM [for age, nonparametric test]).
Including genital, anorectal, and/or oral diagnosis at current consultation.
The denominator includes only the tested individuals.
HIV diagnosed at current consultation or known HIV positive.
More than 1 agent could be named during a consultation.
Mainly metronidazole and (some) clindamycin.
FIG 2.
Share of named antibiotic classes among all named types reported by people visiting an STI clinic for Chlamydia trachomatis and Neisseria gonorrhoeae screening, reported by sexual orientation. There were 587 named agents in 541 STI clinic consultations.
Prevalence of C. trachomatis and association with antibiotic use.
The overall prevalence of C. trachomatis was 10.8% (95% CI, 10.3% to 11.3%) (Table 1), and recent antibiotic use was reported by 10.8% (173/1,597) of patients who received C. trachomatis diagnoses. The association between antibiotic use and C. trachomatis differed between women, heterosexual men, and MSM (overall P interaction term, 0.019). In univariate analyses, any recent antibiotic use was associated with a lower C. trachomatis prevalence in heterosexual men and in MSM but not in women (Table 2). When adjusting for age and HIV status, the risk estimate for heterosexual men attenuated somewhat and became non-statistically significant; however, the risk remained for MSM (Table 2). After stratifying analyses of MSM by anatomic site, it appeared that the inverse association between antibiotic use and C. trachomatis was observed only for anorectal C. trachomatis in MSM (odds ratio [OR] adjusted for HIV and age, 0.42; 95% CI, 0.19 to 0.94) and not for genital C. trachomatis in MSM (adjusted OR, 0.58; 95% CI, 0.27 to 1.23) or oral C. trachomatis in MSM (adjusted OR, 1.10; 95% CI, 0.24 to 4.98).
TABLE 2.
Proportions of Chlamydia trachomatis and Neisseria gonorrhoeae positive screening diagnoses and association with preceding use of antibiotics from a total of 14,775 STI clinic consultations
| Group |
C. trachomatis |
N. gonorrhoeae |
||||
|---|---|---|---|---|---|---|
| % (n) | ORa (95% CI) |
% (n) | OR (95% CI) |
|||
| Univariate | Adjustedb | Univariate | Adjusted | |||
| Women | ||||||
| No antibiotic use (n = 6,320) | 10.6 (667) | 1 | 1 | 1.1 (67) | 1 | 1 |
| Antibiotic use (n = 1,099) | 11.0 (121) | 1.05 (0.85–1.29) | 1.09 (0.89–1.34) | 1.4 (15) | 1.29 (0.73–2.29) | 1.23 (0.70–2.18) |
| Heterosexual men | ||||||
| No antibiotic use (n = 4,579) | 12.1 (556) | 1 | 1 | 1.0 (48) | 1 | 1 |
| Antibiotic use (n = 428) | 8.6 (37) | 0.69 (0.49–0.97)* | 0.76 (0.54–1.08) | 0.9 (4) | 0.93 (0.33–2.59) | 0.83 (0.29–2.33) |
| MSM | ||||||
| No antibiotic use (n = 2,075) | 9.7 (201) | 1 | 1 | 6.4 (132) | 1 | 1 |
| Antibiotic use (n = 274) | 5.5 (15) | 0.54 (0.29–1.00)* | 0.49 (0.26–0.89)* | 6.6 (18) | 1.04 (0.64–1.69) | 0.91 (0.59–1.47) |
Risk estimates for the total group are not presented since C. trachomatis estimates significantly differed between women and heterosexual men (P interaction = 0.039) and between women and MSM (P interaction = 0.024). For N. gonorrhoeae the risk estimates did not differ between sexual orientation groups and the overall univariate OR (1.09; 95% CI, 0.77 to 1.54). Analyses accounted for repeated measurements. *, P < 0.05.
Adjusted for age and HIV status.
We also evaluated associations with specific agents, thereby excluding from our analyses the consultations with patients who reported antibiotic use but failed to name the agent used (Table 3). Additional analyses demonstrated that whether or not a specific antibiotic agent was reported was not associated with C. trachomatis (or with N. gonorrhoeae), nor was the reason for antibiotic use (UTI, RTI, or other) (data not shown). As no interactions were observed between the evaluated agents and sexual orientation (all P > 0.20), this paper presents overall models assessing C. trachomatis and N. gonorrhoeae (rather than separate models for women, heterosexual men, and MSM). In both univariate and multivariate analyses, tetracycline use was inversely associated with C. trachomatis (Table 3). Further analyses showed the associations between tetracycline use and specific anatomic sites: for genital C. trachomatis (OR adjusted for gender, sexual orientation, age, and HIV status, 0.25; 95% CI, 0.06 to 1.03) and for anorectal C. trachomatis (adjusted OR, 0.26; 95% CI, 0.04 to 1.88). Oropharyngeal C. trachomatis was not evaluated due to small numbers.
TABLE 3.
Proportions of C. trachomatis and N. gonorrhoeae positive screening diagnoses and association with preceding use of specific agents of antibiotics from a total of 13,515 STI clinic consultations
| Antibiotic use |
C. trachomatis |
N. gonorrhoeae |
||||
|---|---|---|---|---|---|---|
| % (n) | ORa (95% CI) |
% (n) | OR (95% CI) |
|||
| Univariate | Adjustedb | Univariate | Adjusted | |||
| Doxycycline | ||||||
| No (n = 13,438) | 10.9 (1,467) | 1 | 1 | 1.9 (254) | 1 | 1 |
| Yes (n = 77) | 3.9 (3) | 0.33 (0.10–1.06)# | 0.37 (0.12–1.20) | 3.9 (3) | 2.10 (0.66–6.72) | 1.19 (0.41–3.41) |
| Azithromycin | ||||||
| No (n = 13,458) | 10.9 (1,461) | 1 | 1 | 1.9 (256) | 1 | 1 |
| Yes (n = 57) | 15.8 (9) | 1.54 (0.76–3.14) | 1.62 (0.80–3.32) | 1.8 (1) | 0.92 (0.13–6.68) | 0.69 (0.09–5.62) |
| Amoxicillin | ||||||
| No (n = 13,375) | 10.9 (1,458) | 1 | 1 | 1.9 (255) | 1 | 1 |
| Yes (n = 140) | 8.6 (12) | 0.77 (0.42–1.39) | 0.81 (0.45–1.50) | 1.4 (2) | 0.75 (0.18–3.03) | 0.54 (0.14–2.15) |
| Any tetracyclines | ||||||
| No (n = 13,416) | 10.9 (1,467) | 1 | 1 | 1.9 (254) | 1 | 1 |
| Yes (n = 99) | 3.0 (3) | 0.26 (0.08–0.81)* | 0.29 (0.09–0.91)* | 3.0 (3) | 1.62 (0.51–5.14) | 0.95 (0.33–2.71) |
| Any macrolides | ||||||
| No (n = 13,446) | 10.9 (1,461) | 1 | 1 | 1.9 (256) | 1 | 1 |
| Yes (n = 69) | 13.0 (9) | 1.23 (0.61–2.49) | 1.29 (0.64–2.60) | 1.4 (1) | 0.76 (0.11–5.49) | 0.63 (0.08–5.02) |
| Any quinolones | ||||||
| No (n = 13,466) | 10.8 (1,461) | 1 | 1 | 1.9 (254) | 1 | 1 |
| Yes (n = 49) | 18.4 (9) | 1.85 (0.88–3.87) | 2.11 (1.01–4.42)* | 6.1 (3) | 3.39 (1.04–11.1)* | 3.16 (0.78–12.77) |
| Any cephalosporins | ||||||
| No (n = 13,503) | 10.9 (1,469) | 1 | 1 | 1.9 (257) | NAc | NAc |
| Yes (n = 12) | 8.3 (1) | 0.75 (0.10–5.86) | 0.68 (0.07–6.56) | 0 (0) | ||
| Any penicillins | ||||||
| No (n = 13,315) | 10.9 (1,455) | 1 | 1 | 1.9 (254) | 1 | 1 |
| Yes (n = 200) | 7.0 (15) | 0.66 (0.39–1.12) | 0.70 (0.41–1.20) | 1.5 (3) | 0.78 (0.25–2.44) | 0.64 (0.20–2.01) |
| Nitrofurantoin/trimethoprim/fosfomycin | ||||||
| No (n = 13,410) | 10.9 (1,460) | 1 | 1 | 1.9 (255) | 1 | 1 |
| Yes (n = 105) | 9.5 (10) | 0.86 (0.45–1.65) | 1.91 (0.47–1.75) | 1.9 (2) | 1.00 (0.25–4.09) | 1.71 (0.41–7.08) |
Risk estimates for the total group are presented as estimates; they did not significantly differ between women, heterosexual men, and MSM (P interaction > 0.05). Analyses accounted for repeated measurements. #, P < 0.10; *, P < 0.05.
Adjusted for age, HIV status, gender, and sexual orientation.
NA, not assessed.
Prevalence of N. gonorrhoeae and association with antibiotic use.
The overall prevalence of N. gonorrhoeae was 1.9% (95% CI, 1.7% to 2.1%) (Table 1), and recent antibiotic use was reported by 13.1% (37/282) of patients who received N. gonorrhoeae diagnoses. Overall antibiotic use was not associated with N. gonorrhoeae, even when assessing associations for genital, anorectal, or oral N. gonorrhoeae (data not shown) or by sexual orientation (Table 2). In univariate analyses, quinolone use was positively associated with N. gonorrhoeae, while the risk estimate attenuated somewhat and became non-statistically significant in multivariate analyses (Table 3).
DISCUSSION
This is the first study to systematically assess the recent consumption of different antibiotic agents before STI testing and the impact of background antibiotic use on a C. trachomatis and N. gonorrhoeae diagnostic test result in a clinical setting. One out of eight clinic patients reported recent antibiotic use, of which only a minority were first-line treatments for C. trachomatis or N. gonorrhoeae. Tetracyclines were the only agents found to be associated with a lower C. trachomatis prevalence. In MSM, overall antibiotic use was associated with a lower anorectal C. trachomatis prevalence. Prior antibiotic use was not associated with N. gonorrhoeae.
The observed recent antibiotic consumption rate (12%) may be higher than expected (5.5%, based on the background antibiotic consumption in the Netherlands of 11.4 DDD per 1,000 persons per day, considering a typical course of 5 days with a daily dose in each course that equals the DDD, depending on distribution of courses among individuals) (11, 13). It is possible that the symptoms that led some patients to visit the STI clinic may have also prompted earlier health care visits (e.g., to their general practitioners). It also has been not uncommon in some communities globally to use antibiotics for prophylactic reasons prior to screening tests (18). The shares of the classes named were in line with reported rates from surveillance networks, with penicillins being most commonly used (13). Tetracyclines (comprised mostly of doxycycline) were associated with a lower C. trachomatis prevalence, confirming the ecological correlation found in other European countries between tetracyclines and a lower C. trachomatis prevalence (11).
While the consumption of macrolides per capita also correlated significantly with a lower chlamydia prevalence in Europe, the absence of an association between macrolides (azithromycin in particular) and C. trachomatis prevalence in our current study was notable. Azithromycin is commonly applied by using 500 mg daily for 3 to 5 days in non-C. trachomatis infections (e.g., in RTI); in C. trachomatis infections, the recommended dosage is higher but the duration is shorter (one 1,000-mg dose). This difference in regimens may explain the absence of an association between background azithromycin use and C. trachomatis. Still, differences in regimen were also present for doxycycline and other macrolides (e.g., the recommended dose for non-C. trachomatis sinusitis is lower than but of equal duration to that for C. trachomatis infections). Although azithromycin is the most commonly used treatment for C. trachomatis in many countries, its efficacy is currently under heavy debate, and several studies have demonstrated substantial posttreatment C. trachomatis detection (19, 20). While C. trachomatis DNA may remain detectable in up to 40% of patients after 3 weeks of treatment with azithromycin, it is unknown whether detection indicates a “persisting” C. trachomatis infection and whether detection rates differ between azithromycin and doxycycline (19, 20). There are no comparison data from other studies, as no agent-specific associations at an individual level have yet been reported (15–17).
We could not confirm earlier Australian observations of an inverse association between overall antibiotic use and C. trachomatis prevalence, except for MSM. This may be due to differences in the study populations; the people in the current study are higher-risk STI clinic attendees who potentially are more prone to acquiring a new STI after recent antibiotic use but before screening, attenuating associations in STI clinic populations. However, STI clinics tend to not test individuals with recent STI exposure (patients are asked to come back after a certain window phase), and therefore, such a possible effect is likely to be minimal. A more likely explanation is that lower shares of consumption of anti-C. trachomatis classes in the Netherlands than in Australia may explain the discrepancy. MSM in our study reported higher shares of tetracycline use than did heterosexual men or women; the latter group reported relatively high rates of use of nitrofurantoin, trimethoprim, or fosfomycin (i.e., agents not considered effective against C. trachomatis). To our knowledge, this is the first report on the association between recent antibiotic use and N. gonorrhoeae. We observed no association between recent antibiotic use and N. gonorrhoeae.
The implications for antibiotic use are broad and range from the contribution to antibiotic resistance, the utility of screening, and their impact on transmission and the epidemiology of infection. The impact of our results on clinical practice should be considered in the light of superfluous testing (of incidentally treated cases) or unwanted effects of subsequent treatments (i.e., treatment interactions or induction of treatment-resistant N. gonorrhoeae isolates). For N. gonorrhoeae, superfluous testing is likely not a problem, as ceftriaxone (first-line N. gonorrhoeae treatment) is infrequently used, and hence its impact on incidentally treated N. gonorrhoeae cases is probably limited. For C. trachomatis, one may argue that patients who had been treated recently (e.g., with azithromycin or doxycycline) should not be screened again for C. trachomatis, as they could be assumed to have been cured (treated) or to have acquired their infection very recently (after antibiotic use). In both cases they would not be eligible for screening. However, macrolide (or azithromycin) use was not associated with a lower C. trachomatis prevalence, and in those patients who had used tetracyclines, the C. trachomatis prevalence was low (3%) but not completely zero. As noted before, it is yet unknown whether detected C. trachomatis DNA really indicates a “persistent” infection in treated cases, since no laboratory tests that can test this exist.
Antimicrobial resistance and overuse of antimicrobials are considered serious threats, especially to the treatment of N. gonorrhoeae (5). In 2011, the European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP) found that 7.6% of isolates were resistant to cefotaxime (2.3% in the Netherlands) (RIVM, GRAS, 2013). Euro-GASP also detected isolates with decreased susceptibility to ceftriaxone for the first time (21, 22). Penicillin-, tetracycline-, quinolone-, and fluoroquinolone-resistant N. gonorrhoeae isolates are now disseminated globally.
Of all the patients with diagnosed N. gonorrhoeae in our study, 13% reported recent antibiotic use. Potential induced resistance to first-line N. gonorrhoeae treatment (ceftriaxone) does not (yet) seem to be an important factor in current clinical treatment of N. gonorrhoeae. However, N. gonorrhoeae seems to retain resistance to several classes of antimicrobials, even when the antimicrobials in question are discontinued. Hence, resistance to other antimicrobials may still be a point of concern.
The prevalence of N. gonorrhoeae and C. trachomatis in patients who used quinolones was higher than in those who did not use quinolones. This finding should be interpreted with caution due to relatively low numbers. Nevertheless, it may reflect the possibility that symptoms related to C. trachomatis and N. gonorrhoeae have caused patients to use quinolones before visiting the STI clinic for further testing. On the other hand, it could be hypothesized that the marginally positive association may reflect possible increased susceptibility due to its effect on protective alternative microbiota (23). Finally, the possible interaction (antagonistic or synergic) effects between treatments should always be carefully considered in clinical practice for persons who are currently using antibiotics. Altogether, the impact of background antibiotic use on STI clinic practice seems relatively limited so far, although this may change with increasing antibiotic consumption and may be different in countries with a higher background antibiotic consumption.
This study has several limitations. First, the information on antibiotic use was based on self-reporting, which is subject to both under- and overreporting biases. Second, the specific antibiotic agent could not be identified in two-thirds of consultations. The reported indications were not attributed to a specific agent, as the first-line regimens and their use in practice did not refer to a single agent or class of antibiotics. This limited our analyses to some extent, especially by largely underestimating the prevalence of use of specific agents in the total population and possibly attenuating observed associations with STI prevalence. We have no reason to assume that potential reporting bias may have affected the share of reported agents (as displayed in Fig. 2). While this study is the only study to date to report on specific agents of background antibiotic use at an individual level in a clinic setting, numbers for some agents were small, limiting statistical analyses for detecting associations. Third, no information was available about the exact dosage, start date, and duration. There was also no information about whether a client currently used antibiotics or had stopped more than a week ago. In clinical practice, it would be useful to know whether treatment was current or less recent. When designing future studies, these issues need to be taken into account, for example, by prospective systematic assessment of antibiotic consumption by a trained interviewer. When available, a review of prescribing records would limit the flaws introduced by self-reports. Fourth, it is likely that nongenital infections were missed as anorectal or oropharyngeal C. trachomatis and that N. gonorrhoeae was not tested in all individuals, possible attenuating associations with antibiotic use. However, potential underestimation of risk estimates is likely minimal, as restricting the data to people tested at all anatomic sites revealed highly similar results (i.e., for women the OR was 0.86 [95% CI, 0.50 to 1.49; total group OR,1.09], for heterosexual men it was 0.86 [95% CI, 0.34 to 2.20; total group OR, 0.74], and for MSM it was 0.42 [95% CI, 0.22 to 0.82; total group OR, 0.49]).
In conclusion, recent antibiotic use is common; one of eight clinic patients reported it before being screened for STI. Tetracyclines are associated with a lower C. trachomatis prevalence, while there is a notable absence of an association between C. trachomatis and azithromycin. Some patients who tested positive for C. trachomatis and N. gonorrhoeae had very recently been treated with antibiotics, so possible interactions with current clinic treatments need to be carefully considered. Still, a large part of the reported antibiotics are not first-line treatments against C. trachomatis or N. gonorrhoeae. The impact of background antibiotic use on STI clinic practice seems to be relatively limited, although that may be different in countries with a higher background antibiotic consumption.
ACKNOWLEDGMENTS
We thank the staff of the STI clinic, Helen Sijstermans (Health Service South Limburg), and Kevin Theunissen (Health Service South Limburg) for assistance in retrieving the data.
There are no conflicts of interest. The corresponding author has had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding was investigator initiated.
N.H.T.M.D.-M. analyzed the data and wrote the manuscript; all authors contributed to the final draft of the manuscript.
REFERENCES
- 1.Turner CF, Rogers SM, Miller HG, Miller WC, Gribble JN, Chromy JR, Leone PA, Cooley PC, Quinn TC, Zenilman JM. 2002. Untreated gonococcal and chlamydial infection in a probability sample of adults. JAMA 287:726–733. doi: 10.1001/jama.287.6.726. [DOI] [PubMed] [Google Scholar]
- 2.Senior K. 2012. Chlamydia: a much underestimated STI. Lancet Infect Dis 12:517–518. doi: 10.1016/S1473-3099(12)70161-5. [DOI] [PubMed] [Google Scholar]
- 3.Davies SC, Fowler T, Watson J, Livermore DM, Walker D. 2013. Annual report of the Chief Medical Officer: infection and the rise of antimicrobial resistance. Lancet 381:1606–1609. doi: 10.1016/S0140-6736(13)60604-2. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. 2012. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 5.Low N, Unemo M, Skov Jensen J, Breuer J, Stephenson J. 2014. Molecular diagnostics for gonorrhoea: implications for antimicrobial resistance and the threat of untreatable gonorrhoea. PLoS Med 11:e1001598. doi: 10.1371/journal.pmed.1001598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price MJ, Ades AE, De Angelis D, Welton NJ, Macleod J, Soldan K, Simms I, Turner K, Horner PJ. 2013. Risk of pelvic inflammatory disease following Chlamydia trachomatis infection: analysis of prospective studies with a multistate model. Am J Epidemiol 178:484–492. doi: 10.1093/aje/kws583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2010. STD treatment guidelines. MMWR Recomm Rep 59:45. [Google Scholar]
- 8.British Association for Sexual Health and HIV. 2010. Chlamydia trachomatis UK testing guidelines. British Association for Sexual Health and HIV, Macclesfield, Cheshire, England: http://www.bashh.org/documents/3352.pdf. [Google Scholar]
- 9.Westrom L. 1980. Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. Am J Obstet Gynecol 138:880–892. [DOI] [PubMed] [Google Scholar]
- 10.O'Rourke KM, Fairley CK, Samaranayake A, Collignon P, Hocking JS. 2009. Trends in Chlamydia positivity over time among women in Melbourne Australia, 2003 to 2007. Sex Transm Dis 36:763–767. doi: 10.1097/OLQ.0b013e3181b12765. [DOI] [PubMed] [Google Scholar]
- 11.Ginige S, Chen MY, Hocking JS, Read TR, Fairley CK. 2006. Antibiotic consumption and chlamydia prevalence in international studies. Sex Health 3:221–224. doi: 10.1071/SH06013. [DOI] [PubMed] [Google Scholar]
- 12.Ferech M, Coenen S, Malhotra-Kumar S, Dvorakova K, Hendrickx E, Suetens C, Goossens H, ESAC Project Group . 2006. European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe. J Antimicrob Chemother 58:401–407. doi: 10.1093/jac/dkl188. [DOI] [PubMed] [Google Scholar]
- 13.European Centre for Disease Prevention and Control. 2014. Surveillance of antimicrobial consumption in Europe 2011. ECDC, Stockholm, Sweden. [Google Scholar]
- 14.Land JA, Van Bergen JE, Morré SA, Postma MJ. 2010. Epidemiology of Chlamydia trachomatis infection in women and the cost-effectiveness of screening. Hum Reprod Update 16:189–204. doi: 10.1093/humupd/dmp035. [DOI] [PubMed] [Google Scholar]
- 15.Walker J, Tabrizi SN, Fairley CK, Chen MY, Bradshaw CS, Twin J, Taylor N, Donovan B, Kaldor JM, McNamee K, Urban E, Walker S, Currie M, Birden H, Bowden F, Gunn J, Pirotta M, Gurrin L, Harindra V, Garland SM, Hocking JS. 2012. Chlamydia trachomatis incidence and re-infection among young women—behavioural and microbiological characteristics. PLoS One 7:e37778. doi: 10.1371/journal.pone.0037778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen MY, Fairley CK, De Guingand D, Hocking J, Tabrizi S, Wallace EM, Grover S, Gurrin L, Carter R, Pirotta M, Garland S. 2009. Screening pregnant women for chlamydia: what are the predictors of infection? Sex Transm Infect 85:31–35. doi: 10.1136/sti.2008.030700. [DOI] [PubMed] [Google Scholar]
- 17.Chen MY, Rohrsheim R, Donovan B. 2007. The differing profiles of symptomatic and asymptomatic Chlamydia trachomatis-infected men in a clinical setting. Int J STD AIDS 18:384–388. doi: 10.1258/095646207781024810. [DOI] [PubMed] [Google Scholar]
- 18.Klausner JD, Aplasca MR, Mesola VP, Bolan G, Whittington WL, Holmes KK. 1999. Correlates of gonococcal infection and of antimicrobial-resistant Neisseria gonorrhoeae among female sex workers, Republic of the Philippines, 1996–1997. J Infect Dis 179:729–733. [DOI] [PubMed] [Google Scholar]
- 19.Dukers-Muijrers NH, Speksnijder AG, Morre SA, Wolffs PF, van der Sande MA, Brink AA, van den Broek IV, Werner MI, Hoebe CJ. 2013. Detection of anorectal and cervicovaginal Chlamydia trachomatis infections following azithromycin treatment: prospective cohort study with multiple time-sequential measures of rRNA, DNA, quantitative load and symptoms. PLoS One 14:e81236. doi: 10.1371/journal.pone.0081236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hathorn E, Opie C, Goold P. 2012. What is the appropriate treatment for the management of rectal Chlamydia trachomatis in men and women? Sex Transm Infect 88:352e354. doi: 10.1136/sextrans-2011-050466. [DOI] [PubMed] [Google Scholar]
- 21.European Centre for Disease Prevention and Control. 2012. Response plan to control and manage the threat of multidrug-resistant gonorrhoea in Europe. ECDC, Stockholm, Sweden. [Google Scholar]
- 22.National Institute for Public Health and the Environment. 2014. Sexually transmitted infections, including HIV, in The Netherlands, 2013. Report number 150002005/2014 National Institute for Public Health and the Environment, Bilthoven, the Netherlands. [Google Scholar]
- 23.Stokholm J, Schjørring S, Eskildsen CE, Pedersen L, Bischoff AL, Følsgaard N, Carson CG, Chawes BL, Bønnelykke K, Mølgaard A, Jacobsson B, Krogfelt KA, Bisgaard H. 2014. Antibiotic use during pregnancy alters the commensal vaginal microbiota. Clin Microbiol Infect 20:629–635. doi: 10.1111/1469-0691.12411. [DOI] [PubMed] [Google Scholar]