Abstract
New regimens based on two or more novel agents are sought in order to shorten or simplify the treatment of both drug-susceptible and drug-resistant forms of tuberculosis. PA-824 is a nitroimidazo-oxazine now in phase II trials and has shown significant early bactericidal activity alone and in combination with the newly approved agent bedaquiline or with pyrazinamide with or without moxifloxacin. While the development of PA-824 continues, a potential next-generation derivative, TBA-354, has been discovered to have in vitro potency superior to that of PA-824 and greater metabolic stability than that of the other nitroimidazole derivative in clinical development, delamanid. In the present study, we compared the activities of PA-824 and TBA-354 as monotherapies in murine models of the initial intensive and continuation phases of treatment, as well as in combination with bedaquiline plus pyrazinamide, sutezolid, and/or clofazimine. The monotherapy studies demonstrated that TBA-354 is 5 to 10 times more potent than PA-824, but selected mutants are cross-resistant to PA-824 and delamanid. The combination studies revealed that TBA-354 is 2 to 4 times more potent than PA-824 when combined with bedaquiline, and when administered at a dose equivalent to that of PA-824, TBA-354 demonstrated superior sterilizing efficacy. Perhaps most importantly, the addition of either nitroimidazole significantly improved the sterilizing activities of bedaquiline and sutezolid, with or without pyrazinamide, confirming the value of each agent in this potentially universally active short-course regimen.
INTRODUCTION
Novel regimens composed mostly or entirely of new drugs having potent activity against Mycobacterium tuberculosis and no cross-resistance with existing agents are needed in order to shorten and simplify treatment for tuberculosis (TB), including multidrug-resistant and more extensively drug-resistant TB (DR-TB). Several novel agents in clinical development have demonstrated the potential to shorten TB treatment in animal models (1–8) and clinical trials (9–11). Among them, the diarylquinoline bedaquiline (BDQ) (formerly known as TMC207) recently received accelerated approval from the U.S. Food and Drug Administration for the treatment of DR-TB. We recently reported promising results in a murine model of TB, with regimens composed of BDQ plus two other new drugs in clinical development, the oxazolidinone PNU-100480 (PNU) (also known as sutezolid) and the nitroimidazole PA-824, with or without clofazimine (CFZ), which have greater sterilizing activities than that of the first-line regimen of rifampin (RIF), isoniazid (INH), and pyrazinamide (PZA) (12). Combinations of BDQ and PA-824 (BDQ+PA-824), BDQ+PNU, or BDQ+CFZ with PZA had even greater treatment-shortening ability, suggesting that 4-drug combinations of BDQ+PZA plus PA-824, PNU, and/or CFZ may offer potential as shorter (i.e., ≤4 months) regimens against PZA-susceptible isolates, while still constituting effective short-course (i.e., ≤6 months) regimens against isolates resistant to PZA (12, 13), which are quite common among DR-TB cases (9, 14, 15). Combinations of BDQ+PZA plus PA-824 and/or CFZ are currently under study in a clinical trial designed to evaluate their early bactericidal activity (EBA) over the first 14 days of treatment (ClinicalTrials.gov registration no. NCT01691534). The two-drug combinations of BDQ+PZA, BDQ+PA-824, and PA-824+PZA have already shown greater EBA in the sputum samples from pulmonary TB patients over the first 14 days of treatment than that with monotherapy with BDQ or PA-824, respectively (11).
While PA-824 continues to be evaluated in trials designed to understand its potential contribution to novel regimens for TB, including DR-TB, the Global Alliance for TB Drug Development has pursued the synthesis and evaluation of >1,000 nitroimidazole analogs (16–18). This process identified TBA-354 (compound 93 in Kmentova et al. [17]) as a potential next-generation nitroimidazole with more potent in vitro activity against M. tuberculosis than that of PA-824, efficacy superior to that of PA-824 when administered at 100 mg/kg of body weight/day in acute and chronic murine infection models of TB, and greater metabolic stability than that of delamanid (17, 33); this indicates a potentially superior pharmacokinetic profile compared to this nitroimidazole, which is currently being administered twice daily in a clinical trial (ClinicalTrials.gov registration no. NCT 01424670).
In the present series of experiments, we sought to compare the dose-ranging activity of TBA-354 to that of PA-824 and delamanid administered alone in several murine models of TB and to compare the contributions of TBA-354 and PA-824 to novel drug regimens containing BDQ in combination with PZA, PNU, PZA+PNU, or PZA+CFZ. The results reveal that TBA-354 is more potent than PA-824 when administered alone or in combination with novel regimens and that either nitroimidazole significantly improves the sterilizing activities of novel drug combinations containing BDQ and PNU.
MATERIALS AND METHODS
Mycobacterial strain.
M. tuberculosis strain H37Rv was passaged in mice, frozen in aliquots, and subcultured prior to infection in Middlebrook 7H9 broth with 10% oleic acid-albumin-dextrose-catalase (OADC) (Fisher, Pittsburgh, PA) and 0.05% Tween 80.
Antimicrobials.
INH, RIF, PZA, BDQ, PA-824, PNU, and CFZ were obtained and formulated for oral administration, as previously described (12, 13, 19–22), except that in experiment 4, CFZ was formulated in the same acidified 20% hydroxypropyl-β-cyclodextrin solution as BDQ. TBA-354 and delamanid were supplied by the Global Alliance for TB Drug Development and were formulated in the same cyclodextrin micelle (CM-2) formulation as PA-824.
Aerosol infection with M. tuberculosis.
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University. Experiments 1, 3, and 4 used a high-dose aerosol infection, as previously described (6). Briefly, 5-to-6-week-old female BALB/c mice (Charles River Laboratories, Wilmington, MA) were infected with M. tuberculosis H37Rv, using the inhalation exposure system (Glas-Col, Terre Haute, IN) and a fresh log-phase broth culture (optical density at 600 nm, 0.8 to 1.0), with the goal of implanting between 3.5 and 4.0 log10 CFU in the lungs of each mouse. Experiment 2 used a low-dose aerosol infection, as previously described (23), to establish a stable and more chronic infection model. BALB/c mice that were 4 to 6 weeks old were infected using a 1:10 dilution of a log-phase culture. Two mice from each aerosol infection run (experiments 1, 3, and 4) or four mice from the only run (experiment 2) were humanely killed 1 day after infection and on the day of treatment initiation (D0) to determine the number of bacteria implanted in the lungs and at the start of treatment, respectively.
Chemotherapy.
The mice were block randomized by run to experimental arms prior to treatment. Treatment was initiated 13 to 14 days after infection in experiments 1, 3, and 4, and treatment began 42 days after infection in experiment 2. The treatment was administered once daily, 5 days per week, by gavage. The drug doses were 10 mg/kg of INH, 10 mg/kg of RIF, 150 mg/kg of PZA, 25 mg/kg of BDQ, 50 mg/kg of PNU, and 20 mg/kg of CFZ (13, 22, 24).
In experiment 1, the control mice received RIF+INH+PZA. The test mice received PA-824 alone (10, 30, 100, 300, or 600 mg/kg) or TBA-354 or delamanid alone (3, 10, 30, or 100 mg/kg). Treatment was administered for up to 8 weeks. In experiment 2, all mice received RIF+INH+PZA for the first 4 weeks of treatment. After that, the negative controls received no treatment, the positive controls received RIF+INH, and the test groups received 50 mg/kg of PA-824 alone or 10 mg/kg of TBA-354 alone. The total treatment duration was 12 weeks.
In experiment 3, the positive-control mice received 8 weeks of RIF+INH+PZA, followed by up to 8 weeks of RIF+INH. The remaining mice received either BDQ+PZA or BDQ+PNU, alone or in combination with either 50 mg/kg of PA-824 or 10 or 50 mg/kg of TBA-354. In experiment 4, the mice received BDQ+PZA+CFZ or BDQ+PZA+PNU, alone or in combination with 50 mg/kg of PA-824 or 25 or 50 mg/kg of TBA-354. An additional group received BDQ+PZA+CFZ+PNU. PA-824 and TBA-354 or a sham treatment with the CM-2 vehicle was administered immediately after the dose of BDQ and/or PZA, which were formulated together, in order to be consistent with prior combination experiments (12, 13). PNU or CFZ was administered ≥4 h later. For the BDQ+PZA+CFZ+PNU regimen, BDQ, PZA, and CFZ were formulated and administered together, and PNU was given 4 h later. The drugs were administered for up to 16 weeks in experiment 3 and up to 8 weeks in experiment 4.
Assessment of treatment efficacy.
Efficacy was assessed on the basis of the lung CFU counts at selected time points during treatment (a measure of bactericidal activity) and the proportion of mice with culture-positive relapse after treatment completion (a measure of sterilizing activity). Quantitative cultures of lung homogenates were performed in parallel on 7H11 agar enriched with OADC (basic agar) and on basic agar supplemented with 0.4% activated charcoal to reduce drug carryover effects (13). The plates were incubated for up to 42 days at 37°C before the final CFU counts were determined. The lung CFU counts were assessed in four or five mice per treatment group at each time point in experiments 1 and 2 or experiment 3, respectively. The proportion of mice with culture-positive relapse was determined by holding cohorts of 15 mice for 12 additional weeks after the completion of treatment and then sacrificing them to determine the proportion with positive lung cultures, defined as ≥1 CFU of M. tuberculosis detected after plating the entire lung homogenate onto five 7H11 plates. Four of the five plates were supplemented with 0.4% activated charcoal. The remaining plate without charcoal was used to assess the effect of carryover at the time of relapse assessment.
Selection of drug-resistant isolates.
After 8 weeks of treatment in experiment 1, the lung homogenates were also plated directly on 7H11 agar containing PA-824, delamanid, or TBA-354 at 1, 0.25, or 0.25 μg/ml, respectively, to assay for drug-resistant mutants. To evaluate for cross-resistance between the nitroimidazoles, resistant colonies isolated from 2 mice each from the 2 highest dose groups were scraped together and homogenized with glass beads. The resulting suspension was left to settle for 30 min before being plated in serial 10-fold dilutions on plates containing 1 μg/ml PA-824 or 0.25 μg/ml delamanid or TBA-354.
Statistical analysis.
The CFU counts (x) were log transformed as (x + 1) before analysis, and the group means were compared by one-way analysis of variance with Dunnett's posttest to control for multiple comparisons. Group relapse proportions were compared using Fisher's exact test, adjusting for multiple comparisons. GraphPad Prism version 4 (GraphPad, San Diego, CA) was used for all analyses. The use of 15 mice per group for relapse assessment provides >80% power to detect 40-percentage-point differences in the relapse rate, after setting α at 0.01 to adjust for up to 5 simultaneous two-sided comparisons. Smaller differences may not be meaningful in terms of shortening the duration of treatment.
RESULTS
Experiment 1. Dose-ranging activity of nitroimidazoles in the initial phase of treatment.
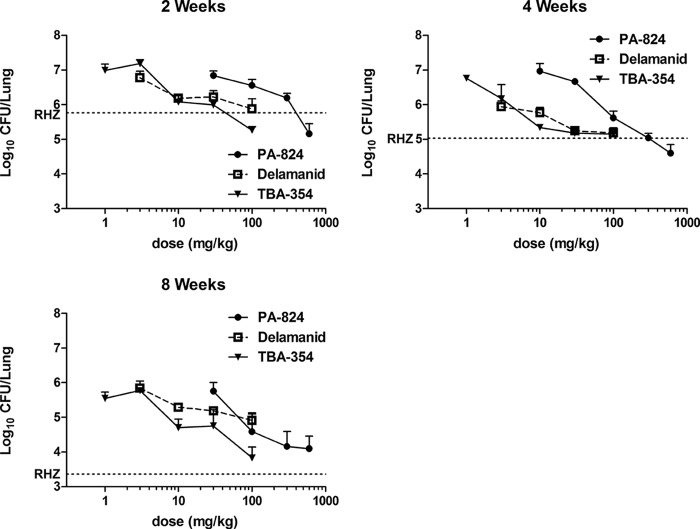
Previous work demonstrated the superior efficacy of TBA-354 compared to that of PA-824 when administered at 100 mg/kg/day, 5 days per week, for 3 weeks in acute and chronic low-dose aerosol models of TB in mice. In the same model of chronic murine TB, TBA-354 demonstrated superior efficacy to that of delamanid when administered at 30 mg/kg/day, 5 days per week, for 8 weeks (17). A separate study performed in a more chronic infection model demonstrated the dose-dependent activity of delamanid that was more potent than that of PA-824 (5). In our initial experiment, we sought to compare the dose-ranging efficacies of these three compounds at 3 time points during the initial phase of treatment, using a high-dose aerosol infection model in BALB/c mice.
In the model used, the typical bacterial burden in the lungs at the start of treatment (D0) is approximately 7.5 log10 CFU. However, a technical error occurred with the plating of D0 lung homogenates, requiring the replating of refrigerated homogenates nearly 4 weeks later. The resultant mean CFU count of 6.19 log10 is lower than the expected result, presumably due to a loss of viability prior to replating. After 8 weeks of treatment with RIF+INH+PZA, the mean lung CFU count fell to 3.36, consistent with the expected 4.0- to 4.5-log kill typically observed in this model (6, 12, 25). All nitroimidazoles displayed time-dependent and dose-dependent bactericidal activities, with TBA-354 and delamanid being approximately 10 times more potent than PA-824 (Fig. 1). The greatest effect of PA-824 (at 600 mg/kg), observed after 8 weeks treatment, was the same as that of TBA-354 (at 100 mg/kg), although drug-resistant mutants were selected and fully replaced the drug-susceptible population by week 8 in the groups receiving 300 to 600 mg/kg PA-824 and 100 mg/kg TBA-354 (Table 1); this limited the bactericidal effect. The greatest effect of delamanid was not as great as that of PA-824 or TBA-354, and this did not appear to be due to the replacement of the susceptible population with resistant mutants, although the selective amplification of mutants susceptible to 0.25 μg/ml delamanid cannot be excluded. Nevertheless, the mutants selected at the highest doses of each nitroimidazole exhibited cross-resistance to all 3 drugs at the concentrations tested (data not shown).
FIG 1.
Lung log10 CFU counts after 2, 4, and 8 weeks of treatment with increasing doses of nitroimidazoles in experiment 1. The dotted line represents the mean CFU count for the RIF+INH+PZA (RHZ) control group. Error bars represent the standard deviation of the mean.
TABLE 1.
Selection of nitroimidazole-resistant mutants after 8 weeks of monotherapy in experiment 1
| Drug, dose (mg/kg) | Mean lung log10 CFU (± SD) | % CFU (no. of mice with isolates) resistant to: |
||
|---|---|---|---|---|
| 1 μg/ml PA-824 | 0.25 μg/ml delamanid | 0.25 μg/ml TBA-354 | ||
| PA-824, 300 | 4.16 ± 0.43 | 48 (1) | ||
| 67–100 (3) | ||||
| PA-824, 600 | 4.17 ± 0.43 | 80–100 (3)a | ||
| Delamanid, 30 | 5.19 ± 0.01 | 0.3–1.3 (3) | ||
| 7 (1) | ||||
| Delamanid, 100 | 4.91 ± 0.21 | 0.01 (1) | ||
| 20–22 (3) | ||||
| TBA-354, 10 | 4.70 ± 0.24 | 2–20 (3)a | ||
| TBA-354, 30 | 4.75 ± 0.47 | 1–10 (4) | ||
| TBA-354, 100 | 3.83 ± 0.32 | 34 (1) | ||
| 87–100 (3) | ||||
The isolate from 1 of 4 mice was unevaluable due to contamination of the culture.
Experiment 2. Comparative activities of TBA-354 and PA-824 in the continuation phase of treatment.
Bacilli surviving into the continuation phase of TB therapy determine the duration of treatment needed to cure TB and may differ in their phenotypic drug susceptibilities compared to those that predominate at the outset of treatment. We further compared the efficacies of TBA-354 and PA-824 following 4 weeks of treatment with RIF+INH+PZA to select for persistent bacteria (20) in BALB/c mice. Both PA-824 and TBA-354 exhibited bactericidal activities during the continuation phase. Consistent with the observations in experiment 1, TBA-354 was ≥5 times more potent than PA-824. However, while PA-824 and TBA-354 each reduced the number of persisting bacilli by ≥1 log10, neither drug was as effective as RIF+INH in this regard (Table 2).
TABLE 2.
Activities of PA-824 and TBA-354 in a continuation phase model in experiment 2
| Treatment (dose [mg/kg]) | Mean ± SD lung log10 CFU on: |
||||
|---|---|---|---|---|---|
| Day −42 | Day 0 | Wk 4 | Wk 8 | Wk 12 | |
| Day 0 to wk 4 | |||||
| RHZ | 2.97 ± 0.02 | 7.00 ± 0.14 | 3.58 ± 0.25 | ||
| Wk 4 to 12 | |||||
| None | 4.94 ± 0.34 | ||||
| RH | 0.80 ± 0.28 | ||||
| PA-824 (50) | 3.19 ± 0.33 | 2.47 ± 0.06 | |||
| TBA-354 (10) | 2.65 ± 0.15 | 2.10 ± 0.08 | |||
Experiment 3. Contribution of TBA-354 and PA-824 to novel combinations based on BDQ+PZA and BDQ+PNU.
To evaluate the ability of TBA-354 to shorten the duration of therapy necessary to prevent relapse in mice, we compared the contributions of TBA-354 and PA-824 to novel three-drug combinations containing BDQ. Based on the superior potency of TBA-354 observed in experiments 1 and 2, 50 mg/kg of PA-824 was compared to 10 mg/kg of TBA-354. A dose of 50 mg/kg of TBA-354 was also included to assess the potential for an increased contribution by that compound at a higher dose.
Lung CFU counts during treatment.
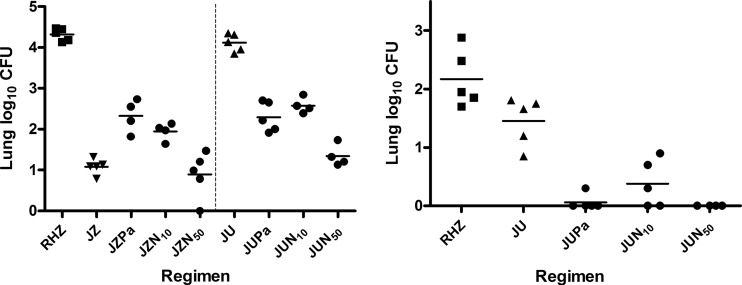
The day after aerosol infection, the mean lung log10 CFU count ± standard deviation (SD) was 3.29 ± 0.12. By D0, the mean ± SD CFU count had increased to 7.08 ± 0.17. The lung CFU counts observed after 4 and 8 weeks of treatment are presented in Fig. 2. After 4 weeks of treatment (W4), RIF+INH+PZA reduced the mean CFU count by 2.76 log to 4.32 log10. The CFU counts were lower in the mice treated with any BDQ+PZA-containing regimen and any 3-drug BDQ+PNU-containing regimen than in those that received RIF+INH+PZA treatment (P < 0.01). The CFU counts were higher among the mice receiving BDQ+PZA+PA-824 (P < 0.01) and BDQ+PZA plus 10 mg/kg of TBA-354 (P < 0.05) compared to BDQ+PZA alone and BDQ+PZA plus 50 mg/kg of TBA-354, indicating the antagonism of PA-824 and TBA-354 on the BDQ+PZA combination, which was not observed when the higher dose of TBA-354 was used. On the contrary, the addition of either nitroimidazole to BDQ+PNU produced lower CFU counts than those with BDQ+PNU alone (P < 0.01). Despite the clear superiority of BDQ+PZA over BDQ+PNU, no BDQ+PZA-containing 3-drug regimen was significantly better than its BDQ+PNU-containing 3-drug counterpart. Indeed, BDQ+PNU plus 50 mg/kg of TBA-354 produced lower CFU counts than BDQ+PZA+PA-824 (P < 0.01) and BDQ+PZA plus 10 mg/kg of TBA-354 (P < 0.01).
FIG 2.
Lung CFU counts following 4 weeks (left) and 8 weeks (right) of treatment in experiment 3. J, bedaquiline; Pa, PA-824; N, TBA-354; U, sutezolid. The subscript numbers in the regimens represent doses in mg/kg. The horizontal lines represent the mean CFU count. The vertical dotted line separates the JZ-containing regimens from the JU-containing regimens.
After 8 weeks of treatment (W8), the lung CFU counts were lower among mice treated with any BDQ+PNU-containing regimen than those treated with RIF+INH+PZA (P < 0.05 for BDQ+PNU, P < 0.01 for 3-drug regimens). Similarly, all 3-drug BDQ+PNU-containing regimens produced lower CFU counts than did BDQ+PNU alone (P < 0.01), and most mice were culture negative. In comparison, all mice remained culture positive after 8 weeks of RIF+INH+PZA plus an additional 4 weeks of RIF+INH.
Relapse after treatment completion.
The relapse results are displayed in Table 3. Regardless of the treatment received, all mice treated for only 4 weeks relapsed, except 1 of 15 mice receiving BDQ+PZA plus 50 mg/kg of TBA-354. While all mice receiving 8 weeks of RIF+INH+PZA and 4 weeks of RIF+INH remained culture positive at the end of treatment, most mice receiving a BDQ+PZA-containing regimen or BDQ+PNU plus 50 mg/kg of TBA-354 survived without relapse after just 8 weeks of treatment. A few more relapses were noted in mice receiving BDQ+PZA+PA-824 than those in BDQ+PZA and BDQ+PZA plus 50 mg/kg of TBA-354, but the difference was not statistically significant. On the other hand, mice receiving BDQ+PZA plus 10 mg/kg of TBA-354 had significantly more relapses than mice receiving BDQ+PZA or BDQ+PZA plus 50 mg/kg of TBA-354 (P < 0.0001). All mice receiving BDQ+PNU alone for 8 and 12 weeks relapsed. The addition of PA-824 or 50 mg/kg of TBA-354 to BDQ+PNU was associated with fewer relapses after 8 or 12 weeks of treatment. The addition of 10 mg/kg of TBA-354 was associated with fewer relapses than with BDQ+PNU alone (P = 0.0006) but more relapses than with BDQ+PNU+PA-824 (P = 0.02) after 12 weeks of treatment.
TABLE 3.
Relapse rates assessed 3 months after treatment completion in experiment 3
| Treatment (dose of PA-824 or TBA-354 [mg/kg]) | Proportion (no./total no. [%]) of mice relapsing after treatment for: |
||
|---|---|---|---|
| 4 wk | 8 wk | 12 wk | |
| BDQ+PZA | 15/15 (100) | 0/15 (0) | |
| BDQ+PZA+PA-824 (50) | 15/15 (100) | 3/15 (20) | |
| BDQ+PZA+TBA-354 (10) | 15/15 (100) | 6/15 (40)a | |
| BDQ+PZA+TBA-354 (50) | 14/15 (93) | 0/15 (0) | |
| BDQ+PNU | 14/15 (93) | 14/14 (100) | |
| BDQ+PNU+PA-824 (50) | 7/15 (47)b | 0/14 (0)c | |
| BDQ+PNU+TBA-354 (10) | 13/15 (87)d | 5/13 (38)e,f | |
| BDQ+PNU+TBA-354 (50) | 1/15 (7)c | 1/14 (7)c | |
P = 0.02 versus BDQ+PZA and BDQ+PZA+TBA-354 (50 mg/kg).
P = 0.002 versus BDQ+PNU.
P < 0.0001 versus BDQ+PNU.
P < 0.0001 versus BDQ+PNU+TBA-354 (50 mg/kg).
P < 0.0006 versus BDQ+PNU.
P = 0.02 versus BDQ+PNU+PA-824.
Experiment 4. Contribution of TBA-354 and PA-824 to novel combinations based on BDQ+PZA+PNU and BDQ+PZA+CFZ.
We previously reported the potent sterilizing activities of novel combinations of BDQ+PZA plus either PNU or CFZ in mice (12). After observing a beneficial effect of adding TBA-354 or PA-824 to the BDQ+PNU combination in experiment 3, we hypothesized that adding these nitroimidazoles to BDQ+PZA+PNU may be beneficial as well. As one of the most active combinations tested previously in mice (12), the BDQ+PZA+CFZ combination was also evaluated with and without a nitroimidazole. Based on the results of experiment 3 suggesting that 10 mg/kg of TBA-354 was not quite as effective as 50 mg/kg of PA-824 in the combinations tested, experiment 4 was used to evaluate the sterilizing activity of 25 mg/kg and 50 mg/kg of TBA-354 compared to that of 50 mg/kg of PA-824.
The day after aerosol infection, the mean lung log10 CFU count ± SD was 4.44 ± 0.10. By D0, the mean ± SD CFU count had increased to 7.95 ± 0.19. The relapse results are displayed in Table 4. Regardless of the treatment received, all mice treated for only 3 weeks relapsed. Treatment with BDQ+PZA+PNU and BDQ+PZA+CFZ for 4 weeks resulted in relapse in 15 (100%) and 13 (87%) of 15 mice, respectively. The addition of PA-824, 25 mg/kg of TBA-354, and 50 mg/kg of TBA-354 to BDQ+PZA+PNU reduced the proportions that relapsed to 10 (67%), 7 (47%), and 6 (40%) of 15 mice (P = 0.04, 0.002, and 0.0007 versus BDQ+PZA+PNU), respectively. On the other hand, the addition of PA-824, 25 mg/kg of TBA-354, and 50 mg/kg of TBA-354 to BDQ+PZA+CFZ did not reduce the proportion that relapsed.
TABLE 4.
Relapse rates assessed 3 months after treatment completion in experiment 4
| Treatment with BDQ+PZA plus the indicated drug(s) (dose of PA-824 or TBA-354 [mg/kg]) | Proportion (no./total no. [%]) of mice relapsing after treatment for: |
|||
|---|---|---|---|---|
| 3 wk | 4 wk | 6 wk | 8 wk | |
| CFZ | 14/14 (100) | 13/15 (87)a | 5/15 (33) | |
| CFZ+PA-824 (50) | ND | 13/14 (93) | 14/15 (93)b | |
| CFZ+TBA-354 (25) | 15/15 (100) | 15/15 (100) | 9/15 (60) | |
| CFZ+TBA-354 (50) | 14/14 (100) | 14/15 (93) | ||
| CFZ+PNU | 15/15 (100) | 13/15 (87) | ||
| PNU | ND | 15/15 (100) | 14/15 (93) | 4/15 (27) |
| PNU+PA-824 (50) | ND | 10/15 (67)c | 1/15 (7)a | 0/15 (0) |
| PNU+TBA-354 (25) | ND | 7/15 (47)d | 0/15 (0)a | |
| PNU+TBA-354 (50) | 15/15 (100) | 6/15 (40)e | 0/15 (0)a | |
P < 0.0001 versus BDQ+PZA+PNU.
P = 0.002 versus BDQ+PZA+CFZ.
P = 0.04 versus BDQ+PZA+PNU.
P = 0.002 versus BDQ+PZA+PNU.
P = 0.0007 versus BDQ+PZA+PNU.
Treatment with BDQ+PZA+PNU and BDQ+PZA+CFZ for 6 weeks resulted in relapse in 14 (93%) and 5 (33%) of 15 mice, respectively. The addition of PA-824 and either dose of TBA-354 to BDQ+PZA+PNU reduced the proportions that relapsed to 1 (7%) and 0 (0%) of 15 mice, respectively (P < 0.0001 versus BDQ+PZA+PNU). On the other hand, the addition of PA-824 and 25 mg/kg of TBA-354 to BDQ+PZA+CFZ increased the proportions that relapsed to 14 (93%) and 9 (60%) of 15 mice, although the difference was statistically significant only for the addition of PA-824 (P = 0.002 versus BDQ+PZA+CFZ). The sterilizing activity of the BDQ+PZA+PNU combination was confirmed by the fact that only 4 (27%) of 15 mice relapsed after just 8 weeks of treatment.
DISCUSSION
In the present study, we compared the activities of PA-824 and the next-generation nitroimidazole derivative TBA-354 as monotherapies and in combination with other novel TB drugs in long-term relapse-based efficacy studies. The results demonstrate that TBA-354 is more potent than PA-824 and that both nitroimidazoles have the potential to contribute important sterilizing activity to novel drug combinations, which, if as effective in humans as in mice, could form the basis for universally active short-course TB regimens irrespective of the resistances to existing drugs.
In the monotherapy experiments, TBA-354 was 5 to 10 times more potent than PA-824, whether in the initial phase of treatment against an infection in which a substantial portion of the bacillary population was actively multiplying, or following 4 weeks of RIF+INH+PZA treatment in a model more representative of the continuation phase of therapy. Delamanid was included in the initial-phase experiment and showed a potency similar to TBA-354 but a smaller maximal effect than that of the other two nitroimidazoles, although this may have been due to a plateau in drug exposure or the selection of drug-resistant mutants, which eluded detection. Importantly, mutants resistant to all 3 nitroimidazoles were selected by each agent when used as monotherapy.
Our previous studies in both acute and chronic BALB/c mouse models have demonstrated an antagonistic effect of PA-824 on the antituberculosis activity of BDQ alone and combinations of BDQ with PZA or CFZ (13). Similar antagonistic effects of PA-824 on BDQ activity have been observed in whole-blood cultures (26). However, the only endpoint for which such antagonism has been demonstrated in mice is the change in the lung CFU count during the first 4 weeks of treatment (13). No significant antagonistic effect on relapse after treatment or on other BDQ-containing regimens has been demonstrated. Experiments 3 and 4 extend previous observations by showing that nitroimidazoles may antagonize the sterilizing activities of BDQ+PZA combinations with or without CFZ in this mouse model. However, the antagonistic effect was statistically significant only in experiment 3 when 10 mg/kg of TBA-354 (but not PA-824 or 50 mg/kg of TBA-354) was added to BDQ+PZA, as well as in experiment 4, when PA-824 (but not 25 or 50 mg/kg of TBA-354) was added to BDQ+PZA+CFZ. Thus, the magnitude of the antagonistic effect on these BDQ+PZA-containing combinations was modest and appeared to be inversely proportional to the dose and potency of the nitroimidazole, suggesting that the antagonism may not be observed as nitroimidazole potency and/or exposure increases. This finding provides further justification for additional trials to identify the nitroimidazole able to produce the greatest effect at its highest well-tolerated dose.
That the addition of a nitroimidazole to a BDQ-containing regimen does not always result in antagonism was also shown by the addition of PA-824 or TBA-354 to the combinations BDQ+PNU or BDQ+PZA+PNU, which significantly improved the bactericidal and sterilizing activities of those regimens in a dose-dependent fashion. These results reinforce and extend previous results showing the sterilizing activity of BDQ+PNU+PA-824 in mice (12) and support ongoing efforts to advance such truly novel regimens in clinical trials. The remarkable difference in the contribution of PA-824 and TBA-354 to the various BDQ-containing regimens studied here is determined by the presence or absence of PNU in the combination. In further support of a positive interaction between PNU and nitroimidazoles, PNU was more effective than CFZ when either drug was combined with BDQ+PZA and PA-824 or TBA-354 in experiment 4. This finding and a similar observation that PNU was more effective than CFZ when combined with BDQ+PA-824 (i.e., without PZA) (27) is all the more remarkable considering that CFZ is more effective than PNU when combined with BDQ+PZA in the absence of PA-824 (27). Taken together, these results indicate that the relative contribution of a nitroimidazole, PNU, and CFZ to BDQ-containing regimens may be regimen specific. The inclusion of PNU or another oxazolidinone may be preferred when a nitroimidazole is included in the regimen. These results also suggest that a nitroimidazole plus PNU or another oxazolidinone, with or without PZA, may be another strong nucleus for novel BDQ-sparing regimens. For example, the PA-824, moxifloxacin (MXF), and PZA regimen, which demonstrated bactericidal activity at least as great as that with standard therapy over the first 14 days in patient sputum samples (11), may further benefit from the addition of PNU or another oxazolidinone. PNU has more potent antituberculosis activity than that of the only currently marketed oxazolidinone, linezolid (LZD), in ex vivo whole-blood cultures and in the first 2 months of treatment in BALB/c mice (22, 28–30). However, our unpublished data indicate that the addition of LZD increases the bactericidal activity of the PZA+PA-824 combination in mice during the first 4 weeks of treatment. Whether regimens containing PA-824 in combination with LZD or other oxazolidinones in development (e.g., AZD 5847) will have sterilizing activity comparable to that of their PNU-containing counterparts warrants further study.
The next-generation nitroimidazole TBA-354 may offer advantages over PA-824 in the treatment of TB (33), and this deserves further study. In the monotherapy studies presented here, TBA-354 was 5 to 10 times more potent than PA-824. However, in the combination studies, 10 mg/kg of TBA-354 tended to underperform 50 mg/kg of PA-824. We have observed similar results when TBA-354 is substituted for PA-824 in the MXF+PZA+PA-824 combination (our unpublished data). Higher doses of TBA-354 (especially 50 mg/kg) outperformed 50 mg/kg of PA-824, including shortening the treatment duration needed to prevent relapse by an additional month when added to BDQ, PNU, and PZA. Therefore, TBA-354 may offer a significant advantage over PA-824 if such efficacious drug exposures are safely achieved in patients. First-in-human studies for TBA-354 will shed light on this potential. Delamanid had a potency similar to that of TBA-354 and superior to PA-824 in experiment 1. However, the serum delamanid concentrations produced by a 200-mg daily dose in TB patients are approximately 10 times lower than that of the same dose of PA-824, and its EBA is no greater (27, 31, 32). Thus, the comparative efficacies of PA-824 and delamanid will require future study in TB patients.
As we discussed recently (12, 13), experiments in these murine models have potentially important limitations related to differences in the pathology of M. tuberculosis infection between mice and humans and the use of a single laboratory strain of M. tuberculosis. The use of initial- and continuation-phase murine models and an evaluation of the nitroimidazoles alone and in combination should contribute to more generalizable results. In addition, the results presented here are in line with observations made previously using other TB models of mice infected with different laboratory strains (5, 17). Work using additional infecting strains and mouse models is ongoing in order to further explore and understand the antagonism between nitroimidazoles and BDQ that has been reported here and previously (23, 29). Additional studies to evaluate the most promising novel combinations in both C3HeB/FeJ mice and guinea pigs are also planned.
In conclusion, these experiments have confirmed and extended prior studies in their demonstration of the potential for truly novel drug combinations containing BDQ, PNU, and a nitroimidazole to provide a strong new foundation for universally active short-course regimens capable of effectively treating TB, irrespective of resistances to existing drugs. When the infecting isolates remain susceptible to PZA, the inclusion of this sterilizing agent is expected to further shorten treatment. While the remarkable treatment-shortening potentials of these regimens in mice cannot be assumed to translate directly to human TB, the results presented here certainly support future clinical trials to investigate the efficacies of these and other similar regimens. This work also confirms previous results indicating the superior potency of TBA-354 compared to that of PA-824. The next-generation nitroimidazole outperformed PA-824 at equivalent doses with monotherapy and combinations of drugs, including in its contribution to sterilizing efficacy. Further study will determine whether TBA-354 will be a more effective nitroimidazole component than PA-824 and delamanid. Future clinical and preclinical work is needed to assess whether sufficient drug exposures for this agent can be reached with safe and well-tolerated doses.
REFERENCES
- 1.Andries K, Gevers T, Lounis N. 2010. Bactericidal potencies of new regimens are not predictive of their sterilizing potencies in a murine model of tuberculosis. Antimicrob Agents Chemother 54:4540–4544. doi: 10.1128/AAC.00934-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibrahim M, Truffot-Pernot C, Andries K, Jarlier V, Veziris N. 2009. Sterilizing activity of R207910 (TMC207)-containing regimens in the murine model of tuberculosis. Am J Respir Crit Care Med 180:553–557. doi: 10.1164/rccm.200807-1152OC. [DOI] [PubMed] [Google Scholar]
- 3.Lenaerts AJ, Hoff D, Aly S, Ehlers S, Andries K, Cantarero L, Orme IM, Basaraba RJ. 2007. Location of persisting mycobacteria in a guinea pig model of tuberculosis revealed by R207910. Antimicrob Agents Chemother 51:3338–3345. doi: 10.1128/AAC.00276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veziris N, Ibrahim M, Lounis N, Andries K, Jarlier V. 2011. Sterilizing activity of second-line regimens containing TMC207 in a murine model of tuberculosis. PLoS One 6:e17556. doi: 10.1371/journal.pone.0017556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumoto M, Hashizume H, Tomishige T, Kawasaki M, Tsubouchi H, Sasaki H, Shimokawa Y, Komatsu M. 2006. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med 3:e466. doi: 10.1371/journal.pmed.0030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuermberger E, Tyagi S, Tasneen R, Williams KN, Almeida D, Rosenthal I, Grosset JH. 2008. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob Agents Chemother 52:1522–1524. doi: 10.1128/AAC.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tasneen R, Tyagi S, Williams K, Grosset J, Nuermberger E. 2008. Enhanced bactericidal activity of rifampin and/or pyrazinamide when combined with PA-824 in a murine model of tuberculosis. Antimicrob Agents Chemother 52:3664–3668. doi: 10.1128/AAC.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams KN, Brickner SJ, Stover CK, Zhu T, Ogden A, Tasneen R, Tyagi S, Grosset JH, Nuermberger EL. 2009. Addition of PNU-100480 to first-line drugs shortens the time needed to cure murine tuberculosis. Am J Respir Crit Care Med 180:371–376. doi: 10.1164/rccm.200904-0611OC. [DOI] [PubMed] [Google Scholar]
- 9.Diacon AH, Pym A, Grobusch M, Patientia R, Rustomjee R, Page-Shipp L, Pistorius C, Krause R, Bogoshi M, Churchyard G, Venter A, Allen J, Palomino JC, de Marez T, van Heeswijk RP, Lounis N, Meyvisch P, Verbeeck J, Parys W, de Beule K, Andries K, McNeely DF. 2009. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med 360:2397–2405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- 10.Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas-Vasquez DE, Gao M, Awad M, Park SK, Shim TS, Suh GY, Danilovits M, Ogata H, Kurve A, Chang J, Suzuki K, Tupasi T, Koh WJ, Seaworth B, Geiter LJ, Wells CD. 2012. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med 366:2151–2160. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- 11.Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, van Niekerk C, Everitt D, Winter H, Becker P, Mendel CM, Spigelman MK. 2012. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet 380:986–993. doi: 10.1016/S0140-6736(12)61080-0. [DOI] [PubMed] [Google Scholar]
- 12.Williams K, Minkowski A, Amoabeng O, Peloquin CA, Taylor D, Andries K, Wallis RS, Mdluli KE, Nuermberger EL. 2012. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother 56:3114–3120. doi: 10.1128/AAC.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tasneen R, Li SY, Peloquin CA, Taylor D, Williams KN, Andries K, Mdluli KE, Nuermberger EL. 2011. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother 55:5485–5492. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mphahlele M, Syre H, Valvatne H, Stavrum R, Mannsåker T, Muthivhi T, Weyer K, Fourie PB, Grewal HM. 2008. Pyrazinamide resistance among South African multidrug-resistant Mycobacterium tuberculosis isolates. J Clin Microbiol 46:3459–3464. doi: 10.1128/JCM.00973-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierre-Audigier C, Surcouf C, Cadet-Daniel V, Namouchi A, Heng S, Murray A, Guillard B, Gicquel B. 2012. Fluoroquinolone and pyrazinamide resistance in multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 16:221–223. doi: 10.5588/ijtld.11.0266. [DOI] [PubMed] [Google Scholar]
- 16.Anderson RF, Shinde SS, Maroz A, Boyd M, Palmer BD, Denny WA. 2008. Intermediates in the reduction of the antituberculosis drug PA-824, (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine, in aqueous solution. Org Biomol Chem 6:1973–1980. doi: 10.1039/b801859f. [DOI] [PubMed] [Google Scholar]
- 17.Kmentova I, Sutherland HS, Palmer BD, Blaser A, Franzblau SG, Wan B, Wang Y, Ma Z, Denny WA, Thompson AM. 2010. Synthesis and structure-activity relationships of aza- and diazabiphenyl analogues of the antitubercular drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824). J Med Chem 53:8421–8439. doi: 10.1021/jm101288t. [DOI] [PubMed] [Google Scholar]
- 18.Palmer BD, Thompson AM, Sutherland HS, Blaser A, Kmentova I, Franzblau SG, Wan B, Wang Y, Ma Z, Denny WA. 2010. Synthesis and structure-activity studies of biphenyl analogues of the tuberculosis drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824). J Med Chem 53:282–294. doi: 10.1021/jm901207n. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal IM, Zhang M, Williams KN, Peloquin CA, Tyagi S, Vernon AA, Bishai WR, Chaisson RE, Grosset JH, Nuermberger EL. 2007. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med 4:e344. doi: 10.1371/journal.pmed.0040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyagi S, Nuermberger E, Yoshimatsu T, Williams K, Rosenthal I, Lounis N, Bishai W, Grosset J. 2005. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob Agents Chemother 49:2289–2293. doi: 10.1128/AAC.49.6.2289-2293.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lounis N, Veziris N, Chauffour A, Truffot-Pernot C, Andries K, Jarlier V. 2006. Combinations of R207910 with drugs used to treat multidrug-resistant tuberculosis have the potential to shorten treatment duration. Antimicrob Agents Chemother 50:3543–3547. doi: 10.1128/AAC.00766-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams KN, Stover CK, Zhu T, Tasneen R, Tyagi S, Grosset JH, Nuermberger E. 2009. Promising antituberculosis activity of the oxazolidinone PNU-100480 relative to that of linezolid in a murine model. Antimicrob Agents Chemother 53:1314–1319. doi: 10.1128/AAC.01182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenthal IM, Tasneen R, Peloquin CA, Zhang M, Almeida D, Mdluli KE, Karakousis PC, Grosset JH, Nuermberger EL. 2012. Dose-ranging comparison of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis. Antimicrob Agents Chemother 56:4331–4340. doi: 10.1128/AAC.00912-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouan MC, Lounis N, Gevers T, Dillen L, Gilissen R, Raoof A, Andries K. 2012. Pharmacokinetics-pharmacodynamics of TMC207 and its N-desmethyl metabolite in a murine model of tuberculosis. Antimicrob Agents Chemother 56:1444–1451. doi: 10.1128/AAC.00720-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nuermberger E, Rosenthal I, Tyagi S, Williams KN, Almeida D, Peloquin CA, Bishai WR, Grosset JH. 2006. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother 50:2621–2625. doi: 10.1128/AAC.00451-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallis RS, Jakubiec W, Mitton-Fry M, Ladutko L, Campbell S, Paige D, Silvia A, Miller PF. 2012. Rapid evaluation in whole blood culture of regimens for XDR-TB containing PNU-100480 (sutezolid), TMC207, PA-824, SQ109, and pyrazinamide. PLoS One 7:e30479. doi: 10.1371/journal.pone.0030479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diacon AH, Dawson R, Hanekom M, Narunsky K, Maritz SJ, Venter A, Donald PR, van Niekerk C, Whitney K, Rouse DJ, Laurenzi MW, Ginsberg AM, Spigelman MK. 2010. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob Agents Chemother 54:3402–3407. doi: 10.1128/AAC.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alffenaar JWC, van der Laan T, Simons S, van der Werf TS, van de Kasteele PJ, de Neeling H, van Soolingen D. 2011. Susceptibility of clinical Mycobacterium tuberculosis isolates to a potentially less toxic derivate of linezolid, PNU-100480. Antimicrob Agents Chemother 55:1287–1289. doi: 10.1128/AAC.01297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallis RS, Jakubiec W, Kumar V, Bedarida G, Silvia A, Paige D, Zhu T, Mitton-Fry M, Ladutko L, Campbell S, Miller PF. 2011. Biomarker-assisted dose selection for safety and efficacy in early development of PNU-100480 for tuberculosis. Antimicrob Agents Chemother 55:567–574. doi: 10.1128/AAC.01179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallis RS, Jakubiec WM, Kumar V, Silvia AM, Paige D, Dimitrova D, Li X, Ladutko L, Campbell S, Friedland G, Mitton-Fry M, Miller PF. 2010. Pharmacokinetics and whole-blood bactericidal activity against Mycobacterium tuberculosis of single doses of PNU-100480 in healthy volunteers. J Infect Dis 202:745–751. doi: 10.1086/655471. [DOI] [PubMed] [Google Scholar]
- 31.Diacon AH, Dawson R, du Bois J, Narunsky K, Venter A, Donald PR, van Niekerk C, Erondu N, Ginsberg AM, Becker P, Spigelman MK. 2012. Phase II dose-ranging trial of the early bactericidal activity of PA-824. Antimicrob Agents Chemother 56:3027–3031. doi: 10.1128/AAC.06125-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diacon AH, Dawson R, Hanekom M, Narunsky K, Venter A, Hittel N, Geiter LJ, Wells CD, Paccaly AJ, Donald PR. 2011. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis 15:949–954. doi: 10.5588/ijtld.10.0616. [DOI] [PubMed] [Google Scholar]
- 33.Upton AM, Cho S, Yang TJ, Kim Y, Wang Y, Lu Y, Wang B, Xu J, Mdluli K, Ma Z, Franzblau SG. 2015. In vitro and in vivo activities of the nitroimidazole TBA-354 against Mycobacterium tuberculosis. Antimicrob Agents Chemother 59:136–144. doi: 10.1128/AAC.03823-14. [DOI] [PMC free article] [PubMed] [Google Scholar]