Abstract
Nitroimidazoles are a promising new class of antitubercular agents. The nitroimidazo-oxazole delamanid (OPC-67683, Deltyba) is in phase III trials for the treatment of multidrug-resistant tuberculosis, while the nitroimidazo-oxazine PA-824 is entering phase III for drug-sensitive and drug-resistant tuberculosis. TBA-354 (SN31354[(S)-2-nitro-6-((6-(4-trifluoromethoxy)phenyl)pyridine-3-yl)methoxy)-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine]) is a pyridine-containing biaryl compound with exceptional efficacy against chronic murine tuberculosis and favorable bioavailability in preliminary rodent studies. It was selected as a potential next-generation antituberculosis nitroimidazole following an extensive medicinal chemistry effort. Here, we further evaluate the pharmacokinetic properties and activity of TBA-354 against Mycobacterium tuberculosis. TBA-354 is narrow spectrum and bactericidal in vitro against replicating and nonreplicating Mycobacterium tuberculosis, with potency similar to that of delamanid and greater than that of PA-824. The addition of serum protein or albumin does not significantly alter this activity. TBA-354 maintains activity against Mycobacterium tuberculosis H37Rv isogenic monoresistant strains and clinical drug-sensitive and drug-resistant isolates. Spontaneous resistant mutants appear at a frequency of 3 × 10−7. In vitro studies and in vivo studies in mice confirm that TBA-354 has high bioavailability and a long elimination half-life. In vitro studies suggest a low risk of drug-drug interactions. Low-dose aerosol infection models of acute and chronic murine tuberculosis reveal time- and dose-dependent in vivo bactericidal activity that is at least as potent as that of delamanid and more potent than that of PA-824. Its superior potency and pharmacokinetic profile that predicts suitability for once-daily oral dosing suggest that TBA-354 be studied further for its potential as a next-generation nitroimidazole.
INTRODUCTION
New tuberculosis (TB) drug regimens that shorten and simplify the current treatment are sorely needed for all patient populations. An ideal new TB drug would be well tolerated, be orally dosed once daily, and have a low cost. It would demonstrate bactericidal and sterilizing efficacy and have a novel mode of action and therefore utility against both drug-sensitive and drug-resistant TB. Until 2012, no new TB drugs had been approved for more than 50 years. However, a resurgence of TB drug discovery over the past 15 years led to two first-in-class drugs with novel modes of action receiving conditional approval for treatment of multidrug-resistant (MDR) TB in 2012 and 2013. One of those drugs is bedaquiline (1, 2, 3, 4), a diarylquinoline inhibitor of Mycobacterium tuberculosis ATP synthase and the other is delamanid (OPC-67683, Deltyba) (5), an antitubercular nitroimidazole. The only other novel compound in late-stage development for TB is PA-824 (Pa), also a nitroimidazole (6). The available clinical data for delamanid and Pa suggest that the nitroimidazole class will make an important contribution to future TB treatment.
The nitroimidazoles have been widely used to treat anaerobic bacterial and protozoal infections since the introduction of metronidazole (Flagyl) (7). These drugs exert at least part of their activity through chemical species formed following bioreduction of the drug within the target pathogen itself. Safe compounds of this class are selectively bioreduced by an enzyme specific to the target pathogen or under specific environmental (e.g., anaerobic) conditions. Many nitroimidazole subclasses have demonstrated activity against members of the M. tuberculosis complex (8). Activity against M. tuberculosis for 2-nitroimidazoles was observed but not pursued due to the lack of selectivity of these more readily reduced compounds. The 5-nitroimidazole metronidazole is active against M. tuberculosis only under anaerobic conditions. Because there is evidence that TB granulomas can be hypoxic, the clinical efficacy of metronidazole has been studied. However, the apparent benefits were short-lived, and toxicity was observed upon extended dosing (9). The identification of bicyclic antitubercular nitroimidazoles, especially CGI-17341 (10), illustrated the potential of these compounds. CGI-17341 demonstrated in vitro activity against drug-sensitive and drug-resistant M. tuberculosis and increased survival of mice with TB in a dose-dependent manner. Mutagenicity precluded development of CGI-17341, but its discovery encouraged the optimization of bicyclic nitroimidazoles for TB, resulting in identification of Pa and delamanid.
Delamanid is a nitroimidazo-oxazole that, following bioreduction within M. tuberculosis, inhibits mycolic acid biosynthesis. It has potent anti-M. tuberculosis activity in vitro and in mice (11) and has demonstrated clinical efficacy both as monotherapy in a trial to measure early bactericidal activity (EBA) and when added to optimized background therapy for MDR TB in a 2-month study that evaluated bacterial CFU in serial sputum samples (5, 12). However, poor absorption of delamanid may pose a challenge for once-daily dosing; in an ongoing phase III trial, delamanid is being administered twice daily for the first 2 months of treatment (clinical trials.gov identifier NCT01424670).
Pa, a nitroimidazo-oxazine, has demonstrated efficacy against TB in several preclinical models (13, 14, 15), and its clinical pharmacokinetic (PK) profile allows for once-daily oral dosing. Elegant work has demonstrated that Pa is activated by a specific M. tuberculosis deazaflavin-dependent nitroreductase (16). The metabolites produced, including reactive nitrogen species, act downstream to inhibit mycolic acid biosynthesis (15) (as for delamanid) and also cause nitrosative damage, including respiratory damage (17). Pa may also act by directing the activity of its activating enzyme away from an endogenous antioxidant role (18). Different effects of Pa may dominate under aerobic (where bacteria are replicating) and hypoxic (where bacteria are not replicating [NR]) conditions. The ultimate clinical efficacy of Pa may be due to targeting of M. tuberculosis residing in both aerobic and hypoxic environments that exist in the lungs of TB patients.
Pa demonstrated clinical EBA as monotherapy (6) and in combination with moxifloxacin (M) and pyrazinamide (Z) (19, 20). Following completion of a 2-month phase II study, the drug regimen PaMZ is scheduled to enter a phase III trial that will enroll patients with TB sensitive to the regimen. PaMZ and other Pa-containing drug regimens have demonstrated the treatment-shortening potential of nitroimidazoles by curing mice with a shorter duration of dosing than the standard of care (21, 22). Some of these shorter nitroimidazole-containing drug regimens include only new TB drugs to which there is no existing resistance and may represent truly transformative treatments for patients with drug-resistant disease.
Pa is not as potent as delamanid, in vitro or in mice, suggesting that the potency of nitroimidazo-oxazines may be further optimized. Seeking to identify a nitroimidazo-oxazine with the potency of delamanid but also suitable for once-daily oral dosing, we previously carried out an extensive medicinal chemistry program in collaboration with the Auckland Cancer Society Research Center. More than 1,000 compounds were synthesized and evaluated with structural variations of the pharmacophore, side chain, and linker. TBA-354 (compound 93 in reference 23) was identified as having particular promise, with lower MICs than Pa, stability in human and mouse liver microsomes, and good bioavailability in rats. Dosed for 3 weeks, 100 mg/kg TBA-354 showed efficacy (CFU reduction) superior to that of 100 mg/kg Pa against acute and chronic murine TB and at 30 mg/kg outperformed delamanid against chronic murine TB when dosed for 8 weeks (23).
Here, we further profile the antimicrobial activity of TBA-354, including in vitro activity against laboratory and clinical strains of M. tuberculosis and efficacy against TB in murine models. We also describe further its PK and absorption, distribution, metabolism, and excretion (ADME) properties.
MATERIALS AND METHODS
Compounds.
Pa, TBA-354, and delamanid were synthesized as previously reported (23).
MICs and MBCs against M. tuberculosis under aerobic and low-oxygen conditions.
The M. tuberculosis H37Rv strain was used.
(i) Aerobic conditions (replicating M. tuberculosis).
MICs were determined using the microplate alamarBlue assay (MABA) as follows. Cultures were incubated in 200 μl 7H12 medium together with the test compound in 96-well plates for 7 days at 37°C. alamarBlue and Tween 80 were added, and incubation was continued for 24 h at 37°C. Fluorescence was determined at excitation/emission wavelengths of 530/590 nm, respectively. The MIC was defined as the lowest concentration effecting a reduction in fluorescence of 90% relative to that in the controls (24, 25, 26).
(ii) Low-oxygen conditions (NR M. tuberculosis).
The low-oxygen recovery assay (LORA) was used, and the MIC (27) was defined as the lowest concentration of the compound which reduces luminescence by 90% after 10 days of exposure to the compound under low oxygen followed by 28 h of aerobic recovery and comparison to the untreated controls. The MABA and LORA minimal bactericidal concentration (MBC) values were the lowest concentrations of test compounds which reduce CFU by 99% after 7 days (aerobic) or 10 days (low-oxygen) exposure relative to CFU at the start of the 7-day (aerobic) or 10-day (low-oxygen) exposure.
MICs against M. tuberculosis H37Rv isogenic monoresistant strains.
MICs were determined against M. tuberculosis H37Rv isogenic strains monoresistant to rifampin (ATCC 35838), isoniazid (ATCC 35822), streptomycin (ATCC 35820), and kanamycin (ATCC 35827) via the MABA as described above.
Spectrum of activity.
Mycobacterium bovis (ATCC 19210), M. scrofulaceum (ATCC 19981), M. kansasii (ATCC 12478), M. gilvum (ATCC 43909), M. fortuitum (ATCC 6841), M. triviale (ATCC 23292), and M. smegmatis (ATCC MC2155) were cultured in Middlebrook 7H9 broth with 0.2% (vol/vol) glycerol, 0.05% Tween 80, and 10% (vol/vol) albumin-dextrose-catalase (BBL Middlebrook ADC enrichment, catalog no. 212352) (7H9-ADC-TG). Staphylococcus aureus (ATCC 29213) and Escherichia coli (ATCC 25922) were cultured in cation-adjusted Mueller-Hinton (CAMH) broth and Candida albicans (ATCC 90028) in RPMI medium until an absorbance at 570 nm of 0.2 to 0.5 was achieved. Cultures were diluted 1:5,000 to 1:10,000 in fresh medium in 96-well plates and incubated at 37°C with test compounds. Incubation times were 3 days for M. smegmatis, 7 days for other mycobacteria, 36 to 48 h for C. albicans, and 16 to 20 h for S. aureus and E. coli. For C. albicans, S. aureus, and E. coli, the MIC was defined as the lowest concentration effecting a reduction of ≥90% in A570 relative to that of untreated cultures. The MABA MICs for mycobacteria were defined as described above.
Effect of serum/albumin on M. tuberculosis MICs.
To test whether protein binding, which can reduce unbound, active compound, altered the apparent activity of TBA-354 and Pa, the MICs of the compounds against M. tuberculosis H37Rv were determined via the MABA (described above) without supplemental protein, in the presence of 4% bovine serum albumin (BSA), and in the presence of 10% fetal bovine serum (26). Note that the basal medium contains 0.5% BSA.
MICs against drug-resistant and drug-susceptible M. tuberculosis clinical isolates.
The M. tuberculosis clinical isolates were obtained from the State Laboratory of Tuberculosis Reference of China. Bacteria were cultured and tested in Middlebrook 7H9 broth with 0.2% (vol/vol) glycerol, 0.05% Tween 80, and 10% (vol/vol) albumin-dextrose-catalase. The MABA MIC was defined as described above.
Frequency of resistance development.
Four replicate cultures of M. tuberculosis H37Rv were prepared in 50-ml flasks, each containing 10 ml Middlebrook 7H9 broth plus glycerol, Casitone and oleic acid-albumin-dextrose-catalase (OADC) supplement at an initial density of M. tuberculosis of <104 CFU/ml and monitored for the presence of preexisting resistant bacteria by plating 100-μl aliquots on medium containing the test compound at 4× MIC. Cultures were then incubated for 2 to 3 weeks with shaking until an A570 of >0.9 (approximately 2 × 109 CFU/ml) was obtained. From each of four cultures, 100-μl aliquots of undiluted and 1:10 diluted suspensions (approximately 1 × 109 CFU/ml and 1 × 108 CFU/ml, respectively) were plated on 10 plates of 7H11 agar with and without 4× MIC of TBA-354. Individual mutation frequencies were calculated for each of the four cultures, and the median value was selected as representative.
In vivo efficacy in mice.
Female ∼20-g BALB/c mice were infected by aerosol with a low dose of M. tuberculosis Erdman as previously described (25). The protocol results in the deposition of approximately 50 to 100 bacilli into the lungs, and the course of infection was then followed by plating homogenates of the lungs on 7H11 agar and determining CFU. Controls consisted of mice treated with the vehicle only. The compounds were prepared weekly by suspension in 0.5% (wt/vol) carboxymethylcellulose (CMC) such that the target dosages were obtained by once-daily dosing by oral gavage of a 200-μl suspension. Groups of 6 or 7 mice were dosed for 5 consecutive days each week. The suspensions were stored at 4°C between daily doses. Mice were sacrificed 3 days after the final dose to minimize carryover from the lung homogenates to the plating medium. Both lungs were homogenized and diluted in Hanks' balanced salt solution (HBSS)-Tween, and aliquots were plated on Middlebrook 7H11 medium. CFU were determined after 3 weeks of incubation at 37°C. For statistical analysis of efficacy data, multiple comparisons among pairs were performed by the Bonferroni method (28).
Bidirectional permeability in Caco-2 cells.
The Caco-2 permeability assay was developed based on the method of Hidalgo et al. (29). In brief, a 96-well multiscreen plate with Caco-2 cells was cultured for 21 to 25 days. TBA-354 at 1 and 10 μM was incubated at 37°C for 40 or 60 min with the Caco-2 cell monolayer in Hanks' balanced salt solution plus HEPES or morpholineethanesulfonic acid (MES) (containing a final concentration of 1% dimethyl sulfoxide [DMSO] from a stock solution), in the absence and presence of ketoconazole (100 μM). The permeability through the cell barrier was measured in duplicate in both directions by taking aliquots from the apical (A) side and the basolateral (B) side. TBA-354 concentrations were analyzed by liquid chromatography-tandem mass spectroscopy (LC-MS/MS) (see “LC-MS/MS analysis methods” below).
Plasma protein binding (30).
TBA-354 binding to proteins of human, monkey, dog, rat, and mouse plasma was conducted with a 96-well equilibrium dialysis apparatus with a 12,000- to 14,000-molecular-weight-cutoff dialysis membrane. TBA-354 was added to the plasma side at 10 μM and was shaken for 8 h at 37°C. Each sample was assessed in duplicate. TBA-354 concentrations were measured by LC-MS/MS as described below.
In vitro metabolism. (i) Metabolism in liver microsomes.
TBA-354 at 1 μM was incubated with rat, mouse, dog, monkey, or human liver microsomes (HLM) (0.3 mg/ml) at 37°C for 1 h in phosphate buffer (pH 7.4) with a NADPH regeneration system (1 mM NADP+, 5 mM glucose 6-phosphate [G6P], 1 U/ml glucose-6-phosphate dehydrogenase [G-6-PDH]). HLM were from a mixed-gender pool; all other species consisted of pooled male microsomes. Upon terminations of the incubations by addition of acetonitrile, each extract was analyzed by LC-MS/MS (see “LC-MS/MS analysis methods” below) to determine the remaining concentration of TBA-354. Positive controls (imipramine, propranolol, terfenadine, and verapamil) showed approximately 30% to 100% losses after the microsomal incubation in all species. To determine the microsomal intrinsic clearance, TBA-354 at 1 μM was incubated with human liver microsomes (0.3 mg/ml) in the presence of a NADPH regeneration system, and the percentages of the parent compound remaining were measured after 0-, 15-, 30-, 45-, and 60-min incubations. The positive controls (terfenadine and verapamil) showed high microsomal clearance (CLint of 124 and 115 ml/min/mg) in this assay.
(ii) Metabolism in hepatocytes.
TBA-354 at 1 μM was incubated with mouse, rat, rabbit, dog, monkey, or human hepatocytes (at ca. 1 × 106 cells/ml) at 37°C for 0, 0.5, 1, 2, and 4 h. The metabolic stability of TBA-354 in human hepatocytes was assessed based on the relative concentrations of the remaining TBA-354 in the 0.5-, 1-, 2-, and 4-h incubations compared to that of the 0-h incubation. The half-life of TBA-354 metabolism in hepatocytes was calculated based on the first-order decay reaction if significant loss of TBA-354 was measured. The results for the positive-control samples demonstrated that the enzymes in the prepared hepatocyte suspensions were active.
(iii) Metabolism by recombinant human CYPs.
The metabolism of TBA-354 by several cytochrome P450 (CYP) isozymes as a function of time was determined by incubating 1 μM TBA-354 with human recombinant CYP2C9, CYP2C19, CYP2D6, and CYP3A4 (0.1 nmol/ml) for 0, 15, 30, 45, and 60 min in the presence of NADPH (1 mM).
Pharmacokinetics of TBA-354 in mice.
The PK of TBA-354 was studied in female BALB/c mice following oral gavage of TBA-354, formulated in 0.5% CMC, at 3, 30, and 100 mg/kg. Plasma samples were collected from three mice per time point over the course of 48 h using sodium heparin as an anticoagulant. In a separate study, mice were administered 2 mg/kg TBA-354 (formulated in 40% hydroxypropyl-β-cyclodextrin and 50 mM citrate buffer at pH 3) by intravenous (i.v.) bolus, and blood samples (0.25 ml) were collected (three per time point) over the course of 24 h into potassium EDTA-containing tubes. Plasma concentrations were determined by LC-MS/MS (see “LC-MS/MS analysis methods” below). Noncompartmental PK analysis was performed with WinNonlin Phoenix v6.2 using composite data.
Inhibition of CYPs.
TBA-354 at 10 μM (for single-concentration assessments) was incubated with individual CYP isoforms at 37°C for 20 to 30 min in pooled HLM (0.25 mg protein/ml), phosphate buffer (100 mM, pH 7.4), magnesium chloride (MgCl2) (5 mM), NADPH (1 mM), and CYP probe substrates (at the approximate Km). The CYP probe substrates and reactions were phenacetin dealkylation for CYP1A2, coumarin 7-hydroxylation for CYP2AD6, bupropion hydroxylation for CYP2B6, paclitaxel 6′-hydroxylation for CYP2C8, diclofenac 4′-hydroxylation for CYP2C9, S-mephenytoin 4′-hydroxylation for CYP2C19, bufuralol 1′-hydroxylation for CYP2D6, chlorzoxazone 6-hydroxylation for CYP2E1, and midazolam 1′-hydroxylation and testosterone 6-hydroxylation for CYP3A4. Experiments were performed in duplicate. Following the addition of acetonitrile and centrifugation at 3,000 to 4,000 rpm for 10 min at 4°C to remove protein, supernatants were analyzed for the formation of CYP probe metabolites by LC-MS/MS, and the percent inhibition was estimated. To determine the 50% inhibitory concentrations (IC50s) of TBA-354 inhibition of CYP2C8, CYP2C19, and CYP3A4 (with two substrates, midazolam and testosterone), TBA-354 (0.01, 0.1, 1, 10, and 30 μM) was incubated with HLM (0.3 mg/ml) and NADPH (1 mM). After 30 min of incubation at 37°C, formation of the metabolite of the CYP-specific substrate in the absence and presence of test compound was measured. Ketoconazole was used as a positive control for CYP3A4 inhibition. For assessment of time-dependent inhibition, HLM were preincubated with TBA-354 for 30 min in the presence of NADPH before the addition of probe substrates to measure CYP activity (preincubation mixture not diluted).
Induction of CYPs.
The potential for induction of CYP enzymes (CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP3A) by 10 μM TBA-354 was evaluated using human hepatocytes (3 donors) cultured in medium containing 2% BSA. Freshly plated human hepatocytes (∼0.15 million cells/well in 48-well plates) were treated with the test compound for 72 h. After induction treatment, CYP enzyme activities were measured by determining the formation of CYP-specific probe metabolites by LC-MS/MS. The fold induction of the CYP enzyme activity compared with that for the vehicle control was calculated.
LC-MS/MS analysis methods.
To measure TBA-354 concentrations in the in vitro Caco-2 permeability assay, a high-performance liquid chromatography (HPLC) 20AD pump (Shimadzu) connected to an API 4000 mass spectrometer (AB Sciex) was used. A Phenomenex Luna C18(2) column (5 μm, 50 by 2 mm) was eluted with a mobile phase of water-acetonitrile (98:2 to 2:98 in 1.7 min of elution time) containing 0.1% formic acid at a flow rate of 0.5 ml/min. TBA-354 was monitored by the positive multiple reaction monitoring (MRM) mode at the transition of m/z 437 to 252. Benzyl nicotinate as an internal standard was monitored at the transition of m/z 214 to 91. In the microsomal metabolism assessments of TBA-354, the same analytical method was used to measure the remaining TBA-354.
In the plasma protein binding assays, the TBA-354 concentration was measured under similar LC-MS/MS conditions. An Agilent HP1100 LC system connected to a Waters Quattro Micro mass spectrometer was used. An Atlantis C18 column (3 μm, 10 × 2.1 mm) was eluted with a mobile phase consisting of water and methanol containing 0.1% formic acid and 10 mM ammonium formate (100% water to 100% methanol in 2 min of elution time). TBA-354 was monitored under the positive MRM mode at the transition m/z of 437.3 to 252.5.
TBA-354 concentrations in hepatocyte metabolism incubations were measured using a Shimadzu liquid chromatograph connected to a Q-Trap 4000 MS/MS system (AB/MDS Sciex). A Phenomenex Luna C18 column (5.0 μm, 50 × 2.0 mm) was used for separation and eluted with a mobile phase consisting of water-acetonitrile (100% water to 100% acetonitrile in 7 min) containing 0.1% formic acid at a flow rate of 1 ml/min. TBA-354 was monitored under the positive ion mode with the transition m/z of 437 to 252. Pa was used as an internal standard monitored at the transition m/z of 360 to 175. The same LC-MS/MS system was used in the CYP inhibition and induction studies.
In the mouse PK study, a Thermo Finnigan LC-TSQ mass spectrometer system was used. TBA-354 was monitored with the single reaction monitoring (SRM) positive mode at the transition of m/z 437.3 to 252.5. The detection linear range was from 10 ng/ml to 1,000 ng/ml.
RESULTS
In vitro antimicrobial profile and frequency of resistance generation.
The MIC of TBA-354 against replicating M. tuberculosis H37Rv is approximately equivalent to that observed with delamanid and about 1 order of magnitude lower than that of Pa (Table 1). All three nitroimidazoles are bactericidal against replicating cultures with MBCs equal to MICs. MIC shifts against replicating M. tuberculosis of all compounds in the presence of 10% fetal bovine serum or 4% BSA (a physiologically equivalent concentration of albumin) were minimal (Table 1), in no case exceeding 3× the MIC obtained in the standard culture medium which contains 0.5% BSA. Although all three compounds are somewhat less active against NR M. tuberculosis (determined under low-oxygen conditions) than against replicating M. tuberculosis, the relative activities of the three compounds against the NR cultures were similar to those observed with the replicating cultures, regardless of whether this was assessed by the recovery of a luminescence signal upon return to normoxia (LORA MIC) or by CFU (LORA MBC) (Table 1).
TABLE 1.
MICs and MBCs against M. tuberculosis H37Rv
| Parameter | MIC or MBC (μM) |
||
|---|---|---|---|
| TBA-354 | Pa | Delamanid | |
| MIC (replicating) | 0.006 | 0.04 | 0.002 |
| MBC (replicating) | 0.006 | 0.061 | 0.003 |
| LORA (NR) MICa | 0.27 | 2.28 | 0.7 |
| LORA (NR) MBC | 3.4 | 17.4 | 4.4 |
| 4% BSAb MIC (replicating) | 0.011 | 0.072 | 0.006 |
| 10% FBSc MIC (replicating) | 0.012 | 0.038 | 0.009 |
Clofazimine, 1.42 μM; thioridazine, 11.74 μM; capreomycin, 5.92 μM, linezolid, 28.42 μM; INH and ethambutol, >1,000 μM.
BSA, bovine serum albumin.
FBS, fetal bovine serum.
Activities for all three compounds are maintained against M. tuberculosis H37Rv isogenic strains resistant to the TB drugs isoniazid (INH), rifampin (RIF), streptomycin (STP), and kanamycin (KAN) (Table 2). For a panel of 10 drug-sensitive and drug-resistant clinical M. tuberculosis isolates sourced from China, the TBA-354 MIC was lower than that of Pa against each individual strain. The MIC range for TBA-354 across the 10 strains tested was <0.02 to 0.36 μM and that of Pa was 0.38 to 1.39 μM (Table 3). Although there was variability in the MICs for TBA-354 and Pa observed among the pansensitive clinical M. tuberculosis isolates, the MICs for the drug-resistant strains did not exceed those obtained for the pansensitive strains.
TABLE 2.
MICs against mono-drug-resistant M. tuberculosis H37Rv strains
| Straina | MIC (replicating) (μM) |
||
|---|---|---|---|
| TBA-354 | Pa (avg, n = 2) | Delamanid | |
| M. tuberculosis H37Rv | 0.004 | 0.034 | 0.004 |
| RIFR | 0.006 | 0.036 | 0.002 |
| INHR | 0.007 | 0.037 | 0.009 |
| STPR | 0.010 | 0.074 | 0.002 |
| KANR | 0.003 | 0.092 | 0.002 |
INHR, isoniazid resistant; KANR, kanamycin resistant; RIFR, rifampin resistant; STPR, streptomycin resistant.
TABLE 3.
MICs versus drug-resistant and drug-susceptible M. tuberculosis clinical isolates
| Isolate and susceptibilitya | MIC (replicating) (μM) |
|||
|---|---|---|---|---|
| TBA-354 | Pa | RMP | INH | |
| 003: pansensitive | 0.14 | 1.39 | 0.12 | 0.37 |
| 018: pansensitive | 0.36 | 0.77 | 0.03 | 0.15 |
| 047: pansensitive | 0.03 | 1.39 | 0.24 | 0.73 |
| 056: pansensitive | 0.28 | 0.73 | 0.06 | 0.18 |
| 002: INH, RIF | 0.03 | 0.65 | >1.51 | 18.22 |
| 054: INH, RIF, RPT | 0.04 | 0.68 | 12.09 | 34.80 |
| 057: INH, RIF, RPT, OFX, LVX | <0.02 | 0.38 | >24.2 | >9.11 |
| 004: INH, STP, RIF, RPT, KAN, OFX | 0.03 | 0.69 | >1.51 | >9.11 |
| 040: INH, RIF, PTO, RPT, OFX, LVX | 0.03 | 0.69 | >24.2 | >145.8 |
| 118: INH, STP, RIF, RPT, PTO, OFX, LVX | 0.03 | 0.59 | >24.2 | >145.8 |
INH, isoniazid; KAN, kanamycin; LVX, levofloxacin; OFX, ofloxacin; PTO, protionamide; RIF, rifampin; RPT, rifapentine; STP, streptomycin.
The MICs of all three compounds were >50 μM against S. aureus, E. coli, and C. albicans (Table 4), suggesting that, similar to Pa and delamanid (11, 15), TBA-354 is a narrow-spectrum agent. TBA-354 and Pa, which were also assessed for activity against nontuberculous mycobacteria, maintained potent activity against M. bovis but not against most of the other mycobacterial species. TBA-354 was at least 7 times more active against M. kansasii than was Pa (Table 4). Spontaneous mutants of M. tuberculosis H37Rv that are resistant to TBA-354 arose at a frequency of approximately 3 × 10−7 (range, 2 × 10−6 to 1 × 10−7) when plated on medium containing 0.025 μM TBA-354, which is similar to the observed frequency of spontaneous mutant generation for Pa (15).
TABLE 4.
Spectrum of activity of TBA-354
| Species | MIC (replicating) (μM) |
||
|---|---|---|---|
| TBA-354 | Pa | Delamanid | |
| M. bovis | <0.179 | <0.217 | NDa |
| M. kansasii | 2.2 | >13.9 | |
| M. scrofulaceum, M. gilvum, M. fortuitum, M. triviale | >11.5 | ||
| M. smegmatis, S. aureus, E. coli, C. albicans | >50 | >50 | >50 |
ND, not done.
ADME and PK profile. (i) In vitro evaluations of ADME characteristics.
An in vitro Caco-2 cell bidirectional permeability assay was used to assess the potential for absorption and P-glycoprotein (P-gp) interactions with TBA-354. TBA-354 demonstrates high permeability at 1 and 10 μM in this assay with an apparent permeability (Papp) apical to basal (A to B) value of >10 × 10−6 cm/s (Table 5). Permeability values were similar in the absence and presence of the P-gp inhibitor ketoconazole, and the efflux ratios for both 1 and 10 μM TBA-354 in the absence and presence of ketoconazole were <2. The result indicates that TBA-354 is not a substrate for the P-gp transporter. Digoxin, as a P-gp substrate positive control, showed a Papp B to A/A to B ratio of 68-fold, which was inhibited to 1.4-fold by ketoconazole. TBA-354 exhibits moderate to high protein binding at 10 μM with mean plasma protein binding values in human, monkey, dog, rat, and mouse plasma of 96.5%, 94.6%, 96.2%, 95.9%, and 92.6%, respectively. The recovery range in the study was 86% to 120%.
TABLE 5.
Permeability of TBA-354 in Caco-2 cells
| Drug, concn | Ketoconazole | Papp A→Ba (10−6 cm/s) | Papp B→A (10−6 cm/s) | B→A/A→B ratio |
|---|---|---|---|---|
| TBA-354, 10 μM | − | 18.2 | 13.9 | 0.76 |
| + | 16.7 | 18.0 | 1.1 | |
| TBA-354, 1 μM | − | 17.2 | 15.6 | 0.9 |
| + | 10.9 | 16.2 | 1.5 | |
| Digoxin, 1 μM | − | 0.15 | 10.2 | 68 |
| + | 1.2 | 1.7 | 1.4 |
A, apical; B, basal; Papp, apparent permeability.
TBA-354 is metabolically stable or moderately metabolized in in vitro incubations with liver microsomes, recombinant cytochrome P450s (CYPs), and hepatocytes. TBA-354 metabolism by the CYPs tested (CYP2C9, CYP2C19, CYP2D6, and CYP3A4) was not measurable. TBA-354 is stable in in vitro microsomal incubations, and no measurable metabolism was detected during a 1-h incubation with human, monkey, dog, rat, or mouse liver microsomes in the presence of a NADPH regeneration system. Metabolism of TBA-354 in the hepatocytes of the six different species was moderate (Fig. 1). After a 4-h incubation of TBA-354 at 1 μM with mouse, rat, rabbit, dog, monkey, and human hepatocytes, unchanged TBA-354 remained as the major component, accounting for 77.1%, 87.7%, 98.6%, 82.9%, 70.7%, and 54.5%, respectively. The estimated half-life of TBA-354 in human hepatocytes was 4.6 h. Metabolite identification following incubation with hepatocytes will be important and is planned.
FIG 1.
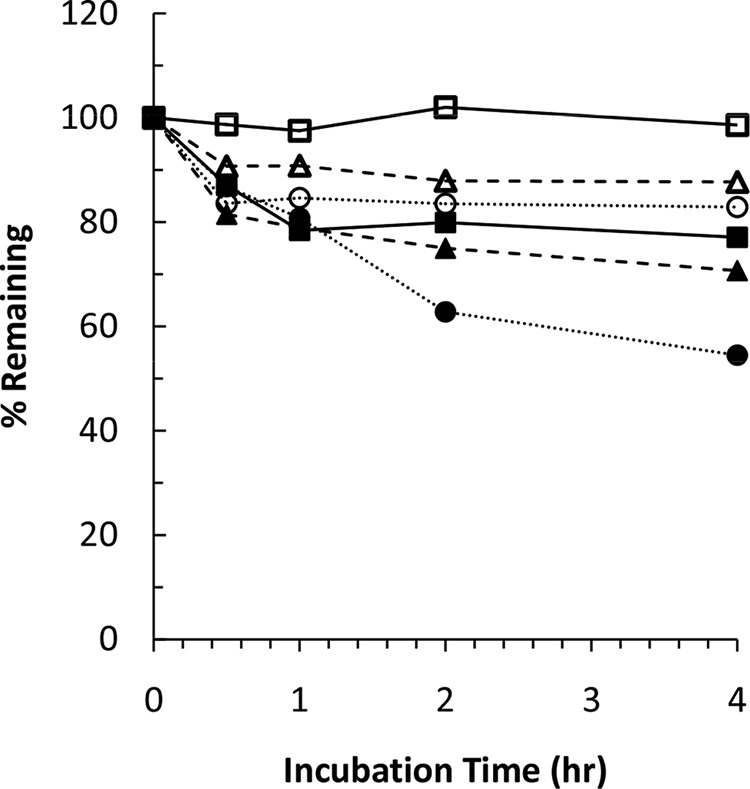
Metabolic stability of TBA-354 in hepatocytes. TBA-354 at 1 μM was incubated with mouse (■), rat (△), rabbit (□), dog (○), monkey (▲), or human (●) hepatocytes (at ca. 1 × 106 cells/ml) at 37°C for 4 h. The metabolic stability of TBA-354 was assessed based on the relative concentrations of the remaining TBA-354 at 0.5, 1, 2, and 4 h compared to TBA-354 at 0 h.
(ii) PK in mice.
PK studies were conducted in mice. The formulation, mouse strain, and dosing route were chosen to match those employed in the mouse efficacy studies. Following oral administration of a single dose of TBA-354 at 3, 30, or 100 mg/kg, the time to maximum concentration of drug in serum (Tmax) ranged from 2 to 6 h and the terminal half-life (t1/2) was relatively long, ranging from 8 to 12 h (Fig. 2 and Table 6). The maximum concentration of drug in serum (Cmax) and the area under the concentration-time curve from 0 h to infinity (AUC0–inf) increased with the increase in dose, but somewhat less than proportionally with AUC0–inf from 22.7 to 242 μg · h/ml and Cmax from 1.6 to 12.8 μg/ml. An i.v. bolus administration of 2 mg/kg TBA-354 permitted assessment of the volume of distribution and an estimate of the absolute bioavailability. A comparison of AUC0–inf values at similar dose levels and concentration ranges (3 mg/kg orally [p.o.] and 2 mg/kg i.v.) indicated that the absolute bioavailability in mice is ∼40%. The apparent volume of distribution (Vz/F) value of 1.61 liters/kg in mice indicates that TBA-354 likely distributes into tissues outside total body water.
FIG 2.
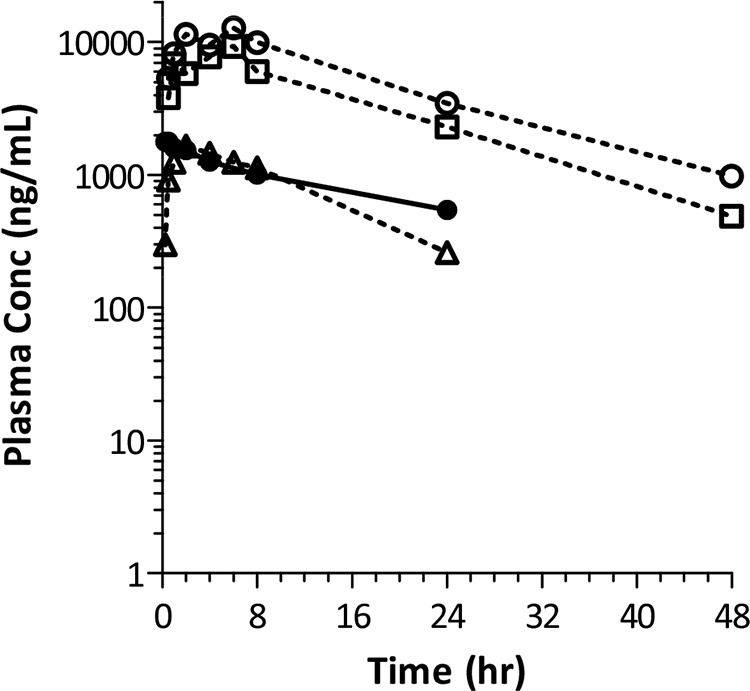
Single-dose pharmacokinetics of TBA-354 in mice. TBA-354 was administered to female BALB/c mice by oral gavage (p.o.) at 3, 30, and 100 mg/kg, formulated in 0.5% carboxymethylcellulose (CMC), and at 2 mg/kg (formulated in 40% hydroxypropyl-β-cyclodextrin and 50 mM citrate buffer at pH 3) by i.v. bolus. Plasma samples were collected from three mice per time point and TBA-354 concentrations were determined by LC-MS/MS.
TABLE 6.
Mean pharmacokinetic parameters for TBA-354 in mice following single doses
| Dose (mg/kg) (route) | Cmax (μg/ml) | Tmax (h) | t1/2 (h) | AUC0–inf (µg·h/ml) | CL/F (liters/h/kg) | Vz/F (liters/kg) |
|---|---|---|---|---|---|---|
| 2 (i.v.) | 1.78 | NRa | 15.6 | 35.3 | 0.073 | 1.61 |
| 3 (p.o.) | 1.65 | 2 | 8.0 | 22.7 | 0.132 | NR |
| 30 (p.o.) | 9.26 | 6 | 11.0 | 153 | 0.196 | NR |
| 100 (p.o.) | 12.8 | 6 | 12.0 | 242 | 0.413 | NR |
NR, not reported.
(iii) Potential for drug-drug interactions.
Direct and time-dependent inhibitions by TBA-354 of CYP2C8, CYP2C19, and CYP3A4 were evaluated in HLM. TBA-354 showed weak inhibition of CYP3A4-catalyzed testosterone 6-hydroxylation (40% at 30 μM), while no inhibition of midazolam 1′-hydroxylation was observed. No other CYPs were directly inhibited by TBA-354; IC50s of TBA-354 inhibition of these CYPs were all >30 μM. In a separate study, inhibition by TBA-354 at 10 μM on CYP enzyme activities in HLM were <10% for CYP2A6, 14% for CYP1A2, 20% for CYP2B6, 33% for CYP2C9, 26% for CYP2D6, and 24% for CYP2E1. Preincubation of TBA-354 with HLM in the presence of NADPH for 30 min resulted in an inhibition of CYP3A4-catalyzed testosterone 6β′-hydroxylation with an IC50 of 24 μM, which may suggest a weak time-dependent inactivation of CYP3A4. However, time-dependent inhibition of CYP3A4-catalyzed midazolam 1′-hydroxylation was not observed. TBA-354 at 10 μM did not significantly induce CYP1A2, CYP2B6, CYP2C9, CYP2C19, or CYP3A (fold induction of less than or close to 2 compared with that for the vehicle control) in any of the three human hepatocyte donors tested. TBA-354 did not significantly affect cell viability under these experimental conditions.
(iv) In vivo activity against murine TB.
We previously reported that all three nitroimidazoles demonstrate efficacy against murine TB, using a low-dose aerosol infection model, when administered during the acute or chronic phase of infection, at 100 mg/kg daily for 5 of 7 days per week for 3 weeks (23). As illustrated in Fig. 3a, TBA-354 and delamanid exhibited significant bactericidal activity (P < 0.001) in that assay when treatment began in the acute phase of infection, reducing lung CFU by approximately 1 log10 relative to that of the pretreatment controls. Both drugs were more active than Pa (P < 0.001); Pa inhibited growth of M. tuberculosis in the lungs of the mice, relative to that in the untreated controls, but did not reduce the number of lung CFU below the pretreatment levels. When treatment was started during the chronic phase of infection (Fig. 3b), the relative activities of the three nitroimidazoles were similar to that observed when treatment was initiated during acute infection (Fig. 3a). However, superior bactericidal activity was demonstrated by TBA-354 and delamanid in the chronic infection model relative to that in the acute infection model, with a 2 to 3 log10 reduction in lung CFU evident relative to the pretreatment CFU following treatment with these compounds. Again, both TBA-354 (P = 0.007) and delamanid (P = 0.018) were more active than Pa but not significantly different from each other (P = 0.538).
FIG 3.
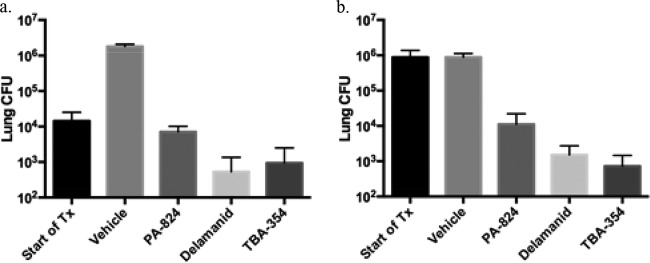
Efficacy of 100 mg/kg TBA-354 against acute and chronic TB in mice. (a) Acute infection model: inoculum was determined 3 days after infection. Treatment with vehicle or 100 mg/kg/day each nitroimidazole for 3 weeks, 5 days per week, commenced at day 10 postinfection (Start of treatment [Tx]) and terminated at day 28 postinfection. CFU were determined, after a 3-day washout period, at day 31 postinfection, for vehicle- and nitroimidazole-treated mice. (b) Chronic infection model: inoculum was determined 4 days postinfection. Treatment with vehicle or 100 mg/kg/day each nitroimidazole for 3 weeks, 5 days per week, started 70 days postinfection (Start of Tx) and ended at day 88 postinfection. CFU were determined, after a 3-day washout period, at day 91 postinfection, for vehicle- and nitroimidazole-treated mice. Data were previously expressed as CFU reduction and relative reduction compared to that for Pa (23).
To confirm and extend those findings, we evaluated the efficacy of TBA-354 against chronic murine TB, using the same low-dose aerosol infection model, with lung CFU determined after 2, 4, and 8 weeks of treatment. In this study, TBA-354 demonstrated dose- and time-dependent killing of M. tuberculosis. Bactericidal activity was significant compared to that of the untreated controls (P < 0.001) at the lowest dose tested (10 mg/kg), and at that dosage, the activity demonstrated by TBA-354 was not significantly less than that demonstrated by delamanid administered at 10 mg/kg or 30 mg/kg and was only significantly less than that shown by delamanid at 100 mg/kg when the treatment duration was 8 weeks (P = 0.033) (Fig. 4). After 8 weeks of treatment, TBA-354 at 30 mg/kg effected a larger reduction in CFU than delamanid at 30 mg/kg (P < 0.001), and this was the only significant difference observed in efficacy between the two compounds administered at equivalent dosages and for the same treatment durations in the experiment. An additional evaluation of TBA-354 in chronically infected mice was performed, in which the untreated controls achieved a bacterial load in lungs that was approximately 1 log10 lower than that for the experiments described above. TBA-354, at the lowest dose of 3 mg/kg, demonstrated a significant reduction in lung CFU after 4 weeks of treatment (P < 0.001), and the magnitudes of killing observed at this dosage and at 10 mg/kg were not significantly different from that of delamanid at 100 mg/kg (data not shown). Finally, no toxicity (as evaluated through clinical observations and monitoring of body weight) was observed during any of these efficacy studies (data not shown).
FIG 4.
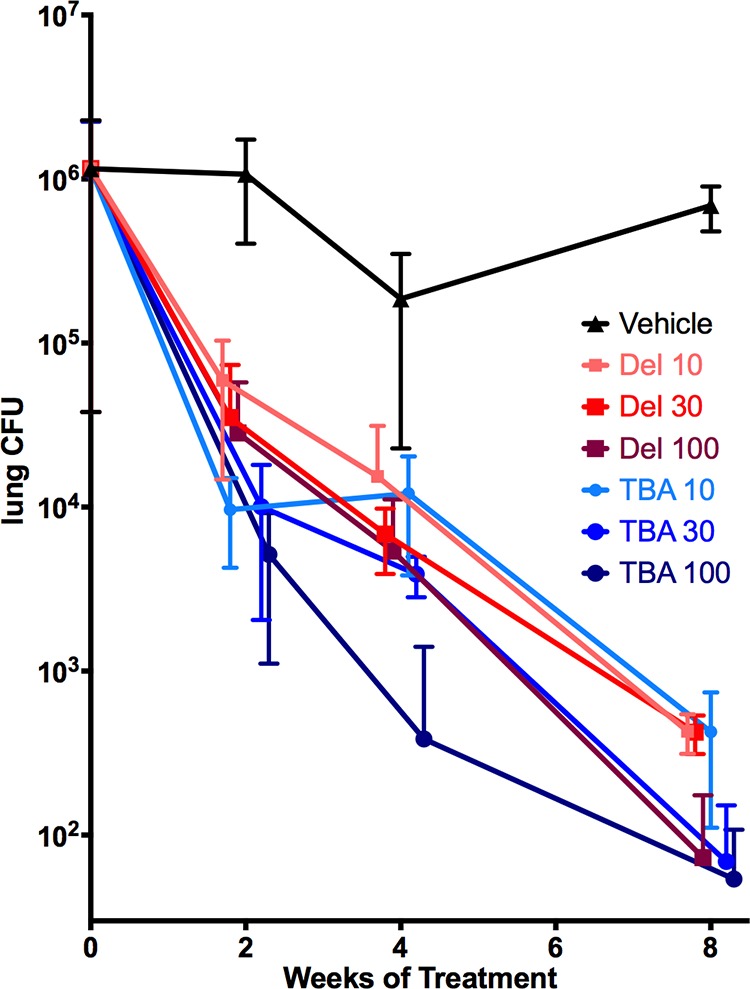
Dose-response and kill kinetics of TBA-354 against chronic TB in mice. Eight weeks of treatment at 10 to 100 mg/kg: treatment initiated on day 49 postinfection. Dosages are in mg/kg. All mice were sacrificed prior to treatment or at 2, 4, and 8 weeks posttreatment. Data points are offset in order to minimize overlap. Del, delamanid; TBA, TBA-354.
DISCUSSION
The bicyclic nitroimidazoles are among the very few novel antitubercular classes with clinical evidence to support their potential to radically improve treatment for drug-sensitive and drug-resistant TB. Because of this, several independent medicinal chemistry programs have aimed to optimize these compounds further (8). Our own medicinal chemistry program, with the Auckland Cancer Society Research Center, identified TBA-354 as a particularly potent nitroimidazo-oxazine with ADME and PK characteristics suggesting suitability for once-daily oral dosing (23). The data we report herein confirm and extend these findings.
The in vitro antimicrobial profile of TBA-354 indicates a spectrum of activity and frequency of resistance development similar to those of Pa and activity against M. tuberculosis superior to that of Pa and similar to that of delamanid. As expected, because the antitubercular mode of action of the nitroimidazoles is different from that of currently used TB drugs, TBA-354, like delamanid and Pa (11, 15), is active against M. tuberculosis clinical isolates with differing drug susceptibility profiles. The potency advantage of TBA-354 compared to that of Pa seen against the H37Rv strain is maintained against these clinical isolates; however, for both Pa and TBA-354, the range of MIC values was relatively large, especially if the MIC for the H37Rv strain is compared to the highest MICs observed against the clinical isolates. Differences in the MICs against M. tuberculosis H37Rv (0.034 μM for Pa and 0.004 μM for TBA-354) and the clinical isolates (0.38 to 1.39 μM for Pa and <0.02 to 0.36 μM for TBA-354) may be due at least in part to minor protocol differences, including the composition of the testing medium, between the University of Illinois (where testing against H37Rv was carried out) and the Beijing Tuberculosis and Thoracic Tumor Research Institute (where the clinical isolates were tested). The magnitudes of the ranges of MICs seen against the clinical isolates tested here (3.6-fold for Pa and >18-fold for TBA-354) are similar to those reported previously for Pa (0.015 to 0.25 μg/ml [16.7-fold]) (15) and for delamanid (0.006 to 0.024 μg/ml [4-fold]) (11). This suggests that variability in MICs across clinical isolates on this order is characteristic of compounds of this class. It will be important to compare the MIC values of Pa and TBA-354 against a much larger panel of M. tuberculosis clinical isolates representative of phylogenetic and geographical diversity, with a range of drug susceptibility profiles, and this is the subject of an ongoing study.
Of note, activity is maintained against NR M. tuberculosis strains which are refractory to many currently used TB drugs (26, 27) and may be present during clinical infection. The mechanism of action of TBA-354 is assumed to be the same as that for Pa as described above; the similar frequency of resistance and the similar relative activities of Pa and TBA-354 against replicating and NR M. tuberculosis support this assumption. Confirmation will require further work, and as part of that, evaluation of the cross-resistance between Pa, TBA-354, and delamanid is planned.
A previous experiment conducted using the same preclinical model of TB as was used in the present work demonstrated that the efficacy of TBA-354 is superior to that of Pa when administered at the same dose and similar to that of delamanid against acute and chronic murine TB (23). Using the same low-dose aerosol infection model, these findings were extended here to demonstrate significant dose- and time-dependent bactericidal activity of TBA-354 against chronic murine TB. In these in vivo experiments, TBA-354 was, in general, at least as potent as delamanid and exhibited significant bactericidal activity at doses as low as 3 mg/kg.
A facile explanation for the superior in vivo potency of TBA-354 over Pa is that, in addition to the lower MIC and MBC of TBA-354 compared to those of Pa, TBA-354 has a longer plasma half-life in mice than Pa and demonstrated a greater plasma AUC following oral dosing to mice at 40 mg/kg (23). The important PK/pharmacodynamic (PD) drivers of efficacy for Pa against murine TB are time/MIC, followed by AUC/MIC (13). Although we have not formally identified the PK/PD drivers of efficacy for TBA-354, if assumed to be the same as for those for Pa and applicable to all of these murine TB models, then the combination of the lower MIC and longer half-life and greater AUC of TBA-354 explain its improved potency in mice. In this study, at the 10-mg/kg dose level, the efficacy demonstrated by TBA-354 was not significantly different from that seen for delamanid given at 30 mg/kg or 100 mg/kg for the same treatment duration, except for 100 mg/kg delamanid at the 8-week time point. This result cannot be easily explained by differences in MICs and MBCs, which are similar for the two compounds and may reflect differences in factors such as metabolism, protein or tissue binding, or plasma or tissue concentrations. Indeed, the PK properties of TBA-354, including the long half-life and a Vz value indicating distribution into tissues, necessitated measures to mitigate a possible confounding “carryover effect,” whereby drug in lung tissues can be carried over to the agar plates used for enumeration of CFU during efficacy studies. A 3-day washout period between the end of dosing and the sacrificing of mice was employed and found to be sufficient; lung homogenates, when plated undiluted and as serial dilutions, always exhibited the expected relationship between the homogenate dilution and CFU, even following administration of the highest dose of compound used for the longest treatment duration.
A limitation of the efficacy studies reported here is that only one preclinical model of TB with one endpoint (lung CFU) is used. A comparison of the efficacy of delamanid, Pa, and TBA-354 against TB in other mouse models, in addition to other preclinical species and with other infecting M. tuberculosis strains will be important to corroborate our findings. These types of evaluations have begun for Pa, providing a baseline for the comparison of TBA-354 and delamanid (13, 31). It will also be of interest to assess the contribution of TBA-354 to the bactericidal and sterilizing efficacy of drug combinations. Toward this, work to measure the time to relapse-free cure of mice by drug combinations, including TBA-354 or Pa, is ongoing through a collaboration between the TB Alliance and Johns Hopkins University. The first of these studies is presented in the accompanying article by Tasneen et al. (32). In the end, only studies of this compound in TB patients will clarify whether the improved potency of TBA-354 seen here in mice is clinically relevant.
In addition to the improved potency of TBA-354 against M. tuberculosis strains compared to with that of Pa, its in vitro ADME and rodent PK profiles predict good clinical bioavailability and a clinical half-life suitable for once-daily dosing. These favorable properties differentiate TBA-354 from delamanid, which is absorption limited. Finally, in vitro studies predict a low risk of CYP-mediated drug-drug interactions, an important attribute for a potential TB drug that is likely to be coadministered with antiretroviral therapies for HIV-coinfected patients, as well as with other TB drugs.
As with all potential TB drugs, information on the safety and toxicology of TBA-354 and on its clinical PK properties are needed to determine whether the clinical plasma levels expected to be required for efficacy are in fact achievable and can be attained safely. Prolongation of the QT interval has been reported for delamanid (3), and this has emerged as a liability for several TB drugs, complicating their administration together as regimens. This and all aspects of the safety profile of TBA-354 need to be carefully considered within the context of possible companion drugs, to allow proper evaluation of the potential utility of this compound. However, the data presently at hand suggest that TBA-354 has the potential to be a next-generation nitroimidazole for TB and should be studied further to allow a more sophisticated assessment. Further work to evaluate its efficacy in preclinical models of TB is ongoing as well as PK, safety, and toxicology studies.
ACKNOWLEDGMENTS
This work was supported by the Bill & Melinda Gates Foundation, the U.S. Agency for International Development (USAID), the U.K. Department for International Development (DFID), the Directorate General for International Cooperation of the Netherlands (DGIS), and Irish Aid.
REFERENCES
- 1.Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 2.Palomino JC, Martin A. 2013. TMC207 becomes bedaquiline, a new anti-TB drug. Future Microbiol 8:1071–1080. doi: 10.2217/fmb.13.85. [DOI] [PubMed] [Google Scholar]
- 3.Diacon AH, Donald PR, Pym A, Grobusch M, Patientia RF, Mahanyele R, Bantubani N, Narasimooloo R, De Marez T, van Heeswijk R, Lounis N, Meyvisch P, Andries K, McNeeley DF. 2012. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother 56:3271–3276. doi: 10.1128/AAC.06126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grosset JH, Ammerman NC. 2013. Dose-ranging activity of the newly registered antituberculosis drug bedaquiline (TMC207). Expert Rev Anti Infect Ther 11:649–651. doi: 10.1586/14787210.2013.811848. [DOI] [PubMed] [Google Scholar]
- 5.Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas-Vasquez DE, Gao M, Awad M, Park SK, Shim TS, Suh GY, Danilovits M, Ogata H, Kurve A, Chang J, Suzuki K, Tupasi T, Koh WJ, Seaworth B, Geiter LJ, Wells CD. 2012. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med 366:2151–2160. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- 6.Diacon AH, Dawson R, du Bois J, Narunsky K, Venter A, Donald PR, van Niekerk C, Erondu N, Ginsberg AM, Becker P, Spigelman MK. 2012. Phase II dose-ranging trial of the early bactericidal activity of PA-824. Antimicrob Agents Chemother 56:3027–3031. doi: 10.1128/AAC.06125-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moffett M, McGill MI. 1960. Treatment of trichomoniasis with metronidazole. Br Med J 2:910–911. doi: 10.1136/bmj.2.5203.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee T, Boshoff H. 2011. Nitroimidazoles for the treatment of TB: past, present and future. Future Med Chem 3:1427–1454. doi: 10.4155/fmc.11.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll MW, Jeon D, Mountz JM, Lee JD, Jeong YJ, Zia N, Lee M, Lee J, Via LE, Lee S, Eum SY, Lee SJ, Goldfeder LC, Cai Y, Jin B, Kim Y, Oh T, Chen RY, Dodd LE, Gu W, Dartois V, Park SK, Kim CT, Barry CE III, Cho SN. 2013. Efficacy and safety of metronidazole for pulmonary multidrug-resistant tuberculosis. Antimicrob Agents Chemother 57:3903–3909. doi: 10.1128/AAC.00753-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashtekar DR, Costa-Perira R, Nagrajan K, Vishvanathan N, Bhatt AD, Rittel W. 1993. In vitro and in vivo activities of the nitroimidazole CGI 17341 against Mycobacterium tuberculosis. Antimicrob Agents Chemother 37:183–186. doi: 10.1128/AAC.37.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto M, Hashizume H, Tomishige T, Kawasaki M, Tsubouchi H, Sasaki H, Shimokawa Y, Komatsu M. 2006. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med 3:e466. doi: 10.1371/journal.pmed.0030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diacon AH, Dawson R, Hanekom M, Narunsky K, Venter A, Hittel N, Geiter LJ, Wells CD, Paccaly AJ, Donald PR. 2011. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis 15:949–954. doi: 10.5588/ijtld.10.0616. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad Z, Peloquin CA, Singh RP, Derendorf H, Tyagi S, Ginsberg A, Grosset JH, Nuermberger EL. 2011. PA-824 exhibits time-dependent activity in a murine model of tuberculosis. Antimicrob Agents Chemother 55:239–245. doi: 10.1128/AAC.00849-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Contreras L, Sung JC, Muttil P, Padilla D, Telko M, Verberkmoes JL, Elbert KJ, Hickey AJ, Edwards DA. 2010. Dry powder PA-824 aerosols for treatment of tuberculosis in guinea pigs. Antimicrob Agents Chemother 54:1436–1442. doi: 10.1128/AAC.01471-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stover CK, Warrener P, VanDevanter DR, Sherman DR, Arain TM, Langhorne MH, Anderson SW, Towell JA, Yuan Y, McMurray DN, Kreiswirth BN, Barry CE, Baker WR. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962–966. doi: 10.1038/35016103. [DOI] [PubMed] [Google Scholar]
- 16.Singh R, Manjunatha U, Boshoff HI, Ha YH, Niyomrattanakit P, Ledwidge R, Dowd CS, Lee IY, Kim P, Zhang L, Kang S, Keller TH, Jiricek J, Barry CE III. 2008. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 322:1392–1395. doi: 10.1126/science.1164571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manjunatha U, Boshoff HI, Barry CE. 2009. The mechanism of action of PA-824: novel insights from transcriptional profiling. Commun Integr Biol 2:215–218. doi: 10.4161/cib.2.3.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurumurthy M, Rao M, Mukherjee T, Rao SP, Boshoff HI, Dick T, Barry CE III, Manjunatha UH. 2013. A novel F(420)-dependent anti-oxidant mechanism protects Mycobacterium tuberculosis against oxidative stress and bactericidal agents. Mol Microbiol 87:744–755. doi: 10.1111/mmi.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson R, Diacon A. 2013. PA-824, moxifloxacin and pyrazinamide combination therapy for tuberculosis. Expert Opin Investig Drugs 22:927–932. doi: 10.1517/13543784.2013.801958. [DOI] [PubMed] [Google Scholar]
- 20.Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, van Niekerk C, Everitt D, Winter H, Becker P, Mendel CM, Spigelman MK. 2012. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet 380:986–993. doi: 10.1016/S0140-6736(12)61080-0. [DOI] [PubMed] [Google Scholar]
- 21.Nuermberger E, Tyagi S, Tasneen R, Williams KN, Almeida D, Rosenthal I, Grosset JH. 2008. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob Agents Chemother 52:1522–1524. doi: 10.1128/AAC.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams K, Minkowski A, Amoabeng O, Peloquin CA, Taylor D, Andries K, Wallis RS, Mdluli KE, Nuermberger EL. 2012. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother 56:3114–3120. doi: 10.1128/AAC.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kmentova I, Sutherland HS, Palmer BD, Blaser A, Franzblau SG, Wan B, Wang Y, Ma Z, Denny WA, Thompson AM. 2010. Synthesis and structure-activity relationships of aza- and diazabiphenyl analogues of the antitubercular drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824). J Med Chem 53:8421–8439. doi: 10.1021/jm101288t. [DOI] [PubMed] [Google Scholar]
- 24.Collins L, Franzblau SG. 1997. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother 41:1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falzari K, Zhu Z, Pan D, Liu H, Hongmanee P, Franzblau SG. 2005. In vitro and in vivo activities of macrolide derivatives against Mycobacterium tuberculosis. Antimicrob Agents Chemother 49:1447–1454. doi: 10.1128/AAC.49.4.1447-1454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franzblau SG, DeGroote MA, Cho SH, Andries K, Nuermberger E, Orme IM, Mdluli K, Angulo-Barturen I, Dick T, Dartois V, Lenaerts AJ. 2012. Comprehensive analysis of methods used for the evaluation of compounds against Mycobacterium tuberculosis. Tuberculosis (Edinb) 92:453–488. doi: 10.1016/j.tube.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Cho SH, Warit S, Wan B, Hwang CH, Pauli GF, Franzblau SG. 2007. Low-oxygen-recovery assay for high-throughput screening of compounds against nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother 51:1380–1385. doi: 10.1128/AAC.00055-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godfrey K. 1985. Statistics in practice. Comparing the means of several groups. N Engl J Med 313:1450–1456. doi: 10.1056/NEJM198512053132305. [DOI] [PubMed] [Google Scholar]
- 29.Hidalgo IJ, Raub TJ, Borchardt RT. 1989. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736–749. [PubMed] [Google Scholar]
- 30.Banker MJ, Clark TH, Williams JA. 2003. Development and validation of a 96-well equilibrium dialysis apparatus for measuring plasma protein binding. J Pharm Sci 92:967–974. doi: 10.1002/jps.10332. [DOI] [PubMed] [Google Scholar]
- 31.Dutta NK, Alsultan A, Gniadek TJ, Belchis DA, Pinn ML, Mdluli KE, Nuermberger EL, Peloquin CA, Karakousis PC. 2013. Potent rifamycin-sparing regimen cures guinea pig tuberculosis as rapidly as the standard regimen. Antimicrob Agents Chemother 57:3910–3916. doi: 10.1128/AAC.00761-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tasneen R, Williams K, Amoabeng O, Minkowski A, Mdluli KE, Upton AM, Nuermberger EL. 2015. Contribution of the nitroimidazoles PA-824 and TBA-354 to the activity of novel regimens in murine models of tuberculosis. Antimicrob Agents Chemother 59:129–135. doi: 10.1128/AAC.03822-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


