Abstract
Hypericin, a natural compound from Hypericum perforatum (St. John's wort), has been identified as a specific inhibitor of Leishmania donovani spermidine synthase (LdSS) using integrated computational and biochemical approaches. Hypericin showed in vitro inhibition of recombinant LdSS enzyme activity. The in vivo estimation of spermidine levels in Leishmania promastigotes after hypericin treatment showed significant decreases in the spermidine pools of the parasites, indicating target specificity of the inhibitor molecule. The inhibitor, hypericin, showed significant antileishmanial activity, and the mode of death showed necrosis-like features. Further, decreased trypanothione levels and increased glutathione levels with elevated reactive oxygen species (ROS) levels were observed after hypericin treatment. Supplementation with trypanothione in the medium with hypericin treatment restored in vivo trypanothione levels and ROS levels but could not prevent necrosis-like death of the parasites. However, supplementation with spermidine in the medium with hypericin treatment restored in vivo spermidine levels and parasite death was prevented to a large extent. The data overall suggest that the parasite death due to spermidine starvation as a result of LdSS inhibition is not related to elevated levels of reactive oxygen species. This suggests the involvement of spermidine in processes other than redox metabolism in Leishmania parasites. Moreover, the work provides a novel scaffold, i.e., hypericin, as a potent antileishmanial molecule.
INTRODUCTION
Leishmaniasis is a widespread tropical disease caused by the protozoan parasite Leishmania, which belongs to the order Kinetoplastida and the family Trypanosomatidae. This parasite is transmitted by the vector sandfly, of the genera Phlebotomus and Lutzomyia. There are three forms of clinical manifestations of leishmaniasis, depending on the type of infecting species, namely, cutaneous, mucocutaneous, and visceral leishmaniasis (1). The most deadly form of the disease is visceral leishmaniasis, also known as kala-azar, which is caused mainly by Leishmania donovani in India. According to WHO statistics, 350 million people in 88 countries are at risk of developing the disease. Every year there are 2 million new cases of the disease, of which 0.5 million are cases of visceral leishmaniasis (2, 3). The treatment of leishmaniasis relies mainly on chemotherapy, as there are no vaccines available. Although many drugs are available on the market, they have several limitations (4, 5), indicating a need for novel drug candidates with specific drug targets.
All aerobic organisms are exposed to oxidative stress and generate toxic reactive oxygen species (ROS) that can degrade DNA, modify proteins, and adversely affect survival of the organism. The amastigote form of the parasite resides and multiplies inside macrophages, which produce large amounts of hydrogen peroxide. Leishmania parasites are reported to be very sensitive to oxidative stress if the functions of trypanothione metabolism enzymes are disrupted (6). The trypanothione system responsible for removal of oxidative stress in Leishmania parasites is unique but is analogous to mammalian host glutathione systems. Trypanothione, bis(glutathionyl)spermidine, is exclusively found in parasitic protozoa of the order Kinetoplastida, such as trypanosomes and Leishmania. Spermidine synthase, a key enzyme for the second step of trypanothione synthesis, catalyzes the formation of spermidine. Glutathione and spermidine are used by trypanothione synthetase for trypanothione synthesis. Thus, inhibition of spermidine synthase is likely to starve the parasite with respect to spermidine for trypanothione synthesis. There are reports showing that the enzyme spermidine synthase is necessary for the survival and virulence of the parasite and shows only 56% amino acid identity with its human counterpart (7). However, it remains unclear whether the death of parasites is because of increased levels of reactive oxygen species due to decreases in the production of spermidine leading to low levels of trypanothione, because of other possible roles of spermidine in parasite survival, or both. It is worth mentioning that spermidine is essential for hypusine modification of the translation factor eIF5A in some organisms. Spermidine performs diverse cellular functions in various other organisms (8). In the case of mammals, spermidine is involved in inhibition of neuronal nitric oxide synthase and transcription of RNA through stimulation of T4 polynucleotide kinase and T7 RNA polymerase activity (9, 10). Spermidine is also reported to be involved in autophagy, a process essential for removal of damaged organelles and potentially toxic protein aggregates, in the case of Caenorhabditis elegans (11). Thus, spermidine starvation in parasites, leading to parasite death, may be due to other factors completely unrelated to ROS generation.
Thus, inhibiting Leishmania donovani spermidine synthase (LdSS) could adversely affect survival of the parasite, making this a potent and effective drug target for leishmaniasis. We have designed a novel specific inhibitor of LdSS and investigated the effects of the inhibitor on Leishmania promastigotes. Overall, the study provides conclusive evidence that the inhibition of spermidine synthase results in spermidine starvation, an increase in ROS levels, and necrosis-like death. However, the necrotic death due to spermidine starvation is not linked to elevated ROS levels in the pathogen.
MATERIALS AND METHODS
Parasites, cell lines, and chemicals.
Leishmania donovani (MHOM/IN/2010/BHU1081) was obtained from Shyam Sundar (Banaras Hindu University, Varanasi, India). The macrophage cell line (J774A.1) used in the study was obtained from the National Centre for Cell Science (NCCS) (Pune, India). The procedures for Leishmania donovani and macrophage cell line J774A.1 were optimized and reported in our previous publications (12–15). Decarboxylated S-adenosylmethionine (dcSAM) was given by Keijiro Sameijima (Tokyo Metropolitan Kamagome Hospital, Tokyo, Japan). Spermidine synthase cDNA was obtained as a clone in pBluescript bacterial vector from Sigrid Roberts (Pacific University School of Pharmacy, Hillsboro, OR). CM-H2DCFDA (chloromethyl 2′,7′-dichlorodihydrofluorescein diacetate acetyl ester) dye and the apoptosis kit were obtained from Life Technologies. Hypericin and all other chemicals used in the experiments were of the highest grade available from Sigma-Aldrich or Merck. Protein molecular weight markers and DNA markers were purchased from New England BioLabs.
Computational methods.
In this study, a previously modeled protein structure of leishmanial spermidine synthase was used to dock large numbers of chemical compounds. Hypericin showed good docking statistics and was selected for further analysis. The details of the structural modeling procedure, structure validation, and structural differences of leishmanial spermidine synthase (LdSS) versus human spermidine synthase (HSS) have already been reported (16). The crystal structure of human spermidine synthase (HSS) was also docked with hypericin, following the same protocol as described above. Molecular dynamics (MD) simulations were also performed, as reported in our earlier publication (16). Minimization, heating, and equilibration of the LdSS-hypericin complex were run using a small force restraint of 10 kcal/mol/Å2 over hypericin.
Cloning, expression, and purification of spermidine synthase.
Spermidine synthase cDNA (900 bp) obtained as a clone in the pBluescript bacterial vector was used as a template to amplify the cDNA. The cDNA was amplified using forward (5′-GGCGCTAGCATGCCAGGCCCCGGTCTT-3′ [underlining indicates an NheI site]) and reverse (5′-CCGGATCCTCGAGCTACTCGTTCAGGTG-3′ [underlining indicates a BamHI site]) primers. The spermidine synthase cDNA was then cloned into the NheI and BamHI restriction sites of the pET-28a(+) vector. This fusion vector was further transformed into BL21(DE3) cells for overexpression of recombinant spermidine synthase. The transformed colonies were picked and allowed to grow at 37°C until an absorbance at 600 nm of 0.5 to 0.6 was obtained. The cells were treated with 1 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG) at 25°C for 8 h and then harvested by centrifugation at 7,000 rpm for 10 min at 4°C. The pellet was dissolved in lysis buffer (50 mM sodium phosphate buffer, 0.1 mM EDTA, 0.1 mM dithiothreitol [DTT], 200 mM sodium chloride [pH 7.4]). The cells were lysed by sonication, and cell debris was separated by centrifugation at 12,000 rpm for 40 min. The supernatant was collected, and protein was purified using nickel-nitrilotriacetic acid (Ni-NTA) affinity chromatography. Enzyme concentrations were determined using the Bradford assay.
Enzymatic assay and inhibition studies.
The spermidine synthase assay mixture (40 μl) contained 5 μg LdSS, substrates of spermidine synthase, i.e., putrescine and decarboxylated S-adenosylmethionine (dcSAM), and 0.1 mM DTT in 100 mM sodium phosphate buffer (pH 7.4). The enzymatic assay was carried out at 37°C for 2 h. Levels of the product formed, spermidine, were estimated using a method reported earlier (17, 18). In brief, 40 μl of the assay mixture was taken and derivatized by adding 40 μl of saturated sodium carbonate and 120 μl of 75 mM dansyl chloride. The reaction mixture was incubated for 2 h at 60°C in the dark. After incubation, the mixture was centrifuged, and 20 μl of the supernatant was analyzed using high-performance liquid chromatography (HPLC) with a C18 column (Dionex Ultimate 3000 system). The dansylated polyamines were detected with excitation and emission wavelengths of 360 nm and 510 nm, respectively. The separation was performed using a gradient of mobile phase A (50 mM ammonium formate [pH 5.0]/acetonitrile, 90:10) and mobile phase B (50 mM ammonium formate [pH 5.0]/acetonitrile, 10:90), with mobile phase B being varied from 55% to 100% over 20 min to separate the polyamines (17, 18). Determination of Km and Vmax for a substrate was performed by varying the concentration of that substrate while keeping a saturating concentration of the other substrate. The concentration of dcSAM was varied from 30 μM to 100 μM, and that of putrescine was varied from 0.1 mM to 2 mM.
The inhibitor identified in silico, hypericin, was assessed against recombinant spermidine synthase. For inhibition studies, the enzyme was incubated with hypericin (100 μM), keeping a saturating concentration of one substrate while varying the concentration of the other substrate in the assay buffer. The data were plotted in a double-reciprocal plot for calculation of the inhibitory constant (Ki).
Cell viability assay.
The effect of hypericin on the survival of Leishmania promastigotes was analyzed by using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (19), as reported in our earlier publications (14, 15). In brief, 200 μl of Leishmania promastigotes (2.5 × 106 cells/ml) was treated with various doses (10 μM, 20 μM, 40 μM, 80 μM, and 100 μM) of hypericin for 24 h at 25°C. Promastigotes were then centrifuged to remove the medium. Subsequently, 200 μl of 0.5 mg/ml MTT was added to the promastigotes, and the mixture was incubated for 4 h. After the incubation period, MTT was removed from the promastigotes, and the formazan formed was dissolved in dimethyl sulfoxide (DMSO). Appropriate blanks were used for each concentration of hypericin-treated and untreated promastigotes.
Reactive oxygen species detection.
Endogenous ROS levels were detected by using CM-H2DCFDA, which is a cell-permeable fluorescent probe. Untreated Leishmania promastigotes (2.5 × 106 cells/ml) were incubated with 20 μM CM-H2DCFDA for 45 min at 25°C. Similarly, reactive oxygen species levels were determined in Leishmania promastigotes treated with hypericin (18 μM) for different time periods (1.5 h, 3 h, 6 h, 12 h, and 24 h). Promastigotes were also pretreated with N-acetylcysteine (NAC) before hypericin treatment, to check the attenuation of ROS development due to hypericin. NAC pretreatment involved treatment with 20 mM NAC for 30 min. ROS production was also monitored in Leishmania promastigotes with trypanothione (0.5 μM) or spermidine (100 μM) supplementation followed by treatment with hypericin (18 μM). The fluorescence of the probe, indicating ROS levels, was assessed and analyzed by using a BD FACSCalibur flow cytometer and CellQuestPro software (12–15).
Analysis of apoptosis/necrosis of Leishmania promastigotes.
Analysis of apoptotic/necrotic features was performed as reported in our earlier publications, using an annexin V-fluorescein isothiocyanate (FITC) apoptosis kit from Life Technologies (14, 15). Untreated promastigotes of Leishmania donovani were used at a cell density of 2.5 × 106 cells/ml, and staining with annexin V-FITC and propidium iodide (PI) was performed according to the instructions given by the manufacturer. The fluorescence of the stained promastigotes was detected with a BD FACSCalibur flow cytometer, and the results were analyzed using CellQuestPro software (14). Similarly, hypericin (18 μM)-treated promastigotes, with or without trypanothione (0.5 μM) or spermidine (100 μM) supplementation, were analyzed for apoptosis/necrosis. Leishmania promastigotes treated with miltefosine (50 μM) for 24 h were used as positive controls for apoptosis. Promastigotes pretreated with NAC and then treated with hypericin were also analyzed, to determine the mode of cell death.
Intracellular thiol detection.
The in vivo thiol (trypanothione and glutathione) levels in Leishmania donovani promastigotes were measured by HPLC, using a method reported earlier (14, 20, 21). In brief, 10 ml of Leishmania promastigotes (2.5 × 106 cells/ml) were treated with hypericin (18 μM) for 12 h or 24 h. These promastigotes were collected and washed twice with phosphate-buffered saline (PBS). Equal numbers of untreated or treated promastigotes (1 × 107 cells) were resuspended in 50 μl of 50 mM HEPES (pH 8.0) containing 5 mM EDTA, followed by the addition of 0.1 ml of 2 mM monobromobimane (dissolved in 100% ethanol). The suspension was then incubated at 70°C for 3 min. After incubation, 0.2 ml of ice-cold 25% trichloroacetic acid solution was added, and the mixture was incubated on ice for 20 min. Finally, the volume of derivatized lysate was 350 μl; therefore, the intracellular thiol levels indicate concentrations in cell lysates of 1 × 107 Leishmania promastigotes diluted to 350 μl in the process of derivatization. Cellular debris and denatured proteins were separated by centrifugation, and acid-soluble thiols were separated using HPLC (Dionex Ultimate 3000 system) with a C18 column and a linear gradient of 50 to 100% methanol in 0.25% acetic acid (pH 3.5). The fluorescence of bimane, with emission and excitation wavelengths of 360 nm and 450 nm, respectively, was used to identify the thiols. The retention times were compared with those of standard thiols after the same treatment. To verify the uptake of thiols after supplementation, Leishmania promastigotes were supplemented with trypanothione (0.5 μM) and treated with hypericin (18 μM) for the aforementioned time intervals and thiol levels were estimated using the same procedure. Thiol levels in untreated Leishmania promastigotes were also determined, for comparison.
Intracellular spermidine detection.
In vivo spermidine concentrations were assessed after hypericin treatment (18 μM). Briefly, 1 ml of Leishmania promastigotes (2.5 × 106 cells/ml) was treated with hypericin for 12 h and 24 h. Equal numbers of untreated and treated promastigotes (1 × 106 cells) were used to compare the spermidine pools. Promastigotes were washed with PBS and centrifuged to collect cells. Subsequently, promastigotes were lysed in 40 μl phosphate buffer and spermidine levels were estimated using methods reported earlier (17, 18). In brief, lysed promastigotes (40 μl) were used to derivatize polyamines by adding 120 μl of 75 mM dansyl chloride and 40 μl of saturated sodium carbonate, bringing the final volume of the derivatized lysates to 200 μl. Therefore, the spermidine levels indicate the concentrations in cell lysates of 1 × 106 Leishmania promastigotes diluted to 200 μl in the process of derivatization. To verify uptake of spermidine as well as changes in the spermidine pools of the parasites after spermidine (100 μM) or trypanothione (0.5 μM) supplementation followed by hypericin (18 μM) treatment, the in vivo spermidine levels were determined using the same procedure for all of these conditions. In vivo spermidine concentrations of untreated promastigotes were also estimated, for comparison. Higher concentrations of spermidine (100 μM) were used for supplementation, as the less efficient spermidine transporter is not able to restore decreased spermidine levels at low concentrations in the time frame used in the study.
Statistical analysis.
The statistical significance of differences between two groups was analyzed with Student's unpaired t test, using SigmaPlot software. Differences with P values of <0.05 and <0.01 were considered significant.
RESULTS
Hypericin was identified as a potential inhibitor of spermidine synthase using a bioinformatic approach.
Natural products from the ZINC database were virtually screened against the predicted protein structure of LdSS. The screened natural products with high scores for docking with LdSS were cross-docked with HSS. The natural products that showed high docking scores with LdSS but failed to dock with HSS or had very low docking scores with HSS were selected for further studies. Molecular docking of LdSS with hypericin yielded a docking score of −8.23 kcal/mol, which signifies high binding affinity between LdSS and hypericin. Hypericin was also docked at the active site of human spermidine synthase (HSS) but it could score just −3.28 kcal/mol with HSS, compared with −8.23 kcal/mol with LdSS. The much lower docking score of hypericin with HSS signifies the very low binding affinity of hypericin for HSS, compared to LdSS.
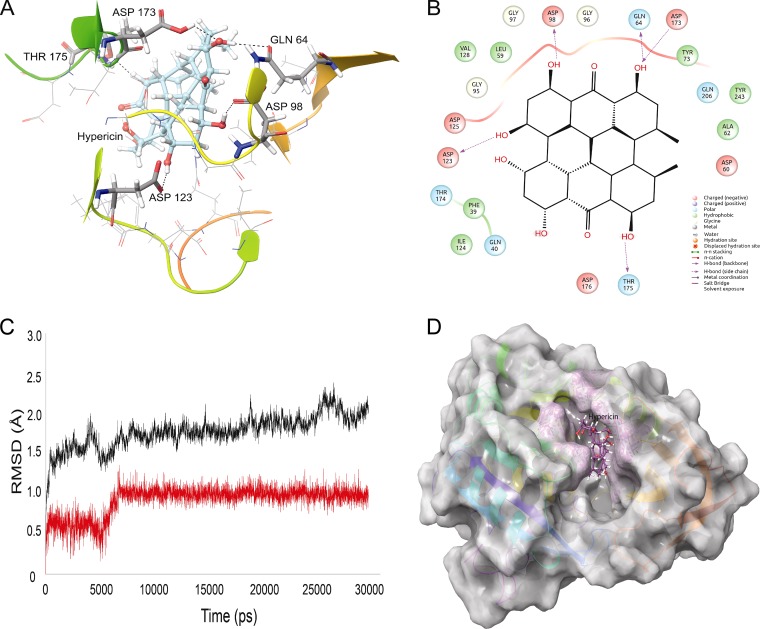
The structures of all spermidine synthases are characterized by the presence of a “gatekeeping loop” that acts as a gatekeeper of the active site and also is responsible for variations in the substrate specificity of the enzyme. In LdSS, the gatekeeping loop extends from residue 173 to residue 180 and surrounds the active site. Despite the gatekeeping loop, the active site of LdSS is composed of negatively charged residues such as Asp98 and Asp123 and hydrophobic residues Leu59, Tyr73, Gly95, Gly96, Gly97, and Ile124. Hence, the active site of LdSS is rich in negatively charged and hydrophobic residues. Hypericin was found to interact with residues Gln64, Asp98, Asp123, Asp173, and Thr175 via hydrogen bonding and with Leu59, Gly96, and Ile124 via hydrophobic interactions (Fig. 1A and B). The presence of 6 hydroxyl groups in hypericin facilitates its hydrogen bonding with polar residues of the active site, while its ring structure adjusts well with hydrophobic residues of the active site of LdSS. Hypericin forms hydrogen bonds with Asp173 and Thr175 (gatekeeping loop residues), which fix the loop movement and allow hypericin to be stabilized at the active site. This stabilized form of hypericin was consistent with 30-ns MD simulations. The protein backbone was found to be highly stable (Fig. 1C), with a low root mean square deviation (RMSD) of around 1.5 to 2 Å throughout the 30-ns MD simulation. A stable backbone signifies that the overall structure of LdSS remains the same, and the active site conformation of LdSS also should be unaffected. The RMSD of hypericin at the active site of LdSS was also very stable, with a consistent value of 1 Å throughout the MD simulation (Fig. 1C). Hypericin remained at the active site during the simulation, surrounded by the gatekeeping loop (Fig. 1D). Hypericin's rich interactions with the LdSS active site and its stability during the MD simulation indicate that it could serve as a potential inhibitor of LdSS.
FIG 1.
Interactions of LdSS with hypericin in molecular docking simulations. (A) Hypericin interacting with the active site residues of LdSS. Hydrogen-bond-forming active site residues are displayed in tube representation and labeled. Asp173 and Thr175 belong to the gatekeeping loop of LdSS, which is a key structure in the opening and closing of the active site of LdSS. (B) Two-dimensional representation of interactions between LdSS and hypericin. (C) RMSD graph of the protein backbone (black) with hypericin at the active site (red). Clearly, overall the protein backbone is highly stable throughout the MD simulation, with very low RMSD values of ∼1.5 to 2.0 Å. Hypericin slightly adjusts its position within the active site of LdSS between 5 and 6 ns and becomes stable until the simulation is completed. (D) Hypericin (purple) buried in the active site of LdSS (white) after 30 ns of MD simulation.
Differential binding of hypericin with LdSS and HSS might be explained on the basis of differences between the active sites. Although the protein sequences of HSS and LdSS exhibit high levels of similarity, there are major differences in the amino acid compositions of their active sites, leading to different sizes, shapes, and charge distributions. Cavity analysis of HSS and LdSS by the SiteMapv3.2 module of Schrodinger revealed that the active sites of both HSS and LdSS are highly hydrophilic (65 to 70% of the total volume). Careful inspection of the hypericin structure suggests that it has a hydrophobic core structure surrounded by oxygen. Hence, it is clear that the binding of hypericin at the active site of LdSS or HSS depends on its ability to form hydrogen bonds with active site residues. During the active sites analysis, it was found that the three-dimensional positions of H-bond donors and acceptors at the active site of HSS and LdSS differ significantly. H-bond donors and acceptors of LdSS are located at the active site to support hypericin via strong H-bonding. Residues Gln40, Gln64, Asp98, Asp123, Ile124, Thr174, Thr175, Gln206, and Tyr243 form a very strong H-bond network with hypericin at the active site of LdSS. At the active site of HSS, however, most of these residues are replaced by other residues or their three-dimensional positions differ. More importantly, the conformation of the gatekeeping loop is different in the two proteins, which plays a major role in substrate recognition in spermidine synthases. In LdSS, the gatekeeping loop allows the formation of a larger active site, which can accommodate large compounds or both the product and the substrate in a single site. In HSS, on the other hand, the gatekeeping loop is too stringent at the active site and splits it into two small sites (substrate and product binding sites). The smaller active site of HSS fails to accommodate hypericin. Overall, all of these factors contribute to the differential binding behavior of hypericin with the active site of LdSS and HSS.
Recombinant LdSS was prepared.
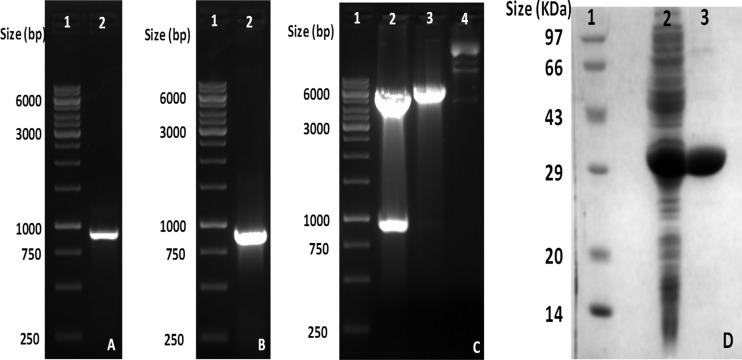
Complete cDNA for Leishmania donovani spermidine synthase obtained as a clone in pBluescript vector was subcloned in pET-28a(+) vector. The amplified cDNA band of LdSS is shown in Fig. 2A. Figure 2B and C presents the clone confirmation results for LdSS cDNA cloned in pET-28a(+) vector, by PCR and restriction digestion. Double-digestion of the clone with NheI and BamHI shows release of a DNA band of 900 bp, confirming insertion of LdSS cDNA into the pET-28a(+) vector. The clone was further confirmed by sequencing using primers for the T7 promoter and T7 terminator. This LdSS clone was transformed into the BL21(DE3) expression strain of Escherichia coli, and protein overexpression was induced using 1 mM IPTG at 25°C for 8 h. The overexpressed recombinant protein was then purified using Ni-NTA affinity chromatography. A purified band of spermidine synthase is shown in Fig. 2D, lane 3.
FIG 2.
Cloning and overexpression of LdSS in the pET28a(+) vector. (A) PCR amplification of LdSS cDNA. Lane 1, 1-kb DNA ladder; lane 2, amplified band of LdSS, showing a size of around 900 bp. (B) Confirmation of the LdSS clone in the pET28a(+) vector by PCR. Lane 1, 1-kb ladder; lane 2, amplified band of 900 bp from the LdSS clone in the pET28a(+) vector. (C) Confirmation of the LdSS clone in the pET28a(+) vector by restriction digestion. Lane 1, 1-kb ladder; lane 2, restriction digestion of the clone with NheI and BamHI, showing release of an insert of 900 bp; lane 3, restriction digestion of the clone with NheI only; lane 4, undigested LdSS clone in the pET28a(+) vector. (D) SDS-PAGE analysis of purified LdSS protein. Lane 1, protein molecular size marker; lane 2, crude supernatant; lane 3, purified recombinant LdSS protein.
Hypericin shows in vitro inhibition of spermidine synthase.
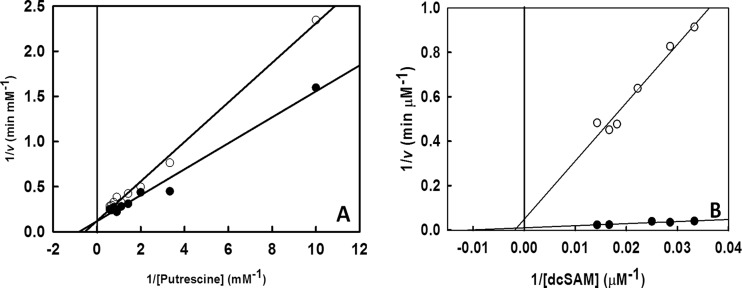
In order to find the optimal conditions for the enzyme assay, the pH (pH 2 to 12) and temperature (20°C to 90°C) were varied over a range, and the optimal values found (pH 8.0 and 37°C) were used for further experiments. The Km and Vmax values were calculated by varying the substrate concentration. The Km with respect to putrescine (KmP) was 1.23 mM, and VmaxP was 8.85 mM min−1 (Fig. 3A). The Km and Vmax with respect to dcSAM (KmS and VmaxS) were calculated to be 90.34 μM and 97.09 μM min−1, respectively (Fig. 3B).
FIG 3.
Inhibition of spermidine synthase by hypericin with various concentrations of different substrates (putrescine and dcSAM). (A) Hypericin shows competitive inhibition of spermidine synthase with respect to putrescine, with KmP = 1.23 mM, VmaxP = 8.59 mM min−1, and KiP = 201.6 mM. (B) Hypericin shows linear mixed inhibition of spermidine synthase with respect to dcSAM, with KmS = 90.9 μM, VmaxS = 97.087 μM min−1, and KiS = 3.68 μM. ●, enzyme assay without inhibitor; ○, enzyme assay in the presence of inhibitor, i.e., hypericin. The data are plotted as averages from three independent experiments.
Hypericin was found to inhibit spermidine synthase activity. The mode of inhibition was assessed with Lineweaver-Burk plots (Fig. 3). Hypericin showed a competitive mode of inhibition with respect to putrescine, since there was no change in Vmax but there was an increase in Km with respect to the control values (Fig. 3A). The inhibitory constant (Ki) with respect to putrescine (KiP) was calculated to be 201.6 μM. Hypericin showed linear mixed inhibition with respect to dcSAM, which was evident from a decrease in Vmax and an increase in Km for the inhibited enzymatic reaction, compared to the uninhibited reaction (Fig. 3B). The Ki with respect to dcSAM (KiS) was 3.68 μM.
Hypericin shows antileishmanial activity but no effect on macrophage cells.
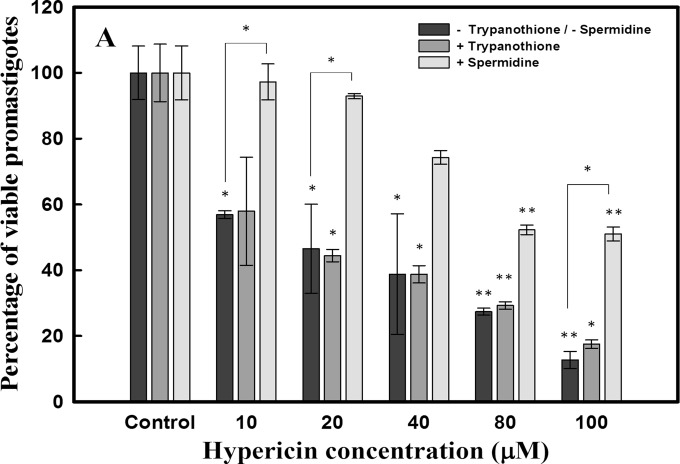
Hypericin was assessed to exhibit antileishmanial effects after 24-h treatment of promastigotes (2.5 × 106 cells/ml) with various doses (10 μM to 100 μM). Hypericin was found to display significant antileishmanial activity, with a 50% inhibitory concentration (IC50) of 18 μM (Fig. 4). This is the first evaluation of the antileishmanial effects of the compound. The novel scaffold may be used for the development of future and better antileishmanial compounds of the class.
FIG 4.
Cell viability assay with Leishmania donovani promastigotes. Leishmania promastigotes (2.5 × 106 cells/ml) were used to check the antileishmanial effects of hypericin. Promastigotes were treated with various concentrations of hypericin for 24 h, and cell viability was checked by using the MTT assay. The IC50 obtained for hypericin was 18.00 μM. Promastigotes were also supplemented with trypanothione (0.5 μM) and spermidine (100 μM) and then treated with hypericin. Promastigotes supplemented with trypanothione showed an IC50 of 17.42 μM, which is comparable to that for treated promastigotes without any supplementation. However, promastigotes supplemented with spermidine showed survival rates of ∼90% with IC50 levels of hypericin. *, P < 0.05; **, P < 0.01.
For a molecule to be an effective and potent drug candidate, it is important to have high specificity with the least adverse effects on patients. With this view, hypericin was evaluated for producing macrophage death but was found to have no significant effect on macrophages even at a concentration 4 times higher than the IC50 dose (data not shown). This indicates the high specificity of hypericin toward LdSS.
Spermidine pools are depleted after treatment with hypericin, showing target specificity of the inhibitor.
Leishmania promastigote cultures (2 ml) with cell densities of 2.5 × 106 cells/ml were treated with the IC50 dose (18 μM) of hypericin for 12 h and 24 h. Intracellular spermidine levels were determined as described in Materials and Methods. Hypericin-treated promastigotes showed diminished levels of spermidine, compared to untreated promastigotes. The amount of spermidine calculated in untreated promastigotes was 34 ± 1 μM, whereas promastigotes treated with hypericin for 12 h and 24 h showed spermidine levels of 3 ± 1 μM and 1 ± 0.1 μM, respectively (Table 1). Promastigotes were also supplemented with trypanothione (0.5 μM) followed by treatment with hypericin. Trypanothione supplementation did not restore the spermidine levels in promastigotes. The concentrations of spermidine after trypanothione supplementation followed by hypericin treatment for 12 h and 24 h were 3 ± 0.5 μM and 2 ± 0.2 μM, respectively. Spermidine levels in untreated promastigotes, treated promastigotes, and promastigotes treated after trypanothione supplementation are presented in Table 1. However, spermidine supplementation resulted in revival of intracellular spermidine pools after 24 h. Spermidine concentrations measured in Leishmania promastigotes supplemented with spermidine (100 μM) and treated with IC50 levels of hypericin for 12 h and 24 h were 7 ± 1 μM and 38 ± 8 μM, respectively (Table 1).
TABLE 1.
Intracellular trypanothione, glutathione, and spermidine levels in Leishmania promastigotes
| Metabolite | Metabolite concentration (μM)a |
||||
|---|---|---|---|---|---|
| Control | With hypericin (18 μM) treatment |
||||
| Without supplementation |
With trypanothione (0.5 μM) supplementation |
||||
| 12h | 24 h | 12 h | 24 h | ||
| Thiols | |||||
| Trypanothione | 317 ± 54 | 43 ± 2 | 39 ± 9 | 235 ± 88 | 478 ± 212 |
| Glutathione | 206 ± 17 | 739 ± 149 | 549 ± 21 | 208 ± 114 | 395 ± 123 |
| Spermidine | |||||
| Without spermidine supplementation | 34 ± 1 | 3 ± 1 | 1 ± 0.1 | 3 ± 0.5 | 2 ± 0.2 |
| With spermidine (100 μM) supplementation | 7 ± 1 | 38 ± 8 | |||
The intracellular thiol levels represent concentrations in a final volume of 350 μl containing cell lysates of 1 × 107 Leishmania promastigotes. The intracellular spermidine levels indicate concentrations of spermidine in a final volume of 200 μl containing cell lysates of 1 × 106 Leishmania promastigotes. The control shows concentrations without any treatment. Other data show concentrations with or without supplementation with treatment with hypericin (18 μM).
Hypericin alters intracellular thiol levels in Leishmania promastigotes.
Spermidine is conjugated with glutathione to form trypanothione in the presence of trypanothione synthetase, so decreases in in vivo spermidine levels should result in nonutilization of glutathione for trypanothione synthesis. This should result in diminished levels of trypanothione and elevated levels of glutathione inside the parasites. Therefore, in order to check the alterations in the thiol pools of the parasite after hypericin treatment, Leishmania promastigote cultures (10 ml) with cell densities of 2.5 × 106 cells/ml were treated with IC50 levels of hypericin for 12 h and 24 h. Intracellular thiol levels were measured by derivatizing thiols, as explained in Materials and Methods. The levels of trypanothione were found to decrease significantly after 12 h and 24 h of treatment, compared to untreated promastigotes. Trypanothione levels in untreated, 12-h-treated, and 24-h-treated promastigotes were 317 ± 54 μM, 43 ± 2 μM, and 39 ± 9 μM, respectively. This further suggests that low spermidine levels due to inhibition of LdSS in hypericin-treated promastigotes cause low in vivo concentrations of trypanothione (Table 1). However, remarkable increases in intracellular glutathione levels were observed after 12 h and 24 h of treatment, in contrast to untreated promastigotes. Glutathione levels in untreated, 12-h-treated, and 24-h-treated promastigotes were 206 ± 17 μM, 739 ± 149 μM, and 549 ± 21 μM, respectively. Thiol levels in untreated and treated promastigotes at different time points are summarized in Table 1.
Since the existence of a trypanothione transporter is evident from the literature (22), trypanothione supplementation can provide deep insight into the mode of cell death for Leishmania promastigotes after hypericin treatment. Supplementing parasites with trypanothione before hypericin treatment should result in the reversal of altered thiol levels in the parasites. In order to check the effects of trypanothione on the in vivo thiol pools of the parasites, 10-ml cultures of Leishmania donovani (2.5 × 106 cells/ml) were supplemented with 0.5 μM trypanothione followed by treatment with an IC50 dose of hypericin for 12 h and 24 h. Compared to unsupplemented promastigotes treated with hypericin, trypanothione levels were restored after trypanothione supplementation, whereas reductions in the levels of glutathione were observed. Trypanothione levels in promastigotes supplemented with trypanothione and treated with hypericin were 235 ± 88 μM and 478 ± 212 μM at 12 h and 24 h, respectively. Intracellular levels of glutathione with trypanothione supplementation were calculated to be 208 ± 114 μM and 395 ± 123 μM at 12 h and 24 h, respectively. Table 1 shows the thiol levels after supplementation with trypanothione.
Trypanothione supplementation is a futile attempt to reduce cell death but spermidine supplementation increases parasite survival rates.
Promastigotes were supplemented with 0.5 μM trypanothione and assessed for the antileishmanial activity of hypericin. The trypanothione supplementation did not prevent the death of the parasites, since the IC50 was found to be consistent with or without supplementation (Fig. 4). The viability of Leishmania promastigotes was also assessed after 100 μM spermidine supplementation followed by treatment with hypericin. Interestingly, spermidine supplementation significantly improved parasite survival rates, and ∼90% of Leishmania promastigotes survived with IC50 levels of hypericin (Fig. 4).
Production of reactive oxygen species is triggered in Leishmania promastigotes after treatment with hypericin.
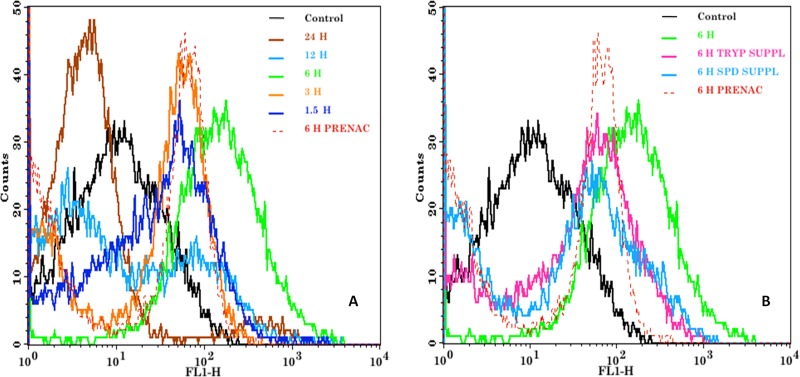
Promastigotes of Leishmania donovani were treated with an IC50 dose of hypericin (18 μM) for various time periods (1.5 h, 3 h, 6 h, 12 h, and 24 h). Compared to untreated Leishmania promastigotes, an increase in ROS levels was observed 1.5 h after treatment, with a slight increase after 3 h and a maximum at 6 h. However, lower levels of reactive oxygen species were seen in the parasites treated for 24 h (Fig. 5A). This may be due to membrane damage of the parasites and subsequent leakage of generated ROS into the medium. Promastigotes pretreated with N-acetylcysteine (NAC), a scavenger of ROS, before hypericin treatment for 6 h displayed significant reduction in the levels of reactive oxygen species, compared to the non-NAC-treated promastigotes. The changes in levels of reactive oxygen species at different time points are shown in Fig. 5A.
FIG 5.
Flow cytometric analysis of the production of reactive oxygen species in promastigotes treated with IC50 levels of hypericin for various times, i.e., 1.5 h, 3 h, 6 h, 12 h, and 24 h. Untreated samples were taken as controls, and pretreated samples were given NAC (20 mM) treatment for 30 min before treatment with an IC50 dose of hypericin for 6 h. Also, the promastigotes were supplemented with trypanothione (0.5 μM) and spermidine (100 μM) and then treated with an IC50 dose of hypericin for 6 h. The samples were stained with CM-H2DCFDA for 45 min to 1 h and then analyzed by using flow cytometry. (A) Comparison of the production of reactive oxygen species in untreated, NAC-pretreated, and treated promastigotes at different time points. (B) Comparison of the production of reactive oxygen species in promastigotes pretreated with NAC, not supplemented, or supplemented with trypanothione and spermidine separately, followed by treatment with hypericin for 6 h.
Trypanothione or spermidine supplementation produces reversal of increased ROS pools in promastigotes treated with hypericin.
Leishmania promastigotes were supplemented with 0.5 μM trypanothione or 100 μM spermidine in the medium. The supplemented promastigotes were then treated with an IC50 dose of hypericin for 6 h. The levels of ROS were reduced significantly after trypanothione or spermidine supplementation, compared to unsupplemented promastigotes treated with hypericin for the same times. The comparison of ROS levels in unsupplemented and supplemented promastigotes is shown in Fig. 5B.
Hypericin stimulates necrosis-like death in parasites.
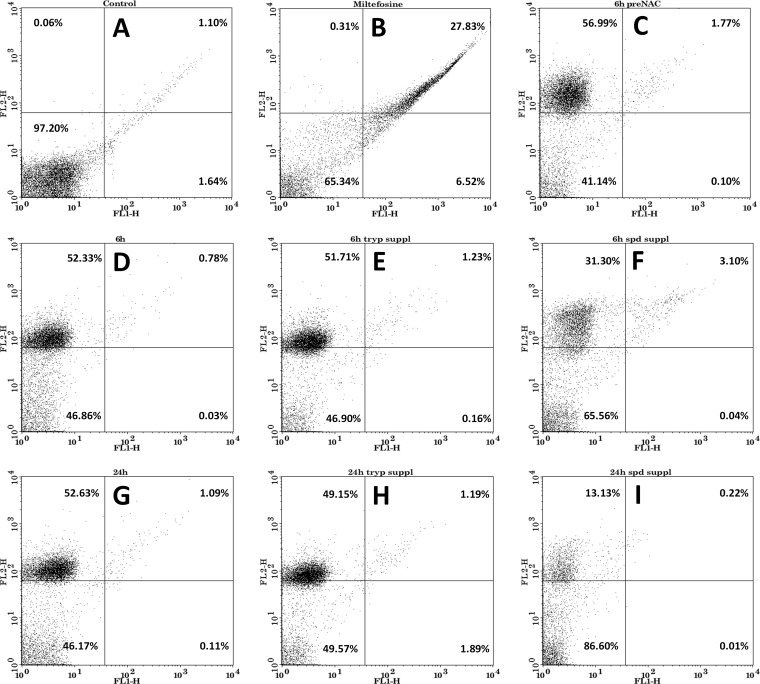
In order to check the mode of cell death after hypericin treatment, flow cytometric analysis of Leishmania donovani was performed after staining with annexin V-FITC and propidium iodide under various conditions. The negative control (promastigotes without any treatment) did not show staining with either annexin V-FITC or propidium iodide. The control for apoptosis with miltefosine (50 μM) treatment showed both annexin V-FITC and propidium iodide binding, indicating apoptosis. Leishmania donovani promastigotes treated with an IC50 dose of hypericin for 6 h and 24 h were not stained with annexin V-FITC but showed positive staining with PI, indicating that promastigotes experience necrosis-like death after hypericin treatment. NAC pretreatment before hypericin treatment did not prevent necrotic death (Fig. 6).
FIG 6.
Analysis of the mode of cell death of Leishmania promastigotes. (A) Promastigotes without any treatment. (B) Promastigotes treated with miltefosine (50 μM) for 24 h. (C) Promastigotes pretreated with N-acetylcysteine (20 mM) before hypericin (18 μM) treatment for 6 h. (D) Promastigotes treated with hypericin (18 μM) for 6 h. (E) Promastigotes supplemented with trypanothione (0.5 μM) before hypericin (18 μM) treatment for 6 h. (F) Promastigotes supplemented with spermidine (100 μM) before hypericin (18 μM) treatment for 6 h. (G) Promastigotes treated with hypericin (18 μM) for 24 h. (H) Promastigotes supplemented with trypanothione (0.5 μM) before hypericin (18 μM) treatment for 24 h. (I) Promastigotes supplemented with spermidine (100 μM) before hypericin (18 μM) treatment for 24 h. Samples were stained with annexin V-FITC and propidium iodide and were analyzed by using flow cytometry. Promastigotes were shown to undergo necrosis after treatment with hypericin.
Leishmania donovani promastigotes supplemented with 0.5 μM trypanothione in the medium were treated with an IC50 dose of hypericin for 6 h and 24 h. Flow cytometric assessment revealed that the trypanothione could not prevent parasite death, and the percentages of promastigotes undergoing necrosis-like death after trypanothione supplementation were comparable to those of promastigotes without supplementation at the respective time points (Fig. 6). Interestingly, spermidine (100 μM) supplementation in the medium before hypericin treatment prevented parasite death to a large extent. Promastigotes supplemented with spermidine showed survival rates of ∼90% with an IC50 level of hypericin. With many-fold higher levels of hypericin, however, growth inhibition was restored only 50 to 60%, suggesting some off-target effects of hypericin at higher concentrations.
DISCUSSION
Inhibiting key redox metabolism enzymes has been a good approach to discovering new drug candidates against leishmaniasis. Here, we have shown the adverse effects of inhibition of one such enzyme, spermidine synthase, on the survival of Leishmania. Hypericin is a naturally occurring anthraquinone derivative obtained from Hypericum perforatum (23). We have identified it as a strong inhibitor of the spermidine synthase of Leishmania donovani by using a computational approach. This in silico analysis was supported by biochemical assessment, which confirmed the inhibition of spermidine synthase by hypericin. Further, the effects of hypericin on Leishmania donovani promastigotes were examined, since promastigotes represent the infective stage of Leishmania. An antileishmanial assay with hypericin suggests that hypericin inhibits the survival of the parasite. Interestingly, the target specificity of hypericin was evident from the insignificant toxicity of hypericin toward macrophages and the decreases in the spermidine pools of the parasites after hypericin treatment.
Spermidine and glutathione together form trypanothione in the presence of trypanothione synthetase. Trypanothione is indispensable for maintaining redox metabolism and hence is crucial for the survival of the parasites (6, 24). Since trypanothione synthesis is directly related to the presence of spermidine, inhibition of spermidine synthesis should result in decreased trypanothione levels and increased glutathione levels inside the parasites, leading to redox imbalance and ultimately death of the parasites. The in vivo trypanothione estimation showed decreased levels of trypanothione after hypericin treatment. This alteration in the thiol pools of the parasites after hypericin treatment was reversed with trypanothione supplementation. Elevated ROS levels were observed in the parasites treated with hypericin, which could be attributed to decreased trypanothione levels in the parasites. If the ROS generation is due to decreased trypanothione levels after hypericin treatment, then supplementing the promastigotes with excess trypanothione should reverse the effects caused by oxidative stress inside the parasites. We observed that supplementation with trypanothione resulted in reduced levels of reactive oxygen species produced after hypericin treatment. However, trypanothione supplementation did not increase the survival rates of the parasites, suggesting that the oxidative stress produced due to decreases in trypanothione levels was not a cause of parasite death. Moreover, cell death due to reactive oxygen species is directed through the apoptotic pathway (25). Our results demonstrated necrosis-like death of the parasites after treatment with hypericin. This confirmed that ROS generated due to trypanothione depletion was insufficient to kill the parasites. The specificity of hypericin was also assessed by measuring the intracellular thiol and spermidine pools of the parasites.
Supplementation of spermidine in the medium allowed survival of the parasites after hypericin treatment, confirming that the death of the parasites is dependent solely on spermidine starvation due to hypericin treatment. Overall, we have determined that, although hypericin specifically targets spermidine synthase (a redox metabolism enzyme), the death of the parasites due to spermidine starvation is not related to a redox imbalance inside the parasites. This suggests the involvement of spermidine in pathways other than redox metabolism, thereby opening the door for the novel investigation of associations of spermidine with its potential interacting molecules.
Promastigotes and axenic amastigotes are capable of transporting spermidine (22). The question of polyamine salvage by parasites in infected macrophages is certainly crucial for the validation of LdSS as a therapeutic target. However, when mice were infected with a spermidine synthase-knockout strain, infectivity was significantly reduced, compared with wild-type parasites (7). This suggests that, although parasites are able to transport spermidine, there is not enough spermidine present in the phagolysosomes for salvage.
Overall, the results suggest hypericin as a potent antileishmanial molecule that inhibits LdSS, resulting in spermidine starvation. As there is no effect on macrophages even at 4 times the IC50, the effect of hypericin seems to be specific to Leishmania. The data indicate that a cellular process other than trypanothione production may be affected by spermidine depletion. Spermidine is essential in all tested eukaryotes for hypusine modification of the translation factor eIF5A, which is the only known essential function of spermidine in all eukaryotes. In fact, the enzyme that transfers the aminobutyl group from spermidine to eIF5A to form deoxyhypusine (deoxyhypusine synthase) is an essential enzyme in Leishmania donovani (26) and, as mentioned, in all tested eukaryotes. The hypusine-modified eIF5A translation factor is required for the translation of mRNA encoding proteins containing polyproline tracts (27). Therefore, it is not surprising that depletion of spermidine leads to parasite death via altered hypusine modification of the translation factor eIF5A. However, further studies are required to confirm this hypothesis. Our study also suggests that spermidine is crucial for the survival of parasites; this crucial need is linked not only to the involvement of spermidine in redox metabolism but also its importance in processes critical for parasite survival. Previous reports suggested the suitability of hypericin for therapeutic uses (23). Taken together, the work provides fundamental insights into the molecular mechanisms of antileishmanial effects of hypericin that may be used for the development of novel drugs against this neglected topical disease.
ACKNOWLEDGMENTS
Research fellowships to S. Singh, M. Das, and R. Bhardwaj by the Indian Institute of Technology Guwahati are acknowledged. Financial support by the Department of Biotechnology, government of India, in the form of a research grant (project BT/04/NE/TBP/2010) to V. K. Dubey and D. Sundar is also acknowledged.
The LdSS cDNA cloned in the pBluescript vector was a kind gift from Sigrid Roberts, Pacific University School of Pharmacy (Hillsboro, OR). We acknowledge Keijiro Sameijima, Tokyo Metropolitan Kamagome Hospital (Tokyo, Japan), for donating dcSAM.
REFERENCES
- 1.Shukla AK, Singh BK, Patra S, Dubey VK. 2010. Rational approaches for drug designing against leishmaniasis. Appl Biochem Biotechnol 160:2208–2218. doi: 10.1007/s12010-009-8764-z. [DOI] [PubMed] [Google Scholar]
- 2.Murray HW, Berman JD, Davies CR, Saravia NG. 2005. Advances in leishmaniasis. Lancet 366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 3.Stockdale L, Newton R. 2013. A review of preventative methods against human leishmaniasis infection. PLoS Negl Trop Dis 7:e2278. doi: 10.1371/journal.pntd.0002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croft SL, Coombs GH. 2003. Leishmaniasis: current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol 19:502–508. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Croft SL, Sundar S, Fairlamb AH. 2006. Drug resistance in leishmaniasis. Clin Microbiol Rev 19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krauth-Siegel RL, Comini MA. 2008. Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochim Biophys Acta 1780:1236–1248. doi: 10.1016/j.bbagen.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Gilroy C, Olenyik T, Roberts SC, Ullman B. 2011. Spermidine synthase is required for virulence of Leishmania donovani. Infect Immun 79:2764–2769. doi: 10.1128/IAI.00073-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tirupathi Pichiah PB, Suriyakalaa U, Kamalakkannan S, Kokilavani P, Kalaiselvi S, SankarGanesh D, Gowri J, Archunan G, Cha YS, Achiraman S. 2011. Spermidine may decrease ER stress in pancreatic beta cells and may reduce apoptosis via activating AMPK dependent autophagy pathway. Med Hypotheses 77:677–679. doi: 10.1016/j.mehy.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Hu J, Mahmoud MI, el-Fakahany EE. 1994. Polyamines inhibit nitric oxide synthase in rat cerebellum. Neurosci Lett 175:41–45. doi: 10.1016/0304-3940(94)91073-1. [DOI] [PubMed] [Google Scholar]
- 10.Wan CY, Wilkins TA. 1993. Spermidine facilitates PCR amplification of target DNA. PCR Methods Appl 3:208–210. doi: 10.1101/gr.3.3.208. [DOI] [PubMed] [Google Scholar]
- 11.Morselli E, Mariño G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, Cabrera S, Bénit P, Rustin P, Criollo A, Kepp O, Galluzzi L, Shen S, Malik SA, Maiuri MC, Horio Y, López-Otín C, Andersen JS, Tavernarakis N, Madeo F, Kroemer G. 2011. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol 192:615–629. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shukla AK, Patra S, Dubey VK. 2011. Evaluation of selected antitumor agents as subversive substrate and potential inhibitor of trypanothione reductase: an alternative approach for chemotherapy of leishmaniasis. Mol Cell Biochem 352:261–270. doi: 10.1007/s11010-011-0762-0. [DOI] [PubMed] [Google Scholar]
- 13.Shukla AK, Patra S, Dubey VK. 2012. Iridoid glucosides from Nyctanthes arbortristis result in increased reactive oxygen species and cellular redox homeostasis imbalance in Leishmania parasite. Eur J Med Chem 54:49–58. doi: 10.1016/j.ejmech.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Saudagar P, Saha P, Saikia AK, Dubey VK. 2013. Molecular mechanism underlying antileishmanial effect of oxabicyclo[3.3.1]nonanones: inhibition of key redox enzymes of the pathogen. Eur J Pharm Biopharm 85:569–577. doi: 10.1016/j.ejpb.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Das M, Saudagar P, Sundar S, Dubey VK. 2013. Miltefosine-unresponsive Leishmania donovani has a greater ability than miltefosine-responsive L. donovani to resist reactive oxygen species. FEBS J 280:4807–4815. doi: 10.1111/febs.12449. [DOI] [PubMed] [Google Scholar]
- 16.Grover A, Katiyar SP, Singh SK, Dubey VK, Sunder D. 2012. A leishmaniasis study: structure-based screening and molecular dynamics mechanistic analysis for discovering potent inhibitors of spermidine synthase. Biochim Biophys Acta 1824:1476–1483. doi: 10.1016/j.bbapap.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Enomoto K, Nagasaki T, Yamauchi A, Onoda J, Sakai K, Yoshida T, Maekawa K, Kinoshita Y, Nishino I, Kikuoka S, Fukunaga T, Kawamoto K, Numata Y, Takemoto H, Nagata K. 2006. Development of high-throughput spermidine synthase activity assay using homogeneous time-resolved fluorescence. Anal Biochem 351:229–240. doi: 10.1016/j.ab.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Porta R, Esposito C, Sellinger OZ. 1981. Rapid assay of spermidine synthase activity by high-performance liquid chromatography. J Chromatogr 226:208–212. doi: 10.1016/S0378-4347(00)84223-1. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Mukhopadhyay R, Dey S, Xut N, Gaget D, Lightbody J, Ouellettet M, Rosen BP. 1996. Trypanothione overproduction and resistance to antimonials and arsenicals in Leishmania. Proc Natl Acad Sci U S A 93:10383–10387. doi: 10.1073/pnas.93.19.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharya A, Biswas A, Das PK. 2009. Role of a differentially expressed cAMP phosphodiesterase in regulating the induction of resistance against oxidative damage in Leishmania donovani. Free Radic Biol Med 47:1494–1506. doi: 10.1016/j.freeradbiomed.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Basselin M, Coombs GH, Barrett MP. 2000. Putrescine and spermidine transport in Leishmania. Mol Biochem Parasitol 109:37–46. doi: 10.1016/S0166-6851(00)00234-6. [DOI] [PubMed] [Google Scholar]
- 23.Kubin A, Wierrani F, Burner U, Alth G, Grünberger W. 2005. Hypericin: the facts about a controversial agent. Curr Pharm Des 11:233–253. doi: 10.2174/1381612053382287. [DOI] [PubMed] [Google Scholar]
- 24.Krauth-Siegel RL, Leroux AE. 2012. Low-molecular-mass antioxidants in parasites. Antioxid Redox Signal 17:583–607. doi: 10.1089/ars.2011.4392. [DOI] [PubMed] [Google Scholar]
- 25.Simon HU, Haj-Yehia A, Levi-Schaffer F. 2000. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418. doi: 10.1023/A:1009616228304. [DOI] [PubMed] [Google Scholar]
- 26.Chawla B, Jhingran A, Singh S, Tyagi N, Park MH, Srinivasan N, Roberts SC, Madhubala R. 2010. Identification and characterization of a novel deoxyhypusine synthase in Leishmania donovani. J Biol Chem 285:453–463. doi: 10.1074/jbc.M109.048850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutierrez E, Shin B-S, Woolstenhulme CJ, Kim J-R, Saini P, Buskirk AR, Dever TE. 2013. eIF5A promotes translation of polyproline motifs. Mol Cell 51:35–45. doi: 10.1016/j.molcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]