Abstract
The bloodstream infection mortality risk score (BSIMRS) predicts the outcome of patients with Gram-negative bloodstream infections (BSI) with high discrimination. This retrospective cohort study examined the impact of inappropriate antimicrobial therapy on mortality in adult patients with Gram-negative BSI admitted to Palmetto Health Hospitals in Columbia, SC, USA, from 1 January 2011 to 31 December 2012 after stratification by predicted prognosis at initial presentation using BSIMRS. A multivariate Cox regression model was used to identify independent risk factors for 28-day mortality overall and within each predefined BSIMRS category (<5, 5 to 9, and ≥10). Relative risk reduction (RRR), absolute risk reduction (ARR), and number needed to treat (NNT) were calculated from a predictive logistic regression model of mortality. Overall, 390 unique patients with first episodes of Gram-negative BSI were identified. The median age was 66 years, and 229 (59%) were women. There was significant association between inappropriate antimicrobial therapy and mortality in patients with BSIMRS of 5 to 9 (adjusted hazard ratio [aHR], 3.55; 95% confidence intervals [CI], 1.22 to 8.31; P = 0.02) and BSIMRS of ≥10 (aHR, 4.99; 95% CI, 1.09 to 22.87; P = 0.04) but not in those with BSIMRS of <5 (aHR, 3.34; 95% CI, 0.17 to 22.77; P = 0.34). RRR, ARR, and NNT were 0.25, 0.02, and 63 for BSIMRS of <5; 0.56, 0.32, and 3 for BSIMRS of 5 to 9; and 0.39, 0.39, and 3 for BSIMRS of ≥10, respectively. There is a significant benefit from appropriate antimicrobial therapy in patients with Gram-negative BSI with guarded (BSIMRS of 5 to 9) and poor (BSIMRS of ≥10) predicted prognosis. Survival difference remains unclear among those with good predicted prognosis (BSIMRS of <5) at initial presentation.
INTRODUCTION
Bloodstream infections (BSI) have a significant impact on morbidity and mortality in the general population. It is estimated that over 500,000 individuals develop BSI annually in the United States, leading to nearly 75,000 deaths (1). Appropriate antimicrobial therapy is essential to reduce the burden of BSI, since inappropriate antimicrobial therapy has been associated with increased mortality (2–5).
Adjustment for the acute severity of illness has been emphasized as an important tool for the examination of the impact of inappropriate antimicrobial therapy in patients with BSI (6). A risk score recently has been derived and validated to predict the outcome of patients at the time of diagnosis of Gram-negative BSI. The BSI mortality risk score (BSIMRS) has a high discriminative ability to predict the prognosis of patients with Gram-negative BSI in both referral and population-based settings (7, 8). The BSIMRS is based on acute severity of illness, as summarized by the Pitt bacteremia score (9), source of infection, and major underlying medical conditions that are independently associated with mortality (Table 1). There is a substantial increase in predicted 28-day mortality following Gram-negative BSI as the BSIMRS increases from 0 to 16 (7, 8).
TABLE 1.
Bloodstream infection mortality risk scorec
| Variable | Point allocation |
|---|---|
| Malignancy | 3 |
| Liver cirrhosis | 4 |
| High-inoculum infectiona | 4 |
| Pitt bacteremia scoreb | |
| 0-1 | 0 |
| 2-3 | 2 |
| ≥4 | 5 |
High-inoculum infection indicates a source of infection other than urinary tract or central venous catheter.
Pitt bacteremia score is calculated based on temperature (35.1-36 or 39.0-39.9°C, 1 point; ≤35 or ≥40°C, 2 points), blood pressure (hypotension, 2 points), mental status (disorientation, 1 point; stupor, 2 points; coma, 4 points), respiratory status (mechanical ventilation, 2 points), and cardiac status (cardiac arrest, 4 points). The worst reading is recorded on the day the first positive blood culture is obtained or the day before for nosocomial bloodstream infections.
Modified from Al-Hasan et al. (8) with permission of the publisher.
The current retrospective cohort study examined the overall impact of inappropriate empirical antimicrobial therapy on 28-day mortality in patients with Gram-negative BSI. The impact of inappropriate antimicrobial therapy was stratified by predicted prognosis at initial presentation using predefined BSIMRS categories. Additionally, a predictive model of mortality was developed based on BSIMRS and appropriateness of empirical antimicrobial therapy.
MATERIALS AND METHODS
Setting.
The study was conducted at Palmetto Health Richland and Baptist Hospitals in Columbia, SC, USA. The two hospitals have a combined total of over 1,100 licensed beds and provide care in a wide variety of medical and surgical subspecialties for local residents of Richland County, as well as for regional referrals from within the state of South Carolina (http://www.palmettohealth.org).
Definitions.
Gram-negative BSI was defined as the growth of any aerobic Gram-negative bacillus in a blood culture. Monomicrobial BSI was defined as the growth of only one species of a microorganism in a blood culture, and polymicrobial BSI was defined as the growth of more than one microorganism in a blood culture. The primary source of BSI was defined according to the Centers for Disease Control and Prevention (CDC) criteria (10). Cancer was defined as a current diagnosis of a malignant tumor, excluding skin basal and squamous cell carcinoma. Liver cirrhosis was defined based on clinical, laboratory, ultrasonography, or histopathology results, when available (11). Mortality was defined as death or discharge to hospice within 28 days of the onset of BSI. Empirical antimicrobial therapy was defined as antimicrobial agents received within the first 48 h following the collection of the first set of positive blood cultures. Empirical antimicrobial therapy was considered appropriate if it met all of the following criteria: (i) patient received at least the minimum recommended dose of an antimicrobial agent according to the medication package insert for creatinine clearance at the time of BSI; (ii) the empirical antimicrobial agent was administered via the intravenous route, with the exception of fluoroquinolones, which were considered appropriate if administered orally in hemodynamically stable patients due to relatively high oral bioavailability; (iii) bloodstream isolate was susceptible to an empirical antimicrobial agent based on in vitro antimicrobial susceptibility testing results using Clinical Laboratory and Standards Institute (CLSI) guidelines. Of note, ampicillin, ampicillin-sulbactam, and first-generation cephalosporins were considered inappropriate for treatment of BSI due to chromosomally mediated AmpC-producing Enterobacteriaceae (CAE). However, third-generation cephalosporins were considered appropriate for CAE if the bloodstream isolate was susceptible to these agents in vitro by CLSI standards. All penicillins and cephalosporins were considered inappropriate for treatment of extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae regardless of in vitro antimicrobial susceptibility testing results. Similarly, all beta-lactams were considered inappropriate for carbapenemase-producing Enterobacteriaceae.
Case ascertainment.
In this retrospective cohort study, we identified all patients with BSI due to aerobic Gram-negative bacilli from 1 January 2011 to 31 December 2012 through microbiology laboratory databases at Palmetto Health. We included 390 unique hospitalized adults with first episodes of monomicrobial BSI caused by aerobic Gram-negative bacilli in the study. Patients <18 years old (n = 62), with polymicrobial BSI (n = 79), with recurrent episodes of BSI (n = 24), and who were treated outside the hospital (n = 23) were excluded.
Statistical analysis.
Descriptive statistics were used to summarize the data: medians and interquartile ranges (IQR) for continuous variables and counts and percentages for categorical variables. Cox proportional hazards regression was used to identify risk factors for mortality. Patients were monitored for 28 days after the onset of BSI or until death or discharge to hospice. This enabled us to censor patients who were lost to follow-up between discharge and 28 days from the date of obtaining initial blood cultures. Patients who were lost to follow-up before 28 days were censored at the date of last health care encounter. In order to identify independent risk factors for mortality, variables were included in a multivariate Cox model if the P value for a univariate association with mortality was ≤0.10. The components of the BSIMRS were analyzed as one continuous variable in the final model according to the total point allocation in the BSIMRS. The proportional hazards assumption was evaluated by plotting the log-integrated hazard versus time from the Kaplan-Meier method. Hazard ratios (HR) with 95% confidence intervals (CI) were presented to demonstrate the strength of association between each risk factor and mortality.
Patients were stratified based on the predicted prognosis at the time of diagnosis of Gram-negative BSI using the BSIMRS (<5, 5 to 9, and ≥10). These three categories were predefined based on the discriminative ability of BSIMRS in a previous study (8). The impact of inappropriate antimicrobial therapy on mortality was examined in each group using Kaplan-Meier survival analysis. The log-rank P value was used to assess the statistical significance in survival between patients who received appropriate and inappropriate empirical antimicrobial therapy in each group. Multivariate Cox proportional hazards regression was used to allow adjustments for age and independent risk factors for mortality other than BSIMRS and antimicrobial therapy, if any.
Finally, logistic regression was used to develop a predictive model of mortality in patients with Gram-negative BSI who were monitored for 28 days based on BSIMRS and the appropriateness of empirical antimicrobial therapy after adjustments for age and other independent risk factors of mortality, if any. Predicted probabilities obtained directly from the logistic regression model were plotted by BSIMRS to visualize the estimated risk of mortality in patients who received appropriate and inappropriate empirical antimicrobial therapy. The predicted probabilities of mortality with 95% CI were calculated from logistic regression models for each of the three BSIMRS categories. Relative risk reduction (RRR), absolute risk reduction (ARR), and number needed to treat (NNT) were reported to estimate the potential benefit from appropriate antimicrobial therapy.
JMP (version 10.0; SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. The level of significance for statistical testing was defined as P < 0.05 (2-sided) unless otherwise specified.
RESULTS
Over the 2-year study period, we identified 390 unique adult patients with first episodes of Gram-negative BSI, 257 (66%) from Palmetto Health Richland Hospital and 133 (34%) from Palmetto Health Baptist Hospital. The median age was 66 years, and 229 (59%) were women. The urinary tract was the most common source of infection, and Escherichia coli was the most common bloodstream isolate (Table 2).
TABLE 2.
Clinical characteristics of patients with Gram-negative bloodstream infection
| Variable | Value (n = 390) |
|---|---|
| Age in years, median (IQR) | 66 (55-78) |
| Female gender, n (%) | 229 (59) |
| Ethnicity, n (%) | |
| White | 191 (49) |
| African-American | 188 (48) |
| Other | 11 (3) |
| Diabetes mellitus, n (%) | 144 (37) |
| End-stage renal disease, n (%) | 38 (10) |
| Liver cirrhosis, n (%) | 6 (2) |
| Cancer, n (%) | 70 (18) |
| Immunocompromised host, n (%) | 62 (16) |
| Chemotherapy | 36 (9) |
| Neutropenia | 15 (4) |
| Transplant recipient | 15 (4) |
| HIV infection | 9 (2) |
| Site of infection acquisition, n (%) | |
| Community acquired | 161 (41) |
| Healthcare associated | 159 (41) |
| Nosocomial | 70 (18) |
| Microbiology, n (%) | |
| Escherichia coli | 207 (53) |
| Klebsiella species | 77 (20) |
| Proteus species | 31 (8) |
| Pseudomonas aeruginosa | 20 (5) |
| Enterobacter species | 19 (5) |
| Other | 36 (9) |
| Primary source of infection, n (%) | |
| Urinary tract | 216 (55) |
| Gastrointestinal tract | 50 (13) |
| Respiratory tract | 12 (3) |
| Central venous catheter | 35 (9) |
| Skin and soft tissue | 17 (4) |
| Other | 8 (2) |
| Unknown | 52 (13) |
| Pitt bacteremia score, median (IQR) | 2 (1-4) |
| BSIMRS, median (IQR) | 4 (2-6) |
| Inappropriate antimicrobial therapy, n (%) | 31 (8) |
| Beta-lactam monotherapya | 12/211 (6) |
| Fluoroquinoloneb or aminoglycoside monotherapya | 5/64 (8) |
| Beta-lactam plus fluoroquinoloneb or aminoglycosidea | 5/104 (5) |
| Other antimicrobial classes or combinationsa | 9/11 (82) |
Data show the number of inappropriate regimens/total per antimicrobial class or combination (percentage of inappropriate regimens).
Including 7 and 4 patients who received oral fluoroquinolones either as monotherapy or in combination with beta-lactams, respectively.
During 28 days of follow-up from the onset of Gram-negative BSI, 53 patients died, 6 were discharged to hospice, 281 survived, and 50 were lost to follow-up. The results of univariate Cox proportional hazards regression for risk factors of mortality are shown in Table 3. Age, BSIMRS, and inappropriate empirical antimicrobial therapy were independently associated with mortality in the multivariate Cox proportional hazards regression model. There was nearly a 40% increase in mortality for each decade increase in age and for each point increase in BSIMRS. There was a nearly 3-fold increase in the risk of mortality in patients with Gram-negative BSI who received inappropriate empirical antimicrobial therapy (Table 4).
TABLE 3.
Univariate Cox model results for risk factors of mortality in Gram-negative bloodstream infection
| Variablea | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age (per decade) | 1.37 | 1.16-1.65 | <0.001 |
| Female gender | 0.67 | 0.40-1.11 | 0.12 |
| White ethnicity | 1.83 | 1.09-3.15 | 0.02 |
| Diabetes mellitus | 0.50 | 0.27-0.89 | 0.02 |
| End-stage renal disease | 0.83 | 0.29-1.86 | 0.67 |
| Transplant recipient | 0.93 | 0.16-2.97 | 0.92 |
| HIV infection | 0.72 | 0.04-3.28 | 0.73 |
| Nursing home resident | 1.47 | 0.76-2.63 | 0.24 |
| Recent hospitalization | 1.25 | 0.71-2.11 | 0.43 |
| Nosocomial acquisition | 1.46 | 0.77-2.60 | 0.23 |
| BSIMRS (per point) | 1.39 | 1.29-1.51 | <0.001 |
| Liver cirrhosis | 4.42 | 1.08-12.0 | 0.04 |
| Cancer | 5.18 | 3.10-8.66 | <0.001 |
| Nonurinary/CVC source | 2.37 | 1.42-3.99 | 0.001 |
| Pitt bacteremia score | |||
| 0-1 | Referenceb | ||
| 2-3 | 3.49 | 1.34-9.72 | 0.005 |
| ≥4 | 10.77 | 4.90-28.40 | <0.001 |
| Inappropriate antimicrobial therapy | 2.94 | 1.40-5.55 | 0.006 |
CVC, central venous catheter.
Pitt bacteremia score was analyzed as a categorical variable, with scores of 2-3 and ≥4 compared to a reference score of 0-1.
TABLE 4.
Multivariate Cox model results for independent risk factors of mortality in Gram-negative bloodstream infection
| Variable | aHR | 95% CI | P value |
|---|---|---|---|
| Age (per decade) | 1.41 | 1.18-1.70 | <0.001 |
| White ethnicity | 1.15 | 0.68-2.00 | 0.60 |
| Diabetes mellitus | 0.65 | 0.34-1.16 | 0.15 |
| BSIMRS (per point) | 1.41 | 1.30-1.54 | <0.001 |
| Inappropriate antimicrobial therapy | 3.54 | 1.67-6.82 | 0.002 |
Among 390 patients with Gram-negative BSI, 214, 158, and 18 had BSIMRS of <5, 5 to 9, and ≥10, respectively. In Kaplan-Meier analyses, inappropriate antimicrobial therapy was associated with increased risk of mortality in patients with BSIMRS of 5 to 9 and ≥10 (P = 0.02 and 0.008, respectively). However, there was no significant association between inappropriate antimicrobial therapy and mortality in patients with BSIMRS of <5 (P = 0.26) (Fig. 1). The same results were demonstrated with the multivariate Cox proportional hazards model after adjustment for age. For patients with BSIMRS of 5 to 9 and ≥10, inappropriate empirical antimicrobial therapy was associated with a nearly 3- and 5-fold increase in mortality, respectively. However, in patients with BSIMRS of <5, there was no significant increase in mortality in association with inappropriate empirical antimicrobial therapy (Table 5).
FIG 1.
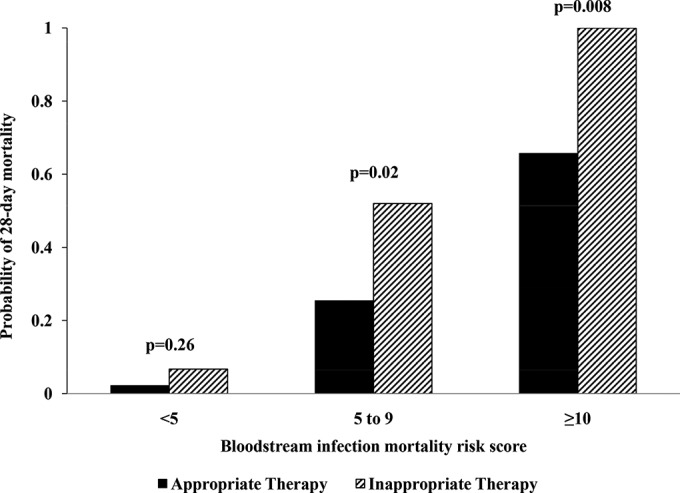
Outcome of patients with Gram-negative bacteremia by bloodstream infection mortality risk score and empirical antimicrobial therapy. P values were calculated using a log-rank test from Kaplan-Meier survival curves.
TABLE 5.
Impact of inappropriate antimicrobial therapy on mortality in Gram-negative bacteremia after stratification by BSIMRS
| BSIMRS | aHR | 95% CI | P value |
|---|---|---|---|
| <5 | 3.34 | 0.17-22.77 | 0.34 |
| 5-9 | 3.55 | 1.22-8.31 | 0.02 |
| ≥10 | 4.99 | 1.09-22.87 | 0.04 |
The predicted probability of mortality at the time of diagnosis of Gram-negative BSI based on BSIMRS and antimicrobial therapy, after age adjustment, is illustrated in Fig. 2. Predicted mortality in patients who received appropriate and inappropriate empirical antimicrobial therapy appears comparable until the BSIMRS exceeds 3. Afterwards, there is a substantial increase in predicted mortality in patients who received inappropriate empirical antimicrobial therapy compared to those who received appropriate therapy.
FIG 2.
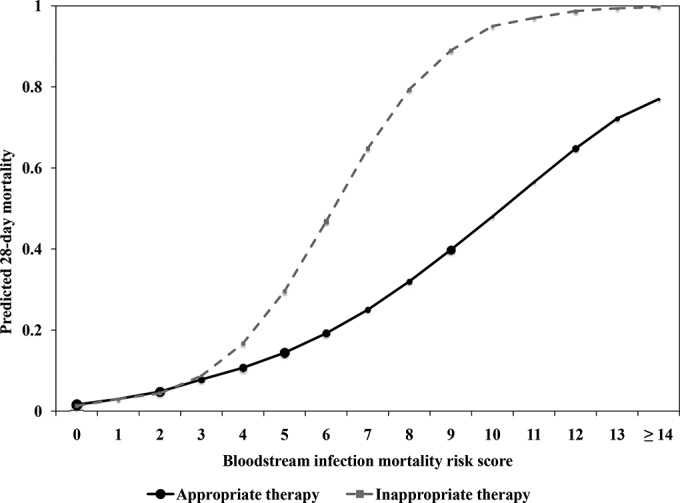
Predicted mortality following Gram-negative bacteremia by bloodstream infection mortality risk score and empirical antimicrobial therapy. The size of the marker for point estimates is weighted approximately by the relative number of subjects with the corresponding risk score.
The overall predicted probability of mortality was low in the BSIMRS <5 category, with overlapping 95% CIs between appropriate and inappropriate antimicrobial therapy (Table 6). On the other hand, there was a substantial reduction in predicted mortality with appropriate antimicrobial therapy in groups with BSIMRS of 5 to 9 and ≥10, as estimated by both RRR and ARR. The NNT with appropriate antimicrobial therapy for Gram-negative BSI was nearly 3 in the latter two BSIMRS groups.
TABLE 6.
Predicted probabilities of mortality with and without appropriate antimicrobial therapy in Gram-negative bacteremia by BSIMRS
| BSIMRS | Mortality (95% CI) by therapy type |
RRR | ARR | NNT | |
|---|---|---|---|---|---|
| Inappropriate | Appropriate | ||||
| <5 | 0.07 (0.04-0.10) | 0.05 (0.04-0.06) | 0.246 | 0.016 | 63 |
| 5-9 | 0.58 (0.46-0.69) | 0.25 (0.22-0.28) | 0.558 | 0.321 | 3.1 |
| ≥10 | 0.99 (0.86-1.00) | 0.61 (0.52-0.69) | 0.392 | 0.390 | 2.6 |
DISCUSSION
This is the third study to demonstrate that Gram-negative BSI is a spectrum of illness with a wide variation in prognosis, as predicted by the BSIMRS. Whereas the initial derivation and validation cohorts in Minnesota included a predominantly white population (7, 8), the current study in South Carolina included a more diverse ethnic population.
Appropriate antimicrobial therapy is essential in patients with Gram-negative BSI (2–5). Adjustment for acute severity of illness has been advocated in prior reviews (6). However, the precise estimation of the impact of appropriate empirical antimicrobial therapy on outcome remains difficult due to the influence of variables other than acute severity of illness and antimicrobial therapy (12). The current study demonstrates that the outcome of patients with Gram-negative BSI is dictated mostly by major host factors, source of infection, and the acute severity of illness at initial presentation, as summarized by the BSIMRS. Appropriate empirical antimicrobial therapy influences the outcome of patients within each BSIMRS category (<5, 5 to 9, and ≥10), but the impact of initial prognosis remains more substantial. For example, patients with a BSIMRS of <5 have a predicted mortality of <10% even if they receive inappropriate empirical therapy. On the other hand, patients with BSIMRS of 5 to 9 and ≥10 have a predicted mortality of nearly 25% and 60%, respectively, when treated with appropriate empirical antimicrobial therapy (Table 6). This difference in predicted prognosis at initial presentation likely explains why some patients survive despite receiving inappropriate antimicrobial therapy, while others expire despite receiving appropriate therapy.
The results of this study confirm that there is an overall survival benefit from appropriate empirical antimicrobial therapy in Gram-negative BSI after adjustments for potential confounders. However, after stratification by predicted prognosis using BSIMRS, most of this benefit is observed in patients with guarded (BSIMRS of 5 to 9) and poor predicted (BSIMRS of ≥10) prognosis. Whether there is a survival difference in patients with good predicted prognosis (BSIMRS of <5) at initial presentation remains unclear. This may explain the lack of significant difference in mortality among patients with BSI who received appropriate and inappropriate empirical antimicrobial agents in prior studies, particularly when the majority of included patients had a urinary source of infection (13–16).
The overall NNT with appropriate antimicrobial therapy in patients with BSI was estimated to be nearly 8 in a recent meta-analysis (3). The current study demonstrated that the NNT varied by predicted prognosis at the diagnosis of BSI. The NNT was nearly 3 in patients with BSIMRS of 5 to 9 and ≥10. On the other hand, in patients with BSIMRS of <5, the NNT with appropriate antimicrobial therapy was 63. In addition, the relative risk reduction also was lower in patients with BSIMRS of <5 compared to those of patients with BSIMRS of 5 to 9 and ≥10 (Table 6). This overall lower benefit from appropriate antimicrobial therapy in patients with BSIMRS of <5 compared to those with BSIMRS of ≥5 should be taken into consideration when making empirical antimicrobial treatment decisions in individual patients or developing clinical management guidelines.
Many medical centers publish institutional antibiograms to help local health care providers choose empirical antimicrobial therapy for treatment of suspected infections, including BSI. However, the choice of an appropriate threshold for the selection of an antimicrobial agent is left to the health care provider. The current work provides an objective tool to enhance the utility of such antibiograms. In an era of increasing antimicrobial resistance and limited availability of effective antimicrobial agents for treatment of serious Gram-negative infections, it is imperative to maximize the benefit-to-risk ratio of antimicrobial therapy in order to improve overall outcomes. Given higher benefit from appropriate antimicrobial therapy in patients with BSIMRS of ≥5 compared to those with BSIMRS of <5, it is reasonable to use an arbitrary susceptibility of ≥95% for BSIMRS of ≥5 and ≥90% for BSIMRS of <5, for example.
In addition, stratification of patients with Gram-negative BSI based on prognosis as predicted by BSIMRS provides a tool to improve the use of empirical antimicrobial agents in patients with suspected BSI due to Gram-negative bacilli that harbor antimicrobial resistance genes. Prior studies have identified patients at risk of BSI due to Pseudomonas aeruginosa and fluoroquinolone-resistant and ESBL-producing Enterobacteriaceae based on the number of individual risk factors for antimicrobial resistance or the summation of the weighted score for each risk factor (17–20). The decision to expand antimicrobial coverage may appear less complex in patients with no or multiple risk factors for antimicrobial resistance. However, many patients fall in the intermediate category where they have only one risk factor or two low-weight risk factors. Using the BSIMRS to stratify patients based on predicted prognosis may enhance the performance and utility of such risk scores, as it incorporates the potential benefit from appropriate antimicrobial therapy with the risk of antimicrobial resistance in order to help make a better treatment decision. For example, in a population with an overall prevalence of 3% for ESBL-producing Enterobacteriaceae among bloodstream isolates, the nonstratified use of carbapenems for the empirical treatment of BSI is a low-yield intervention in patients with BSIMRS of <5. It would require treating 2,100 (63/0.03) patients with carbapenems in this population to potentially save one life. On the other hand, if carbapenems were used in patients with BSIMRS of ≥5 and intermediate or high risk for BSI due to ESBL-producing Enterobacteriaceae (15% and 30% probabilities, respectively, as previously estimated [20]), the NNT with carbapenems may be as low as 20 (3/0.15) and 10 (3/0.3), respectively.
The inclusion of patients with BSI from two large medical centers that provide health care for a large proportion of residents of Richland County, SC, USA, is a strength of this study. The diversity of the Richland County population increases the generalizability of the study results. Stratification of patients based on predicted prognosis at initial presentation is a unique feature of this study, as it provides a scientific method to precisely estimate the effect of empirical antimicrobial therapy on the outcome of patients with Gram-negative BSI.
Our study has limitations. First, it is a retrospective cohort study with variables collected retrospectively. However, all variables in the study, including those in the BSIMRS, were clearly predefined prior to data collection. Second, although two hospitals were included, both were located in the same geographical area and belong to the same health care system. Including patients from multiple hospitals in different areas would add variety to the population and prescription practices. Third, it remains possible that the study is underpowered in the detection of a difference in mortality in patients with BSIMRS of <5 who received appropriate and inappropriate antimicrobial therapy, and a larger sample size is required to reexamine this group in future studies. However, given the low mortality in patients with BSIMRS of <5, an adequately powered study to detect a relatively small difference in mortality between appropriate and inappropriate therapy in this group would require the enrollment of thousands of patients (850 patients in the inappropriate therapy arm, assuming 80% power and 5% alpha error). Alternatively, softer endpoint variables could be used instead of mortality to examine the outcome of patients with BSIMRS of <5, such as length of stay in the hospital or predefined criteria for treatment cure (21, 22). It would be useful to standardize these definitions across the medical literature and use clinical cure criteria that correlate with either survival or shorter length of hospitalization.
In summary, the BSIMRS is an excellent tool to predict the prognosis of patients with Gram-negative BSI based on acute severity of illness, primary source of infection, and major host comorbidities. Appropriate empirical antimicrobial therapy is essential to improve the outcome of patients with Gram-negative BSI. However, the impact of appropriate therapy seems higher in patients with guarded (BSIMRS of 5 to 9) and poor (BSIMRS of ≥10) predicted prognoses than in those with good predicted prognosis (BSIMRS of <5) at initial presentation. The BSIMRS provides an objective tool to improve the selection of empirical antimicrobial therapy, in addition to local antimicrobial resistance rates and risk factors for antimicrobial resistance.
ACKNOWLEDGMENTS
We thank the Antimicrobial Stewardship and Support Team at Palmetto Health in Columbia, SC, USA, for their help in facilitating the conducting of this study.
S.E.C. and M.N.A. have full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the analysis.
S.E.C., J.K., H.A., and M.N.A. have no conflicts of interest to report.
P.B.B. received research funding from Cubist Pharmaceuticals.
REFERENCES
- 1.Goto M, Al-Hasan MN. 2013. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 19:501–509. doi: 10.1111/1469-0691.12195. [DOI] [PubMed] [Google Scholar]
- 2.Bryan CS, Reynolds KL, Brenner ER. 1983. Analysis of 1,186 episodes of Gram-negative bacteremia in non-university hospital: the effects of antimicrobial therapy. Rev Infect Dis 5:629–638. doi: 10.1093/clinids/5.4.629. [DOI] [PubMed] [Google Scholar]
- 3.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. 2010. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 54:4851–4863. doi: 10.1128/AAC.00627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Retamar P, Portillo MM, López-Prieto MD, Rodríguez-López F, de Cueto M, García MV, Gómez MJ, del Arco A, Muñoz A, Sánchez-Porto A, Torres-Tortosa M, Martín-Aspas A, Arroyo A, García-Figueras C, Acosta F, Corzo JE, León-Ruiz L, Escobar-Lara T, Rodríguez-Baño J, SAEI/Bacteremia Group SAMPAC . 2012. Impact of inadequate empirical therapy on the mortality of patients with bloodstream infections: a propensity score-based analysis. Antimicrob Agents Chemother 56:472–478. doi: 10.1128/AAC.00462-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul M, Fraser AA, Leibovici L. 2007. Propensity-matched analysis of appropriate empirical antibiotic treatment. Clin Infect Dis 44:1251–1252. doi: 10.1086/513585. [DOI] [PubMed] [Google Scholar]
- 6.McGregor JC, Rich SE, Harris AD, Perencevich EN, Osih R, Lodise TP Jr, Miller RR, Furuno JP. 2007. A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin Infect Dis 45:329–337. doi: 10.1086/519283. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. 2013. Predictive scoring model of mortality in Gram-negative bloodstream infection. Clin Microbiol Infect 19:948–954. doi: 10.1111/1469-0691.12085. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hasan MN, Juhn YJ, Bang DW, Yang H-J, Baddour LM. 2014. External validation of Gram-negative bloodstream infection mortality risk score in a population-based cohort. Clin Microbiol Infect 20:886–891. doi: 10.1111/1469-0691.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, Bonomo RA, Rice LB, Wagener MM, McCormack JG, Yu VL. 2004. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 140:26–32. doi: 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 10.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. 1988. CDC definitions for nosocomial infections. Am J Infect Control 16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 11.Chen SY, Tsai CL, Lin CH, Lee CC, Chiang WC, Wang JL, Ma MH, Chen SC, Chen WJ, Chang SC. 2009. Impact of liver cirrhosis on mortality in patients with community-acquired bacteremia. Diagn Microbiol Infect Dis 64:124–130. doi: 10.1016/j.diagmicrobio.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Sexton DJ, Miller BA, Anderson DJ. 2011. Measuring the effect of inappropriate initial antibiotic therapy on outcomes of patients with Gram-negative sepsis: an imprecise science. Crit Care Med 39:199–200. doi: 10.1097/CCM.0b013e318202e68f. [DOI] [PubMed] [Google Scholar]
- 13.Horcajada JP, Shaw E, Padilla B, Pintado V, Calbo E, Benito N, Gamallo R, Gozalo M, Rodríguez-Baño J, ITUBRAS Group, Grupo de Estudio de Infección Hospitalaria, Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica . 2013. Healthcare-associated, community-acquired and hospital-acquired bacteraemic urinary tract infections in hospitalized patients: a prospective multicentre cohort study in the era of antimicrobial resistance. Clin Microbiol Infect 19:962–968. doi: 10.1111/1469-0691.12089. [DOI] [PubMed] [Google Scholar]
- 14.Yang YS, Ku CH, Lin JC, Shang ST, Chiu CH, Yeh KM, Lin CC, Chang FY. 2010. Impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae on the outcome of community-onset bacteremic urinary tract infections. J Microbiol Immunol Infect 43:194–199. doi: 10.1016/S1684-1182(10)60031-X. [DOI] [PubMed] [Google Scholar]
- 15.Thom KA, Schweizer ML, Osih RB, McGregor JC, Furuno JP, Perencevich EN, Harris AD. 2008. Impact of empiric antimicrobial therapy on outcomes in patients with Escherichia coli and Klebsiella pneumoniae bacteremia: a cohort study. BMC Infect Dis 8:116. doi: 10.1186/1471-2334-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SS, Kim Y, Chung DR. 2011. Impact of discordant empirical therapy on outcome of community-acquired bacteremic acute pyelonephritis. J Infect 62:159–164. doi: 10.1016/j.jinf.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Schechner V, Nobre V, Kaye KS, Leshno M, Giladi M, Rohner P, Harbarth S, Anderson DJ, Karchmer AW, Schwaber MJ, Carmeli Y. 2009. Gram-negative bacteremia upon hospital admission: when should Pseudomonas aeruginosa be suspected? Clin Infect Dis 48:580–586. doi: 10.1086/596709. [DOI] [PubMed] [Google Scholar]
- 18.Cheong HS, Kang CI, Wi YM, Kim ES, Lee JS, Ko KS, Chung DR, Lee NY, Song JH, Peck KR. 2008. Clinical significance and predictors of community-onset Pseudomonas aeruginosa bacteremia. Am J Med 121:709–714. doi: 10.1016/j.amjmed.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Koliscak LP, Johnson JW, Beardsley JR, Miller DP, Williamson JC, Luther VP, Ohl CA. 2013. Optimizing empiric antibiotic therapy in patients with severe β-lactam allergy. Antimicrob Agents Chemother 57:5918–5923. doi: 10.1128/AAC.01202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tumbarello M, Trecarichi EM, Bassetti M, De Rosa FG, Spanu T, Di Meco E, Losito AR, Parisini A, Pagani N, Cauda R. 2011. Identifying patients harboring extended-spectrum-beta-lactamase-producing Enterobacteriaceae on hospital admission: derivation and validation of a scoring system. Antimicrob Agents Chemother 55:3485–3490. doi: 10.1128/AAC.00009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shorr AF, Micek ST, Welch EC, Doherty JA, Reichley RM, Kollef MH. 2011. Inappropriate antibiotic therapy in Gram-negative sepsis increases hospital length of stay. Crit Care Med 39:46–51. doi: 10.1097/CCM.0b013e3181fa41a7. [DOI] [PubMed] [Google Scholar]
- 22.Micek S, Johnson MT, Reichley R, Kollef MH. 2012. An institutional perspective on the impact of recent antibiotic exposure on length of stay and hospital costs for patients with gram-negative sepsis. BMC Infect Dis 12:56. doi: 10.1186/1471-2334-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]


