Abstract
Gram-negative bacteria are normally resistant to the antibiotic vancomycin (VAN), which cannot significantly penetrate the outer membrane. We used Escherichia coli mutants that are partially sensitive to VAN to study synergies between VAN and 10 other antibiotics representing six different functional categories. We detected strong synergies with VAN and nitrofurantoin (NTR) and with VAN and trimethoprim (TMP) and moderate synergies with other drugs, such as aminoglycosides. These synergies are powerful enough to show the activity of VAN against wild-type E. coli at concentrations of VAN as low as 6.25 μg/ml. This suggests that a very small percentage of exogenous VAN does enter E. coli but normally has insignificant effects on growth inhibition or cell killing. We used the results of pairwise interactions with VAN and the other 10 antibiotics tested to place VAN into a functional category of its own, as previously defined by Yeh et al. (P. Yeh, A. I. Tschumi, and R. Kishony, Nat Genet 28:489–494, 2006, http://dx.doi.org/10.1038/ng1755).
INTRODUCTION
Vancomycin (VAN) has proved to be an effective antibiotic against certain multidrug-resistant Gram-positive pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA). However, the large size of this glycopeptide precludes it from being useful against Gram-negative bacterial infections, since the outer membrane of Gram-negative bacteria acts as a barrier to its entry into the cell (1). Finding agents that act synergistically with VAN against Gram-negative bacteria would be valuable in treating infections caused by multidrug-resistant pathogens, such as carbapenem-resistant Klebsiella pneumoniae, a leading cause of hospital-acquired pneumonia in the United States (2). Certain mutants of Escherichia coli with gene knockouts that affect outer membrane assembly (surA [3] and smpA [4] mutants) display increased sensitivity to VAN. Recently, we have found that E. coli mutants lacking deoxycytidine deaminase (DCD) also are more sensitive to VAN than wild-type (WT) strains (5). In the work reported here, we have used surA and dcd mutants to study drug interactions between VAN and a series of antibiotics in E. coli. We detected strong synergies between VAN and nitrofurantoin (NTR) and between VAN and trimethoprim (TMP). We then tested different concentrations of each of these two antibiotics both alone and in combination with VAN in the wild-type background and demonstrated that wild-type cells are sensitized to relatively low concentrations of VAN in the presence of subinhibitory concentrations of either NTR or TMP. We discuss the possible implications of these results for combination drug therapy.
MATERIALS AND METHODS
E. coli strains.
The DCD-deficient and SurA-deficient strains used here are from the Keio Collection, described by Baba et al. (6), and were made from the starting strain BW25113 (7). This starting strain (lacIq rrnBT14 ΔlacZWJ16 hsdR514 ΔaraBADAH33 ΔrhaBADLD78) is used as the WT in the experiments reported here, unless otherwise stated. BW25113 is closely related to MG1655, as both are derived from the strain W1485 background (6). The dcd mutant and the surA mutant carry a complete deletion of the dcd gene and the surA gene, respectively, with a kan kanamycin resistance gene insert being used in place of the gene.
Media.
The following media (8) were used: LB medium (10 g tryptone, 5 g yeast extract, 10 g NaCl per liter) and minimal medium [minimal A medium; 10.5 g K2HPO4, 4.5 g KH2PO4, 1 g (NH4)2SO4, 0.5 g sodium citrate·2H2O]. For growth medium, minimal A medium is supplemented with 10 ml of 20% glucose, 1 ml of 1 M MgSO4, and 0.5 ml of 1% thiamine hydrochloride (vitamin B1) per liter.
E. coli genetic methods.
Unless otherwise stated, all genetic methods are as described by Miller (8). The dcd mutant and the surA mutant were purified from single colonies from the Keio Collection. Experiments were started by inoculation of bacteria from a culture made from a single colony and stored in glycerol (8) at −80°C. A sample of the frozen glycerol culture was inoculated into LB medium and grown for 6 h in a water bath at 37°C before being used to seed overnight cultures with approximately 103 cells. This was achieved by inoculating 2-ml cultures with 50 μl of a 10−4 dilution of the overday culture. After 18 h incubation at 37°C on a rotor at 50 rpm, the optical density at 600 nm (OD600) was measured. Graphs of these data display percent growth versus that in LB (see also reference 9).
Determination of single drug concentrations.
Ten different antibiotics were selected as representations of six functional groups in terms of their killing mechanisms, as previously defined by Yeh et al. (9). Overnight cultures containing a range of concentrations of a given antibiotic (usually from the reported MIC with 2-fold intervals) were seeded with approximately 103 cells by inoculating 2-ml cultures with 50 μl of a 10−4 dilution of the overday culture. After 18 h incubation at 37°C on a rotor at 50 rpm, the OD600 was measured. Subinhibitory concentrations were typically chosen to be those that resulted in a 60% to 80% reduction in growth compared to the growth of the controls in LB medium only.
Drug interaction assay.
Cell cultures were prepared with the same method described above for the determination of single drug concentrations, using LB medium supplemented with vancomycin and a second antibiotic at subinhibitory concentrations. Bar graphs were used to compare the effects of the paired drugs with those of the corresponding single drugs at the same dose and with the effect of no treatment for the control grown in LB medium only.
Classification of drug interactions.
Classifications of drug interactions are from previous work (9). Additivity is defined as Wxy = WxWy, where Wx is the proportion of growth relative to that for the control with no drug with drug X, Wy is the proportion of growth with drug Y, and Wxy is the proportion of growth with the drugs combined. For example, if drug X has a residual growth of 0.6 of that of the control with no drug and drug Y has a residual growth of 0.7, the additive expectation of the growth obtained with the two drugs together would be 0.42. There is a range around 0.42, for example, 0.43, that would still be considered additive. We discuss how to calculate this range below. Anything above this range would be antagonistic, and anything below this range would be synergistic. More formally, a deviation from additivity is defined by ε̃, which is calculated from the formulas below. When ε̃ falls within the range of −1 to −0.5, we classify it as synergistic; when ε̃ is between −0.5 and 0.5, we classify it as additive; when ε̃falls between 0.5 and 2, we classify it as antagonistic. ε̃ is calculated as (Wxy − WxWy)/(W̃xy − WxWy|), where W̃xy is equal to min[Wx, Wy] for Wxy > WxWy and 0 otherwise. If W̃xy is greater than min[Wx, Wy], then ε̃ is equal to {(Wxy − min[Wx, Wy])/(1 − min[Wx, Wy])} + 1.
Killing assay.
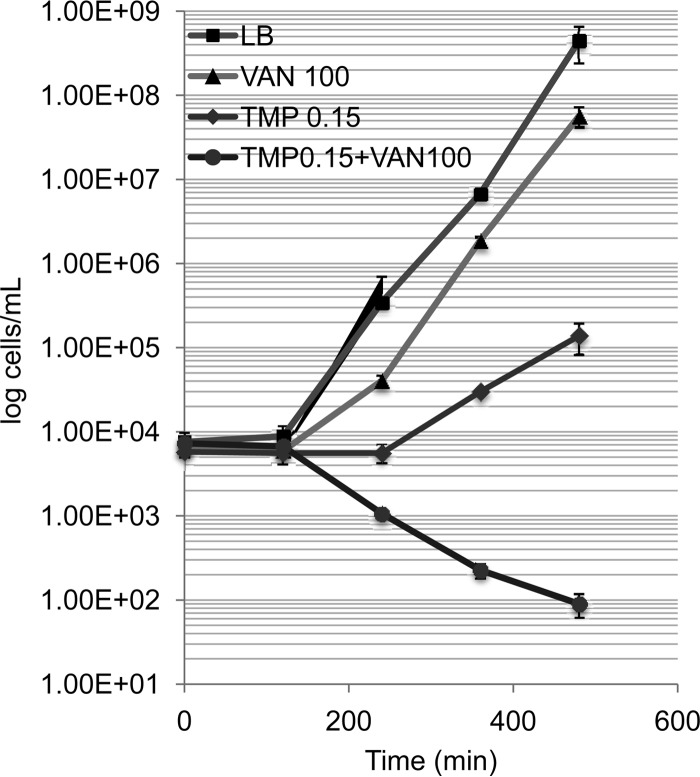
Wild-type E. coli cultures were inoculated with approximately 1 × 104 CFU/ml from a resuspension in MC buffer (0.1 M MgSO4, 0.005 M CaCl2) stored at 4°C and placed into LB medium containing 100 μg/ml of vancomycin, 0.15 μg/ml of trimethoprim, the two drugs combined, and a control with LB medium only. Aliquots of the culture were removed at five time intervals (0, 2, 4, 6, and 8 h) of incubation on a rotor at 50 rpm, and dilutions were prepared as needed for determination of the titers. The numbers of viable cells were determined by colony counts on LB medium plates after 24 h of incubation at 37°C. The assay was performed in triplicate cultures and was repeated at least twice. The values represent means ± standard deviations.
Antibiotics.
Cefoxitin (FOX), chloramphenicol (CHL), ciprofloxacin (CPR), clindamycin (CLI), erythromycin (ERY), nitrofurantoin (NTR), streptomycin (STR), tetracycline (TET), tobramycin (TOB), trimethoprim (TMP), and vancomycin (VAN) were purchased from Sigma (St. Louis, MO).
RESULTS
Pairwise drug combinations.
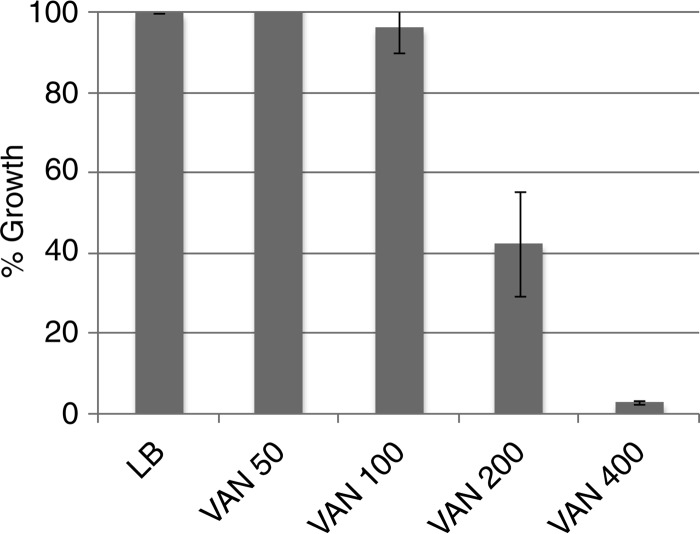
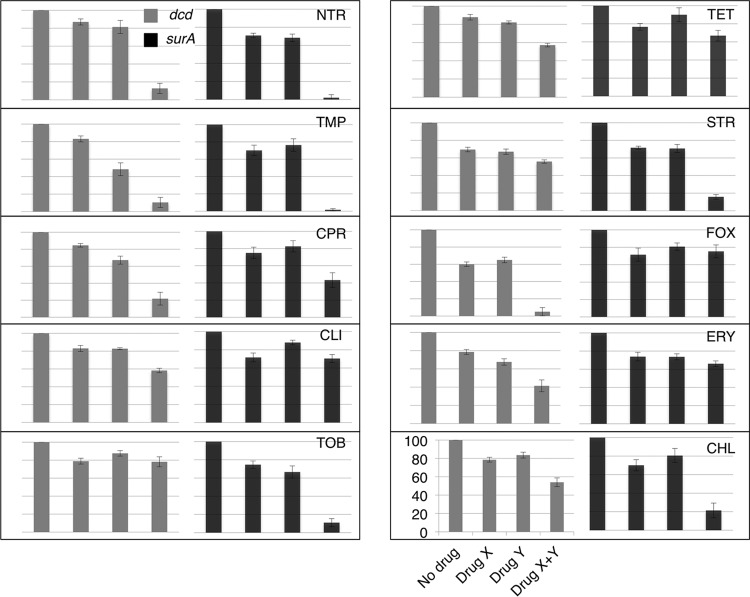
We initially tested a set of 10 antibiotics with a subinhibitory concentration of VAN in each of two backgrounds, a dcd mutant and a surA mutant. Whereas the MIC for VAN for the wild type is 400 μg/ml (Fig. 1), under the conditions of the experiment (see Materials and Methods), the MIC for the dcd mutant was 50 μg/ml and that for the surA mutant was 8 μg/ml. We used 25 μg/ml as a subinhibitory concentration for the dcd mutant and 2 μg/ml as a subinhibitory concentration for the surA mutant. Table 1 lists the antibiotics used in this study and the concentrations used in the initial tests for synergy. They were determined by defining the concentrations which gave between 60% and 90% residual growth, allowing one to best observe synergistic interactions. The antibiotics were chosen as representatives of the basic families of antibiotics used in a prior study (9). Figure 2 displays the results in the format employed by Yeh and coworkers (9). The latter study characterized the interactions as additive, suppressive, antagonistic, and synergistic (see Materials and Methods for a fuller explanation). Here, percent residual growth versus growth in LB medium without antibiotic was plotted for each single antibiotic and for the pair of antibiotics. Synergistic effects are those that are significantly greater than simple additive effects. From Fig. 2, for the surA mutant background, we assigned merely additive effects (no synergy) to the combinations of VAN with CLI, ERY, FOX (which had no effect), and TET; weak synergies with VAN and CIP or CHL; stronger synergies with VAN and STR or TOB; and very strong synergies with VAN and either NTR or TMP. The two backgrounds gave similar but not identical results, with the biggest difference being the lack of synergy between VAN and the aminoglycosides STR and TOB in the dcd mutant background.
FIG 1.
Vancomycin susceptibility of WT E. coli in LB medium. WT E. coli was grown in the presence of the different concentrations (μg/ml) of vancomycin indicated. At each concentration, growth percentages were calculated by comparison with the growth in LB medium (see Materials and Methods).
TABLE 1.
Antibiotics used in the study with their dosages and primary targets
| Antibiotic | Abbreviation | Dose (μg/ml) used for: |
Primary target | |
|---|---|---|---|---|
| dcd mutant | surA mutant | |||
| Cefoxitin | FOX | 1 | 2 | Cell wall synthesis |
| Chloramphenicol | CHL | 0.25 | 0.75 | Protein synthesis, 50S ribosomal subunit |
| Ciprofloxacin | CPR | 0.01 | 0.01 | DNA gyrase |
| Clindamycin | CLI | 8 | 15 | Protein synthesis, 50S ribosomal subunit |
| Erythromycin | ERY | 5 | 10 | Protein synthesis, 50S ribosomal subunit |
| Nitrofurantoin | NTR | 0.5 | 0.8 | DNA |
| Streptomycin | STR | 0.6 | 0.2 | Protein synthesis, 30S ribosomal subunit |
| Tetracycline | TET | 0.075 | 0.15 | Protein synthesis, 30S ribosomal subunit |
| Tobramycin | TOB | 2 | 0.6 | Protein synthesis, 30S ribosomal subunit |
| Trimethoprim | TMP | 0.1 | 0.15 | Folic acid biosynthesis |
| Vancomycin | VAN | 25 | 2 | Cell wall synthesis |
FIG 2.
Interactions of different drugs with vancomycin in dcd and surA mutants. A representative graph is labeled to indicate that the bars indicate the results, from left to right, for no drug, drug X (vancomycin), drug Y (abbreviated on the top right corner of each panel), and the combination of drug X and Y, respectively, plotted against the growth percentage on the y axis (where the line at the bottom represents 0% and the line at the top represents 100%). Refer to Table 1 for the concentrations used.
Synergies in the wild-type background.
The strengths of the synergies with VAN and NTR and with VAN and TMP suggest that the effects might be strong enough to be seen in the wild-type background. This was indeed the case, as Fig. 3 and 4 demonstrate. The degrees of synergy between different concentrations of VAN and NTR are displayed in Fig. 3. As the concentration of VAN decreased, an increasing concentration of NTR was required for strong growth inhibition. Strong synergistic effects were seen with concentrations of VAN as low as 12.5 μg/ml. The combination of VAN and TMP showed effects with VAN concentrations as low as 6.25 μg/ml (Fig. 4).
FIG 3.
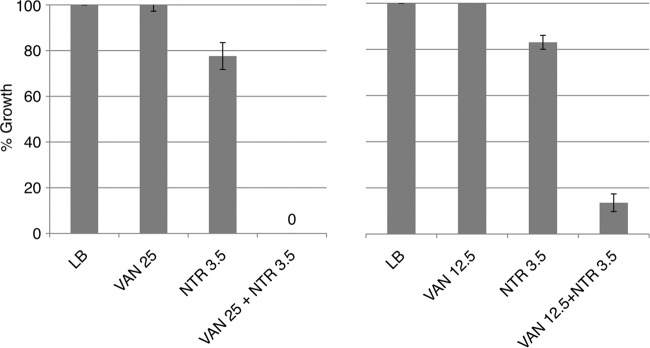
Synergistic interaction between vancomycin and nitrofurantoin with WT E. coli in LB medium. WT E. coli was grown in the presence of vancomycin, nitrofurantoin, and the drugs combined at the concentrations (μg/ml) indicated. Growth percentages at each concentration were calculated by comparison with the growth in LB medium (see Materials and Methods).
FIG 4.

Synergistic interaction between vancomycin and trimethoprim with WT E. coli in LB medium. WT E. coli was grown in the presence of vancomycin, trimethoprim, and the drugs combined at the concentrations (μg/ml) indicated. Growth percentages at each concentration were calculated by comparison with the growth in LB medium (see Materials and Methods).
Classification of vancomycin on the basis of pairwise interactions.
We applied the findings of Yeh et al. (9) to place VAN within the context of the groupings on the basis of their mechanisms of action (Fig. 5). Vancomycin did not cluster into any of the existing groups monochromatically; that is, if vancomycin were placed in any existing group, there would have been some interactions that were synergistic, while the interactions with the other drugs were antagonistic, or vice versa. As a result, we placed vancomycin within its own group. The majority of the interaction network shown here was based on the wild-type strain E. coli MG1655 (9), and the vancomycin growth experiments were conducted in surA or dcd mutant derivatives of the closely related strain BW25113 (see Materials and Methods). However, in these strains of E. coli (MG1655 and BW25113 surA or dcd), drug-drug interaction networks yielded very similar clustering, and in both cases, vancomycin was a distinct group by itself (data not shown).
FIG 5.
Time-kill kinetics of vancomycin (100 μg/ml), trimethoprim (0.15 μg/ml), and the two drugs combined in wild-type E. coli compared to that of a no-drug control consisting of LB medium only. Cultures were started with an inoculum of 1 × 104 cells/ml, and viable cells were measured by plating every 2 h for 8 h. Viable cell counts were plotted against time durations on a semilogarithmic graph.
DISCUSSION
The proliferation of multidrug-resistant bacteria is a major problem in public health. The challenge is to find new approaches to overcome antibiotic resistance and, particularly, antibiotic-resistant Gram-negative bacteria, whose outer membrane already precludes the use of many drugs that are effective against Gram-positive pathogens. Thus, some investigators are focusing on natural products derived from nonconventional sources, such as plants (10, 11), marine microorganisms (12), and insects (13). Another strategy involves making new chemical derivatives of existing antibiotics, on the basis of the rational design of the compounds using information such as the three-dimensional structure of the antibiotic (14, 15), or linking drugs to peptides that can pass through membranes to facilitate their entry into the cell (16).
Combinatorial strategies for antibiotic use offer an expanded repertoire of drug therapies (17). Of particular interest are potentiators of existing antibiotics, or codrugs, a number of which have been used in both laboratory (18–23) and clinical (24–26) settings. Also, Collins and coworkers have demonstrated that specific metabolites can potentiate aminoglycosides acting on E. coli and Staphylococcus aureus biofilms (27). One desired potentiator would sensitize Gram-negative bacteria to vancomycin (VAN), a glycopeptide antibiotic that blocks peptidoglycan polymerization by binding to the peptidyl-d-alanyl-d-alanine termini of peptidoglycan precursors (28) but that cannot penetrate the outer membrane (1, 29). In this regard, Collins and coworkers have shown that silver sensitizes E. coli to a series of antibiotics, including VAN (30), and Wink and coworkers have reported that EDTA potentiates the activity of the combination of VAN plus thymol (31).
In the work reported here, we utilized mutant E. coli strains (dcd and surA mutants) that have increased sensitivity to VAN to study pairwise synergies between VAN and a set of antibiotics (Table 1). We examined our results in the context of a previous, larger study of drug interactions in E. coli (9). This previous study examined drug-drug interactions and classified them as synergistic, antagonistic, or additive (no interaction). The drugs can then be clustered on the basis of these drug interactions: all drugs in each group can interact only with all other groups either synergistically or antagonistically, but not both. (Additive interactions can be found between any of these groups.) As it turns out, these groups that are clustered on the basis of drug interactions with other groups also cluster according to mechanism of action: for example, all folic acid biosynthesis inhibitor drugs cluster in one group and all DNA synthesis inhibitors cluster in a different group. This means that we can use a simple, phenotypic assay to determine the mechanism of action of any drug. A drug that does not cluster into any of the known groups would end up clustering by itself in a new group, and such drugs would be candidates for drugs that have new mechanisms of action.
Placing our own current data in the context of data from this previous study, we show that VAN can be clustered by itself, in its own functional group, as depicted in Fig. 6. This means that VAN likely has a mechanism of action different from that of all other drugs evaluated here (including the other drugs that affect cell wall synthesis) or it has multiple mechanisms of action. Upon closer inspection of our current data and previously published data, this is not surprising. For example, VAN and trimethoprim together are highly synergistic (Fig. 2 to 4). When other cell wall synthesis inhibitors, such as penicillin and its derivatives, are combined with trimethoprim, the drugs exhibit highly antagonistic interactions (9). Indeed, all the cell wall synthesis inhibitors tested in that study (ampicillin, piperacillin, and cefoxitin) exhibited highly antagonistic interactions with trimethoprim. Thus, this is good evidence to support the idea that VAN is operating within bacterial cells in a very fundamentally different way than other cell wall synthesis inhibitors, revealing a complexity of interaction patterns that ultimately have implications for understanding the mechanism of action of drugs.
FIG 6.

Vancomycin does not cluster monochromatically with other known drug groups in E. coli. By clustering drugs according to how they interact with all other drugs (synergistic, antagonistic, or additive) and keeping all clusters interacting with each other as all synergistic/additive or antagonistic/additive, we can elucidate the underlying mechanism of action of the drug within a bacterial cell. Red lines, synergistic interactions; green lines, antagonistic interactions; solid line, data from a previous study (9); dashed lines, the types of interaction of vancomycin with these other drugs. In the surA strain, vancomycin clusters by itself in its own group. This result suggests that vancomycin either has a mechanism of action unique in comparison with that of other drugs or has multiple mechanisms of action.
It is understandable that SurA-deficient mutants have increased susceptibility to VAN (3, 4), since outer membrane assembly is defective (3). However, the mechanism of increased sensitivity of DCD-deficient cells (5) is not as straightforward. One possibility is that a very small percentage of exogenous VAN does enter the cell (Fig. 1) and this can act synergistically with certain other processes. The mechanism of synergy is poorly understood, but an attractive possible mechanism of synergy also would explain why DCD-deficient cells are more susceptible to VAN. Cells starved for thymidine build up irreparable lesions (32) that ultimately result in thymineless death (33). In DCD-deficient cells, thymidine is limiting, and this situation is exacerbated by the addition of cytidine (5). Collins and coworkers have postulated that bactericidal antibiotics ultimately generate hydroxyl radicals that damage DNA, resulting in cell killing (34). Several recent studies have challenged this idea (35–38), although these have been critiqued in a recent review (39). Thus, if small amounts of VAN generate some DNA damage, that damage may be synergistic with the DNA damage caused by the partial thymineless state of DCD-deficient cells. This predicts that agents that directly damage DNA will show strong synergy with VAN, as will agents that starve the cell of thymidine, such as trimethoprim (TMP). From Fig. 2, it is clear that the strongest synergies with VAN are seen with TMP and also with nitrofurantoin (NTR), an agent that directly damages DNA (40). The respective synergies are strong enough that even in wild-type E. coli, concentrations of VAN as low as 12.5 μg/ml can display an effect when used in combination with NTR (Fig. 4). Moreover, concentrations of VAN of 12.5, 6.25, and even 3.13 μg/ml can show effects when used in combination with TMP (Fig. 5). Therefore, the hydroxyl radical route of cell killing defined by Collins and coworkers (34) would interact synergistically with other DNA lesions caused by certain agents or conditions. The use of combinations of approved drugs that are already in use does offer some advantages over screening for new potentiators, as combinations of approved drugs can be applied in a clinical setting with many fewer delays.
Ultimately, our study aimed to show how vancomycin can be used in conjunction with other drugs against Gram-negative bacteria. Our data also support the idea that vancomycin is operating in a manner that is fundamentally different from that of other cell wall synthesis inhibitor antibiotics. While our research is focused on understanding basic biological principles, there are potentially significant clinical issues that can addressed with further study on this topic. Importantly, there is a pressing need for new antibiotics, especially for Gram-negative bacteria, and our study suggests ways in which we can begin thinking about using vancomycin against such bacteria.
ACKNOWLEDGMENTS
C.B. was supported by an NIH CARE scholars fellowship. P.Y. was supported by the Hellman Fellows Fund and a UCLA Faculty Career Development Award.
REFERENCES
- 1.Nikaido H. 1989. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother 33:1831–1836. doi: 10.1128/AAC.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lockhart SR, Abramson MA, Beekmann SE, Gallagher G, Riedel S, Diekema DJ, Quinn JP, Doern GV. 2007. Antimicrobial resistance among gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol 45:3352–3359. doi: 10.1128/JCM.01284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Justice SS, Hunstad DA, Harper JR, Duguay AR, Pinkner JS, Bann J, Frieden C, Silhavy TJ, Hultgren SJ. 2005. Periplasmic peptidyl prolyl cis-trans isomerases are not essential for viability, but SurA is required for pilus biogenesis in Escherichia coli. J Bacteriol 187:7680–7686. doi: 10.1128/JB.187.22.7680-7686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamae C, Liu A, Kim K, Sitz D, Hong J, Becket E, Bui A, Solaimani P, Tran KP, Yang H, Miller JH. 2008. Determination of antibiotic hypersensitivity among 4,000 single gene knockout mutants of Escherichia coli. J Bacteriol 190:5981–5988. doi: 10.1128/JB.01982-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang TM, Yuan J, Zhou A, Beppler C, Miller JH. 2014. Deoxycytidine deaminase-deficient Escherichia coli strains display acute sensitivity to cytidine, adenosine, and guanosine and increased sensitivity to a range of antibiotics, including vancomycin. J Bacteriol 196:1950–1957. doi: 10.1128/JB.01383-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame single-gene knockout mutants: the Keio Collection. Mol Syst Biol 2:2006 0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller JH. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria, p 194–195. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 9.Yeh P, Tschumi AI, Kishony R. 2006. Functional classification of drugs by properties of their pairwise interactions. Nat Genet 28:489–494. doi: 10.1038/ng1755. [DOI] [PubMed] [Google Scholar]
- 10.Appendino G, Gibbons S, Giana A, Pagani A, Grassi G, Stavri M, Smith E, Rahman MM. 2008. Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod 71:1427–1430. doi: 10.1021/np8002673. [DOI] [PubMed] [Google Scholar]
- 11.Shiu WK, Rahman MM, Curry J, Stapleton P, Zioh M, Malkinson JP, Gibbons S. 2012. Antibacterial acylphloroglucinols from Hypericum olympicum. J Nat Prod 75:336–343. doi: 10.1021/np2003319. [DOI] [PubMed] [Google Scholar]
- 12.Jang KH, Nam SJ, Locke JB, Kauffman CA, Beatty DS, Paul LA, Fenical W. 2013. Anthracimycin, a potent anthrax antibiotic from a marine-derived actinomycete. Angew Chem Int Ed Engl 52:7822–7824. doi: 10.1002/anie.201302749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freitak D, Knorr E, Vogel H, Vilcinskas A. 2012. Gender- and stressor-specific microRNA expression in Tribolium castaneum. Biol Lett 8:308–311. doi: 10.1098/rsbl.2011.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee RE, Hurdle JG, Liu J, Bruhn DF, Sherman MS, Vaddady PK, Zheng Z, Qi J, Akbergenov R, Das S, Madhura DB, Rathi C, Trivedi A, Villellas C, Lee RB, Rakesh, Waidyarachchi SL, Sun D, McNeil MR, Ainsa JA, Boshoff HI, Gonzalez-Juarrero M, Meibohm B, Boettger EC, Lenaerta AJ. 2014. Spectinamidea: a new class of semisynthetic antituberculosis agents that overcome native drug efflux. Nat Med 20:152–158. doi: 10.1038/nm.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carney DW, Schmitz KR, Truong JV, Sauer RT, Sello J. 2014. Restriction of the conformational dynamics of the cyclic acyldepsipeptide antibiotics improves their antibacterial activity. J Am Chem Soc 136:1922–1929. doi: 10.1021/ja410385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geller BL, Greenberg DE. 2014. Peptide-conjugated phosphorodiamidate morpholino oligomers: a new strategy for tackling antibiotic resistance. Ther Deliv 5:243–245. doi: 10.4155/tde.13.145. [DOI] [PubMed] [Google Scholar]
- 17.Cottarel G, Wierzbowski J. 2007. Combination drugs, an emerging option for antibacterial therapy. Trends Biotech 25:547–555. doi: 10.1016/j.tibtech.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Lomovskaya O, Warren MS, Lee A, Galazzo J, Fronko R, Lee M, Blais J, Cho D, Chamberland S, Renau T, Ishida H, Lee VJ. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob Agents Chemother 45:105–116. doi: 10.1128/AAC.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson ML, Levy SB. 1999. Reversal of tetracycline resistance by different bacterial tetracycline resistance determinants by an inhibitor of the Tet(B) antiport protein. Antimicrob Agents Chemother 43:1719–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conlon BP, Nakayasu ES, Flack LE, LaFleur MD, Isabella VM, Coleman K, Leonard SN, Smith RD, Adkins JN, Lewis K. 2013. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 503:365–370. doi: 10.1038/nature12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y, Coates AR. 2013. Enhancement by novel anti-methicillin-resistant Staphylococcus aureus compound HT61 of the activity of neomycin, gentamicin, mupirocin, and chlorhexidine: in vitro and in vivo studies. J Antimicrob Chemother 68:374–384. doi: 10.1093/jac/dks384. [DOI] [PubMed] [Google Scholar]
- 22.Ejim L, Farha MA, Falconer SB, Wildenhain J, Coombes BK, Tyers M, Brown ED, Wright G. 2011. Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy. Nat Chem Biol 7:348–350. doi: 10.1038/nchembio.559. [DOI] [PubMed] [Google Scholar]
- 23.Marks LR, Clementi EA, Hakansson A. 2012. The human milk protein-lipid complex HAMLET sensitizes bacterial pathogens to traditional antimicrobial agents. PLoS One 7:e43514. doi: 10.1371/journal.pone.0043514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buynak JD. 2006. Understanding the longevity of the β-lactam antibiotics and of antibiotic/β-lactamase inhibitor combinations. Biochem Pharmacol 71:930–940. doi: 10.1016/j.bcp.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Hikida M, Kawashima K, Yoshida M, Mitsuhashi S. 1992. Inactivation of new carbapenem antibiotics by dehydropeptidase-1 from porcine and human renal cortex. J Antimicrob Chemother 30:129–134. doi: 10.1093/jac/30.2.129. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman J, Trimble J, Brophy GM. 2009. Safety of imipenem/cilastin in neurocritical care patients. Neurocrit Care 10:403–407. doi: 10.1007/s12028-008-9170-z. [DOI] [PubMed] [Google Scholar]
- 27.Allison KR, Brynildsen MP, Collins JJ. 2011. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473:216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barna JC, Williams DH. 1984. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu Rev Microbiol 38:339–357. doi: 10.1146/annurev.mi.38.100184.002011. [DOI] [PubMed] [Google Scholar]
- 29.Pages JM, James CE, Winterhalter M. 2008. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat Rev Microbiol 6:893–903. doi: 10.1038/nrmicro1994. [DOI] [PubMed] [Google Scholar]
- 30.Morones-Ramirez JR, Winkler JA, Spina CS, Collins JJ. 2013. Silver enhances antibiotic activity against Gram negative bacteria. Sci Transl Med 5:190ra81. doi: 10.1126/scitranslmed.3006276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamoud R, Zimmermann S, Reichling J, Wink M. 2013. Synergistic interactions in two-drug and three drug combinations (thymol, EDTA, and vancomycin) against multi drug resistant bacteria including E. coli. Phytomedicine 21:443–447. doi: 10.1016/j.phymed.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Kuong KJ, Kuzminov A. 2010. Stalled replication fork repair and misrepair during thymineless death in Escherichia coli. Genes Cells 15:619–634. doi: 10.1111/j.1365-2443.2010.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohkawa T. 1975. Studies of intracellular thymidine nucleotides. Thymineless death and the recovery after re-addition of thymine in Escherichia coli K12. Eur J Biochem 60:57–66. [DOI] [PubMed] [Google Scholar]
- 34.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 35.Ezraty B, Vergnes A, Banzhaf M, Duverger Y, Huguenot A, Brochado AR, Su SY, Espinosa L, Loiseau L, Py B, Typas A, Barras F. 2013. Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science 340:1583–1587. doi: 10.1126/science.1238328. [DOI] [PubMed] [Google Scholar]
- 36.Keren I, Wu Y, Inocencio J, Mulcahy LR, Lewis K. 2013. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science 339:1213–1216. doi: 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Imlay JA. 2013. Cell death from antibiotics without the involvement of reactive oxygen species. Science 339:1210–1213. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahoney TF, Silhavy TJ. 2013. The Cpx stress response confers resistance to some, but not all, bactericidal antibiotics. J Bacteriol 195:1869–1874. doi: 10.1128/JB.02197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dwyer DJ, Collins JJ, Walker GC. 10 September 2014. Unraveling the physiological complexities of antibiotic lethality. Annu Rev Pharmacol Toxicol doi: 10.1146/annurev-pharmtox-010814-124712. [DOI] [PubMed] [Google Scholar]
- 40.Tu Y, McCalla DR. 1975. Effect of activated nitrofurans on DNA. Biochim Biophys Acta 402:142–149. doi: 10.1016/0005-2787(75)90032-5. [DOI] [PubMed] [Google Scholar]