Abstract
Alpha-toxin (AT) is a major virulence determinant in Staphylococcus aureus skin and soft tissue infection models. We previously demonstrated that prophylactic administration of 2A3, an AT-neutralizing monoclonal antibody (MAb), prevents S. aureus disease in a mouse dermonecrosis model by neutralizing AT-mediated tissue necrosis and immune evasion. In the present study, MEDI4893*, an affinity-optimized version of 2A3, was characterized for therapeutic activity in the dermonecrosis model as a single agent and in combination with two frontline antibiotics, vancomycin and linezolid. MEDI4893* postinfection therapy was found to exhibit a therapeutic treatment window similar to that for linezolid but longer than that for vancomycin. Additionally, when combined with either vancomycin or linezolid, MEDI4893* resulted in reduced tissue damage, increased neutrophil and macrophage infiltration and abscess formation, and accelerated healing relative to those with the antibiotic monotherapies. These data suggest that AT neutralization with a potent MAb holds promise for both prophylaxis and adjunctive therapy with antibiotics and may be a valuable addition to currently available options for the treatment of S. aureus skin and soft tissue infections.
INTRODUCTION
Staphylococcus aureus is the leading cause of skin and soft tissue infections (SSTI) in the United States (1, 2). Although these are often mild infections, they can lead to severe invasive diseases such as bacteremia, endocarditis, osteomyelitis, and sepsis. S. aureus infections can also be difficult to completely eradicate even when the infecting isolate is susceptible to antibiotics (3, 4). This is further complicated by the emergence and spread of methicillin-resistant S. aureus (MRSA) along with an increasing incidence of resistance to macrolides, aminoglycosides, and fluoroquinolones (5) and more recently linezolid (LZD) (6, 7). This has led to the search for alternative, nonantibiotic options to either treat or prevent serious S. aureus infections and to preserve the active antibiotics currently available to manage these infections.
One possible strategy to improve treatment outcomes is to combine antibiotic therapy with an approach designed to enhance the host immune response against the offending pathogen. A protective immune response against S. aureus SSTI is characterized by an interleukin-1β (IL-1β)-dependent proinflammatory cytokine response leading to immune cell influx and neutrophilic abscess formation (8). Alpha-toxin (AT), a 33-kDa cytolytic pore-forming toxin produced by a majority of S. aureus clinical isolates, has been reported to blunt this protective immune response. In a dermonecrosis model, it has been demonstrated that mice infected with S. aureus isogenic mutants defective for AT expression mount a robust inflammatory cytokine response (e.g., IL-1β, keratinocyte chemoattractant [KC], IL-6, and IL-17) with accompanying immune cell infiltration, abscess formation, and significant disease attenuation compared to the case for mice infected with wild-type S. aureus (9, 10). We reported similar results following prophylactic administration of the AT-neutralizing monoclonal antibody (MAb) 2A3, the MEDI4893* precursor (9). Additionally, Fritz et al. reported that patients with an S. aureus SSTI and high serum anti-AT IgG titers were less likely to have a recurrent infection than patients with low anti-AT titers, providing evidence for a role for AT in human disease (11).
MEDI4893 is an extended-half-life, high-affinity AT-neutralizing MAb currently under clinical development (www.clinicaltrialsregister.eu) and was recently shown to neutralize 11 different AT sequence variants expressed by 200 different S. aureus clinical isolates (45). MEDI4893 was generated by introducing the YTE mutations into the previously reported anti-AT MAb LC10 (12–14). The YTE mutations increase IgG half-life (t1/2) in humans but significantly reduce serum exposure in mice and prevent its use in mice (15, 16). Since LC10 and MEDI4893 have the same amino acid sequence except for the YTE mutations, LC10 is referred to as MEDI4893*. It is possible that addition of a molecule which neutralizes the cytotoxic and immunosuppressive effects of AT to standard antibiotic therapies may result in improved disease outcomes. In fact, coadministration of MEDI4893* with vancomycin (VAN) and linezolid resulted in improved disease outcomes relative to those with therapy with antibiotics alone in a murine pneumonia model (13). To more fully characterize MEDI4893*, we evaluated its activity when administered therapeutically, alone and in combination with vancomycin or linezolid, in a murine S. aureus dermonecrosis model to gain an understanding of the value that treatment with an AT-neutralizing MAb, such as clinical candidate MEDI4893 (www.clinicaltrialsregister.eu), may provide over antibiotic monotherapy.
MATERIALS AND METHODS
Bacterial strains, antibiotics, and antibodies.
Methicillin-resistant Staphylococcus aureus SF8300 (USA300) was generously provided by Binh Diep (University of California, San Francisco). Vancomycin (VAN) was obtained from Sigma-Aldrich (St. Louis, MO). Linezolid (LZD) was obtained from Tecoland Corporation (Edison, NJ). Vancomycin was prepared in 5% dextrose (d5w), and linezolid was dissolved in 5% aqueous hydroxypropyl-β-cyclodextrin (HPβCD) (Sigma-Aldrich, St. Louis, MO). Antibiotics were prepared fresh daily and refrigerated between doses. MEDI4893* is an anti-S. aureus AT-neutralizing human IgG1 (13). R347 is a human anti-HIV gp120 IgG1 that was used as an isotype control. Monoclonal antibodies were prepared daily by dilution into sterile phosphate-buffered saline (PBS), pH 7.2 (Invitrogen, Carlsbad, CA).
In vitro susceptibility testing.
MICs were determined by the broth microdilution method, according to CLSI guidelines. The MIC was defined as the lowest antibiotic concentration that prevented visible growth after an incubation of 16 to 20 h (17).
In vivo models.
All animal studies were approved by the MedImmune Institutional Animal Care and Usage Committee and were conducted in an Association for Accreditation and Assessment Laboratory Animal Care (AAALAC)-accredited facility, in compliance with U.S. regulations governing the housing and use of animals.
S. aureus dermonecrosis model.
The S. aureus dermonecrosis model was conducted and bacteria prepared as previously described (18). Briefly, 6- to 8-week-old female BALB/c mice (Harlan Laboratories, Frederick, MD) were shaved and inoculated intradermally (i.d.) with 4 × 107 to 5 × 107 CFU of SF8300 diluted into 50 μl PBS. To determine the optimal therapeutic vancomycin, linezolid, and MEDI4893* doses, mice were infected with S. aureus SF8300 and then treated with vancomycin (intraperitoneally [i.p.]) or linezolid (per os [p.o.]) at 1 and 8 h postinfection or with MEDI4893* (i.p.) at 1 h postinfection. The IgG1 isotype control R347, which gave lesions similar to those with d5w, HPβCD, or PBS, was used as the negative control. Skin lesions were measured with Fowler digital calipers (Sylvac Systems, Crissier, Switzerland) at 24 h postinfection. The area of the lesion was then calculated as length × width.
To quantify S. aureus CFU in infected skin, mice (n = 5) were euthanized at 24, 72, and 168 h postinfection. The infected skin was disinfected with isopropanol, removed, placed into sterile tubes, homogenized in 1 ml PBS (pH 7.4) (Omni Prep multisample homogenizer; Omni International, Marietta, GA), and serially diluted for CFU determination on Trypticase soy agar (TSA) plates. Comparative studies with TSA and CHROMagar (BD, Paris, France) with infected and naive mice demonstrated that no other skin bacteria were present (data not shown).
Single-dose pharmacokinetics (PK).
Mice were infected as described above, and at 3 h postinfection they were dosed i.p. with vancomycin (100 mg/kg) or per os with linezolid (5 mg/kg). At 0.125, 0.25, 0.5, 1, 2, 4, and 6 h after dosing (5 animals/time point), mice were euthanized and blood collected by cardiac puncture into lithium heparin tubes (BD Sciences, Franklin, NJ). Plasma was obtained from whole blood by centrifugation.
For linezolid determination, samples were precipitated in acetonitrile and centrifuged. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses were performed as described previously (19) with modification to afford 7-min cycle times through a steep solvent gradient. Samples containing vancomycin were precipitated in 30% (wt/vol) trichloroacetic acid solution and centrifuged. Supernatants (10 μl) were injected onto a Phenomenex (Torrance, CA) 50- by 2-mm (inner diameter) Jupiter C18 column packed with particles of 5 μm in size and 300 Å in pore diameter. The column temperature and flow rate were 30°C and 0.25 ml/min, respectively. The eluent was mixed from 0.1% (vol/vol) aqueous formic acid solution (solvent A) and acetonitrile containing 0.1% (vol/vol) formic acid (solvent B), with the composition initially set at 5% solvent B. After sample injection, solvent B was increased linearly to 95% in 1 min and held for 2.5 min before finally returning in 0.1 min to 5% solvent B to equilibrate for 3.4 min before the subsequent run. The effluent was diverted to waste for 2 min after injection to reduce the amount of inorganic salt entering the ion source of the mass spectrometer. The LCQ-Deca's electrospray ionization (ESI) source was operated in the positive-ion mode. Nitrogen was used as both the sheath and auxiliary gases at pressures of 80 and 20 units, respectively. The spray voltages were set at 5.0 kV and 4.5 kV for the analysis of linezolid and vancomycin, respectively. Selected reaction monitoring (SRM) was used for quantification, and matrix-matched calibrations using colistin B as an internal standard were performed using blank mouse plasma and homogenate of infected mouse skin collected from untreated subjects. SRM transitions were m/z 1472→1167 and 1178→1134 for vancomycin and colistin B, respectively.
Pharmacokinetic parameters were determined using PK Solutions 2.0 (Summit Research Services, Montrose, CO).
IL-1β detection.
Mice were infected and dosed as described above. At 24 h postinfection, groups of 10 animals were euthanized and skin lesions harvested, weighed, and frozen in liquid nitrogen. Each lesion was homogenized with a mortar and pestle and digested for 2 h at 4°C in Reporter lysis buffer (Promega, Madison, WI) supplemented with complete mini-protease inhibitor tablets (Roche Diagnostics, Indianapolis, IN). Supernatants were stored at −80°C and quantified for IL-1β content with the Mesoscale 7-Plex proinflammatory mouse cytokine kit (Mesoscale, Gaithersburg, MD). Values were normalized to pg/mg skin.
Flow cytometry.
Skin lesions were harvested at 24, 72, or 168 h postinfection, minced with a razor blade, transferred to 50-ml conical tubes, and incubated with shaking (150 rpm) for 2 h at 37°C in 10 ml Glutamax RPMI 1640 medium (Invitrogen, Grand Island, NY) containing 0.3 mg/ml Liberase TL (Roche Applied Sciences, Indianapolis, IN), 0.01% DNase (Sigma-Aldrich), 100 U/ml penicillin, 100 μg/ml streptomycin (Gibco-Invitrogen), 0.05 mM β-mercaptoethanol (Gibco-Invitrogen), and 100 μM nonessential amino acid (Gibco-Invitrogen). Digested tissues were filtered through a 40-μm cell strainer (BD Falcon, Franklin Lakes, NJ) to obtain single-cell suspensions. Cell pellets were incubated with 3 ml ACKACK buffer (Invitrogen) for 3 min on ice and then washed twice with 50 ml supplemented Glutamax RPMI containing 10% fetal bovine serum (FBS) (Invitrogen) to lyse red blood cells. Neutrophils (polymorphonuclear leukocytes [PMNs]) and macrophages were quantified as previously described (18). Briefly, 100 μl of cell suspension was transferred to a U-bottom 96-well plate (VWR International, Radnor, PA), incubated with anti-CD16/32 blocker (clone 93; eBioscience, San Diego, CA) for 15 min at 4°C, and washed once with PBS–1% bovine serum albumin (BSA) after washing with 200 μl PBS–0.1% BSA. Cells were stained with the Hoechst stain (Life Technologies, Grand Island, NY) for live/dead gating, washed once with PBS–1% BSA, and then incubated with phycoerythrin (PE)-Cy7-Gr-1b (eBioscience, clone RB6-8C5), allophycocyanin (APC)-F4/80 (eBioscience, clone BM8), and fluorescein isothiocyanate (FITC)-CD11b (eBioscience, clone M1/70) Abs for 1 h at 4°C. Following a PBS-FBS wash, samples were acquired using an LSR-II flow cytometer (BD Sciences) and data analyzed with FlowJo software (Tree Star, Inc., Ashland, OR). The percentage of neutrophils and macrophages were calculated as percent Gr-1b+/CD11b+ and percent F4/80+/CD11b+, respectively. Total cell numbers for each population were then calculated based on cell counts from the initial skin single-cell suspension.
Histopathology.
At 24, 72, and 168 h postchallenge, mice were euthanized and skin lesions were measured, photographed, removed, trimmed to approximately 4 mm, and placed into 10% neutral buffered formalin (VWR) in tissue cassettes. Skin samples were processed and paraffin embedded, on edge. Paraffin sections of 4 μm were stained with hematoxylin (50% Gills I and 50% Gills II) and eosin (Leica Microsystems, Richmond, IL) (H&E). All histology sections were reviewed by a pathologist who was blinded to the experimental conditions.
Statistical analyses.
Data were analyzed using t tests and analysis of variance (ANOVA) followed by the Sidek or Dunnett test or using the Kruskal-Wallis test followed by Dunn's test where appropriate. All statistical analyses were performed using GraphPad Prism v.5.0. A P value of <0.05 was considered statistically significant.
RESULTS
Antibiotic and MEDI4893* treatment window.
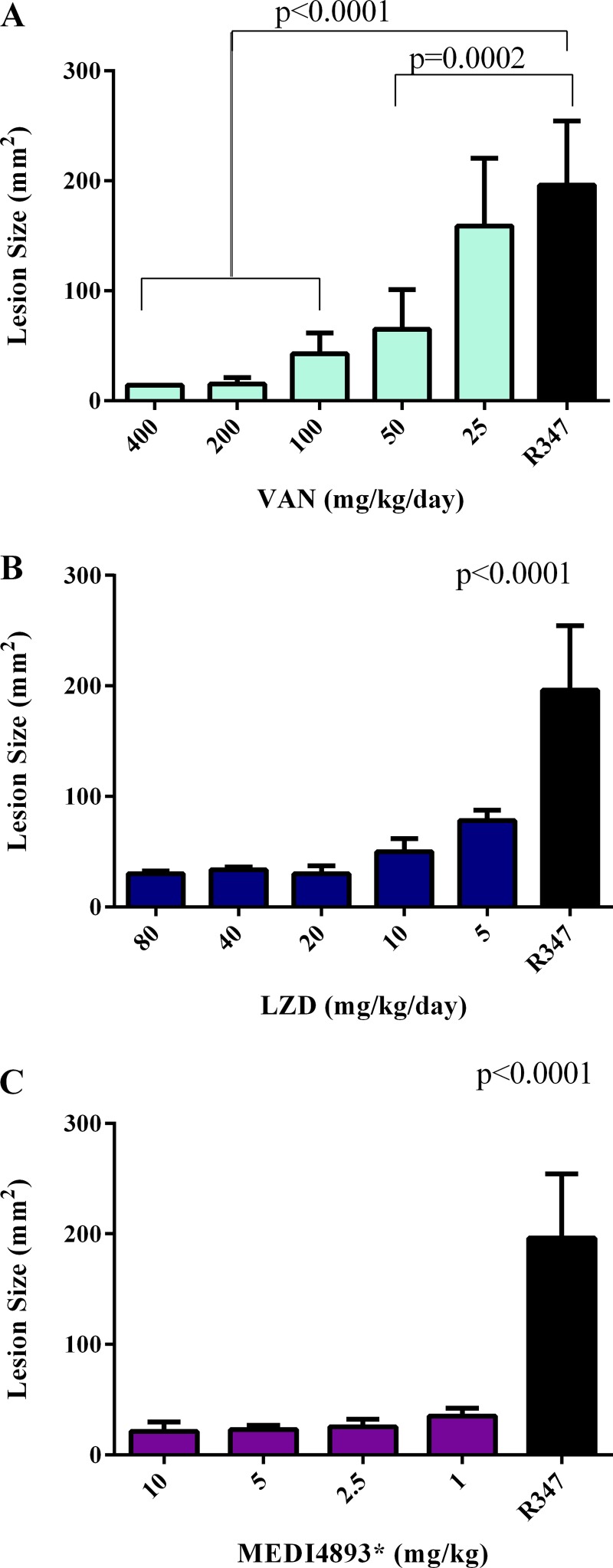
We previously reported on the prophylactic efficacy and mechanism of action of an anti-AT MAb, 2A3, in the murine dermonecrosis model (9, 18). To assess the potential of an AT-neutralizing MAb as a therapeutic for S. aureus skin and soft tissue infections, the therapeutic activity of LC10 (MEDI4893*), an affinity-optimized version of 2A3 (13), was compared with those of two frontline antibiotics, vancomycin and linezolid, in the murine dermonecrosis model. We first conducted dose-ranging studies for the individual antibiotics and MEDI4893* administered at 1 h postinfection to identify effective therapeutic doses. Mice were challenged by intradermal inoculation with SF8300. AT 1 h postinfection, MEDI4893* was administered in a single i.p. injection and vancomycin (VAN) and linezolid (LZD) were administered twice a day (BID) every 8 h (q8h) by i.p. injection and per os (p.o.), respectively. Lesion sizes were measured at 24 h postinfection. Doses of 200 mg/kg/day vancomycin, 10 mg/kg/day linezolid, and 2.5 mg/kg MEDI4893* resulted in minimal lesion sizes and were defined as effective treatment doses (Fig. 1). To determine the plasma pharmacokinetic parameters for these antibiotic doses, the maximal free-drug concentrations (fCmax) and exposures for unbound drug in plasma samples were calculated (Table 1). A single-dose PK study with 100 mg/kg vancomycin resulted in an fCmax of 121.5 μg/ml and a plasma free-drug infinite area under the curve (fAUC∞) value of 224.4 μg/ml. The fAUC/MIC ratio of 448.8 is similar to the AUC/MIC ratio of 400 required for a positive clinical outcome in humans (20). A single-dose PK study with a 5-mg/kg linezolid dose yielded an fCmax of 14.3 μg/ml and an fAUC∞ of 24.3 μg/ml. The mouse fCmax value of 14.3 μg/ml is similar to that of humans (11 μg/ml); however, the mouse fAUC∞ (24.3 μg/ml) and AUC/MIC (24.3) are lower than the observed clinical fAUC∞ (80.80 μg/ml) and AUC/MIC (100) for a positive clinical outcome (20). In spite of these reduced values in mice compared to humans, linezolid at 5 mg/kg administered BID q8 h is an effective dose in this murine model. MEDI4893* exhibited PK similar to those for a standard human MAb in mice, with an 8- to 12-day half-life and ∼17% dose-dependent distribution into the skin (data not shown).
FIG 1.
Anti-AT MAb and antibiotics reduce lesion size in a dose-dependent manner. BALB/c mice (n = 5) were infected i.d. with 5 × 107 CFU S. aureus SF8300. Treatment with vancomycin (VAN) at 25 mg/kg/day to 200 mg/kg/day (A), linezolid (LZD) at 5 mg/kg/day to 80 mg/kg/day (B), or MEDI4893* at 10 mg/kg to 1 mg/kg (C) was initiated 1 h postinfection. Antibiotics were also dosed again at 8 h postinfection. Lesion sizes were measured at 24 h postinfection. Results with the isotype control R347 (black bars) are shown for comparison. Lesion size differences were calculated with a one-way ANOVA and considered statistically significant if the P value was <0.05 (*). Results are representative of 3 independent experiments.
TABLE 1.
Plasma pharmacokinetic/pharmacodynamic parameters for vancomycin and linezolid
| Drug | Dose (mg/kg) | MIC (μg/ml) | Tmaxa (h) | t1/2 (h) | fCmaxb (μg/ml) | fAUC0–∞b (μg · h/ml) | fAUC/MIC |
|---|---|---|---|---|---|---|---|
| Vancomycin | 100 | 0.5 | 0.5 | 0.45 | 121.5 | 224.4 | 448.8 |
| Linezolid | 5 | 1.0 | 0.25 | 0.95 | 14.4 | 24.3 | 24.3 |
Tmax, time to maximum concentration of drug in plasma.
The free-drug exposure following a single 100-mg/kg i.p. dose of vancomycin or a single 5-mg/kg subcutaneous dose of linezolid was determined from the total drug plasma levels corrected for protein binding.
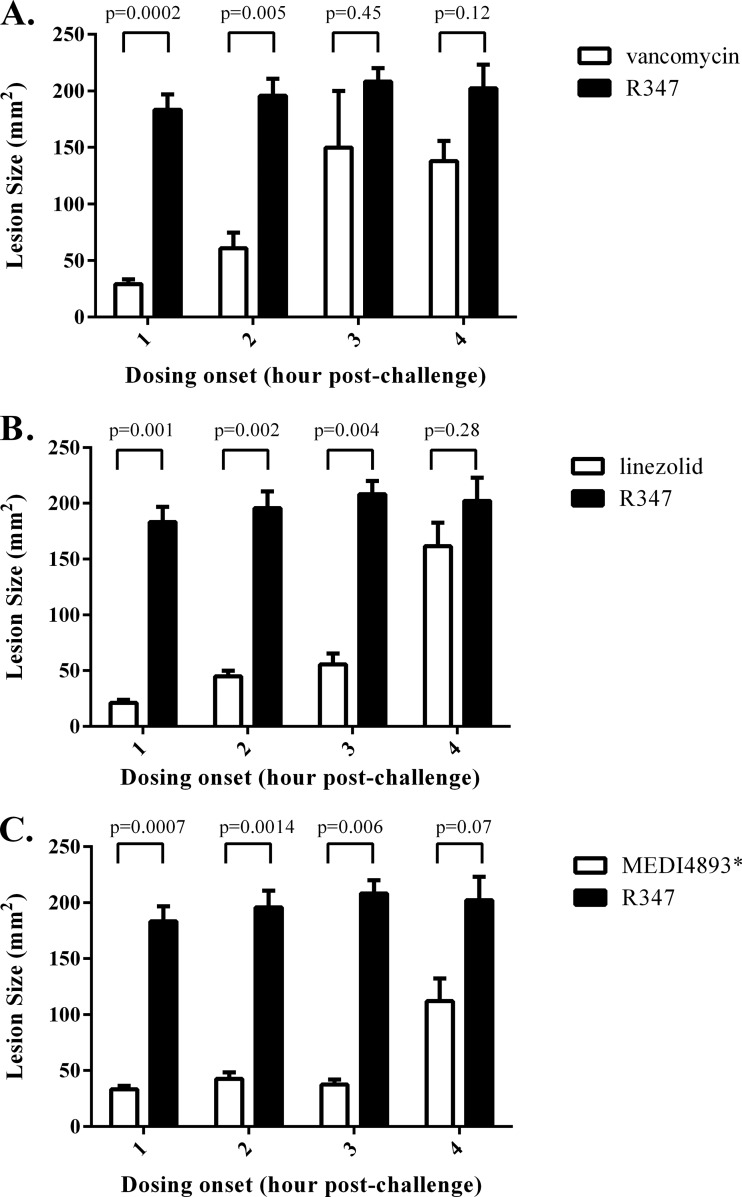
The therapeutic window was next determined for each molecule by varying the initial dosing time (1 to 4 h postinfection) and measuring lesion size at 24 h postinfection (Fig. 2). Treatment with vancomycin at 200 mg/kg/day significantly reduced lesion size when administration was initiated at 1 and 2 h postinfection, but it lost efficacy when the initial administration was delayed to 3 or 4 h postinfection. Linezolid (10 mg/kg/day) and MEDI4893* (2.5 mg/kg) therapies were fully effective when administration was delayed out to 3 h postinfection. When administration was not initiated until 4 h postinfection, neither linezolid nor MEDI4893* resulted in a statistically significant lesion size reduction in comparison to the negative control (R347). These results indicate that MEDI4893* and linezolid provide a larger therapeutic window than vancomycin in this model.
FIG 2.
Lesion size increases as the time between infection and therapy increases. BALB/c mice (n = 5) were infected i.d. with 5 × 107 CFU S. aureus SF8300. Treatment with VAN (A), LZD (B), or MEDI4893* (C) was initiated at 1, 2, 3, or 4 h postinfection. Antibiotics were also dosed again at 8 h postinfection. Lesion sizes were measured at 24 h postinfection. Results with the isotype control R347 (black bars) are shown for comparison. Lesion size differences were calculated with an unpaired t test and considered statistically significant if the P value was <0.05 (*). Results are representative of 3 independent experiments.
MEDI4893*-antibiotic combination therapy.
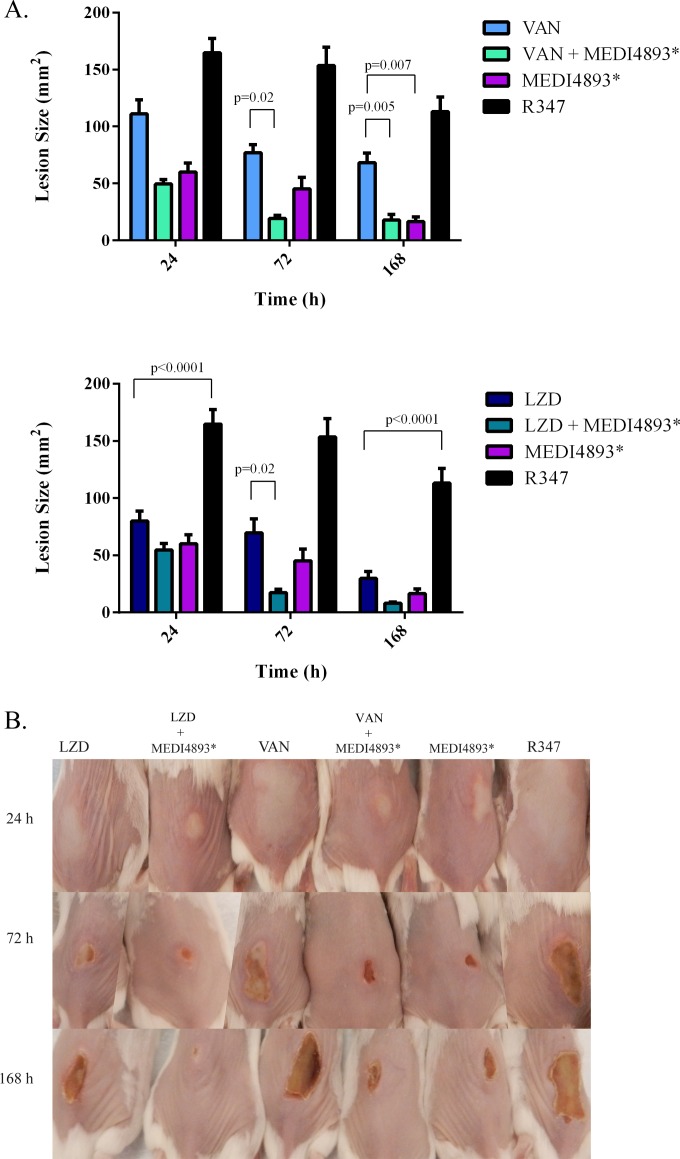
MEDI4893* was previously reported to provide benefit in adjunctive therapy with either vancomycin or linezolid over treatment with the antibiotics alone in a murine pneumonia model (13). To evaluate MEDI4893*-antibiotic combination therapy in the dermonecrosis model, mice were treated at 3 h after S. aureus infection with either vancomycin (200 mg/kg/day), linezolid (10 mg/kg/day), or MEDI4893* (2.5 mg/kg) or with MEDI4893* plus either vancomycin or linezolid. Lesion size was assessed at 24, 72, and 168 h postinfection. Mice receiving MEDI4893* plus vancomycin exhibited reduced lesion sizes relative to those in vancomycin-treated mice, suggesting that adjunctive therapy of MEDI4893* with vancomycin provides a benefit over vancomycin monotherapy in this model. However, the vancomycin-MEDI4893* combination was no more effective at reducing lesion size than MEDI4893* alone (Fig. 3). There was no apparent effect on bacterial CFU at 24 h postinfection in any of the treatment groups relative to the R347 isotype control. Similar to other reports, lesion size in this model is not dependent on bacterial burden (Table 2) but is primarily AT mediated (9, 21–24). However, treatment with vancomycin or MEDI4893*-vancomycin accelerated bacterial burden reduction, resulting in significant CFU reductions at 3 (P < 0.05) and 7 (P < 0.05) days postinfection relative to R347. MEDI4893*-vancomycin adjunctive therapy had the greatest effect on reducing bacterial load, resulting in a significant reduction (P < 0.05) in CFU at 7 days postinfection relative to treatment with vancomycin or MEDI4893* alone. MEDI4893*-linezolid adjunctive therapy resulted in a more rapid reduction in lesion size (P = 0.02) relative to that with linezolid alone at 72 h postinfection, but MEDI4893*-linezolid adjunctive therapy did not accelerate bacterial clearance relative to that with either linezolid or MEDI4893* monotherapy (Table 2). These results in the murine dermonecrosis model indicate a potential benefit of anti-AT adjunctive therapy over monotherapy with the antibiotics linezolid and vancomycin for the treatment of S. aureus skin infections.
FIG 3.
Adjunctive therapy with MEDI4893*-vancomycin or MEDI4893*-linezolid reduces lesion size. BALB/c mice (n = 10) were infected i.d. with 5 × 107CFU S. aureus SF8300 and treated at 3 h postinfection with VAN (200 mg/kg/day), LZD (10 mg/kg/day), MEDI4893* (2.5 mg/kg), R347 (15 mg/kg), or a combination of VAN (200 mg/kg/day) plus MEDI4893* (2.5 mg/kg) or LZD (10 mg/kg/day) plus MEDI4893* (2.5 mg/kg). MEDI4893* was administered once, and antibiotic treatment was continued at 8, 24, and 32 h postinfection. Lesions were measured (A) and photographed (B) at 24, 72, and 168 h postinfection. Lesion size differences were calculated with Student's t test and considered statistically significant if the P value was <0.05 (*). Results from 2 independent studies were pooled.
TABLE 2.
CFU recovery in skin homogenates following SF-8300 challenge and treatment with vancomycin, linezolid, MEDI4893*, combination therapy, or R347
| Drug (dose, mg/kg/day)a | MAb (dose, mg/kg/day)a | Log10 CFU/ml ± SEM at postchallenge time (h): |
||
|---|---|---|---|---|
| 24 | 72 | 168 | ||
| Vancomycin (200) | None | 8.44 ± 0.30 | 7.92 ± 0.20b,d | 7.54 ± 0.11b |
| MEDI4893* (2.5) | 8.49 ± 0.06 | 7.63 ± 0.12b,d | 6.18 ± 0.25b,c,d | |
| Linezolid (10) | None | 8.52 ± 0.16 | 8.40 ± 0.07 | 7.21 ± 0.44b |
| MEDI4893* (2.5) | 8.58 ± 0.31 | 8.13 ± 0.10b | 7.23 ± 0.15b | |
| None | MEDI4893* (2.5) | 8.00 ± 0.01 | 8.98 ± 0.15e | 7.66 ± 0.31b |
| R347 (15.0) | 8.52 ± 0.11 | 8.76 ± 0.16 | 8.53 ± 0.19 | |
Vancomycin and linezolid were dosed at 3, 8, 24, and 32 h postinfection. MEDI4893* and R347 were dosed at 3 h postinfection. See Materials and Methods for further details.
The CFU value is significantly lower than that for the R347 control (P < 0.05).
Vancomycin combination therapy is significantly different from vancomycin monotherapy (P < 0.05).
Significantly different from MEDI4893* monotherapy (P < 0.05).
Significantly different from vancomycin monotherapy and vancomycin-MEDI4893* therapy (P < 0.05).
Effect of adjunctive therapy on the inflammatory immune response.
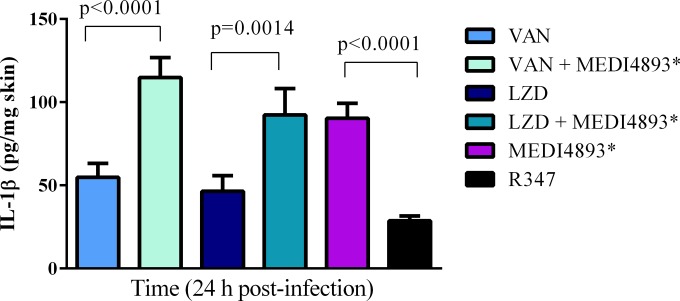
Recent publications have described a critical requirement for IL-1β-mediated immune cell infiltration and abscess formation to effectively combat S. aureus skin and soft tissue infections (25, 26). Additionally, a robust inflammatory response is also required for an effective healing process following skin injury (27). AT expression is key for S. aureus to evade this protective innate immune response (9, 10, 28). To determine the effect of adjunctive therapy on the local inflammatory response, IL-1β levels were assessed at 24 h postinfection in the lesions from mice treated with the monotherapies (vancomycin, linezolid, or MEDI4893*) or with MEDI4893* plus vancomycin or linezolid. At this time point, vancomycin and linezolid monotherapy resulted in IL-1β levels in the lesions that were similar to those with R347 (Fig. 4). In contrast, treatment with vancomycin or linezolid in combination with MEDI4893* resulted in IL-1β levels that were significantly higher than those with vancomycin (P < 0.0001) or linezolid (P < 0.0014) monotherapy and similar to those in mice treated with MEDI4893* alone (Fig. 4). These results indicate that treatment with linezolid or vancomycin in combination with an AT-neutralizing MAb leads to a more robust protective proinflammatory response in the skin than treatment with antibiotics alone.
FIG 4.
The addition of MEDI4893* to antibiotic therapy increases IL-1β in skin lesions. Mice were challenged and treated as described in the legend to Fig. 3. At 24 h postinfection, skin lesions were collected and processed as described in Materials and Methods to measure IL-1β levels. Lesion size differences were calculated with Student's t test and considered statistically significant if the P value was <0.05 (*). Results are representative of 3 independent experiments.
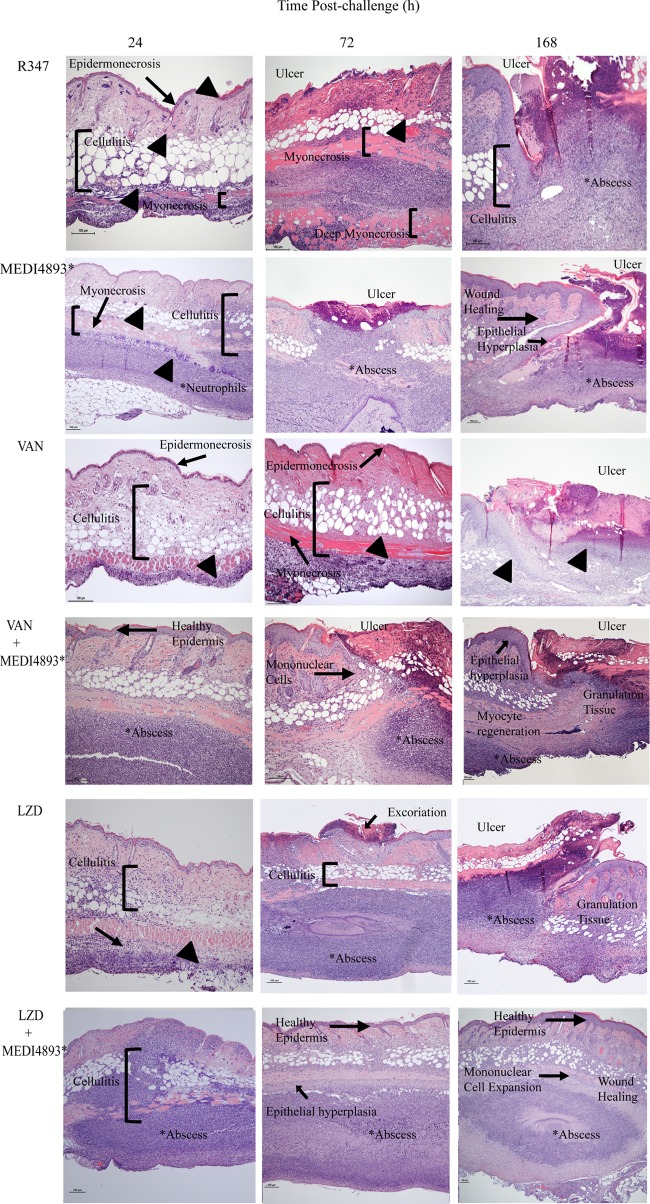
Histopathology.
To further evaluate the inflammatory response and to examine the tissue damage and healing following S. aureus infection, the pathology of skin lesions from infected mice treated with the monotherapies or the MEDI4893*-antibiotic combinations was examined. Twenty-four hours postinfection, skin lesions from R347-treated mice exhibited extensive epidermal and soft tissue necrosis with minimal to no neutrophil infiltration despite the presence of bacterial colonies, whereas MEDI4893*-treated mice had moderate to mild epidermal necrosis with some neutrophilic and mononuclear cell infiltrate (Fig. 5). The skin lesions from the vancomycin-treated mice exhibited a large area of epidermal necrosis with marked cellulitis and soft tissue and muscle necrosis, with limited apparent neutrophilic infiltrate. Linezolid-treated mice exhibited slight epidermal necrosis and cellulitis and moderate myonecrosis with some neutrophilic infiltration. Mice receiving the MEDI4893*-antibiotic combination therapies exhibited increased neutrophilic and mononuclear cell infiltrate along with reduced epidermal necrosis relative to those with their respective antibiotic monotherapies, indicating an accelerated and extensive immune response to bacterial infection that was not observed in mice treated with antibiotics alone or in R347-treated mice.
FIG 5.
MEDI4893* reduces AT-mediated tissue damage and promotes healing of skin lesions. BALB/c mice were infected with SF8300 and treated with VAN, LZD, MEDI4893*, R347, or antibiotic-MEDI4893* combinations at 3 h postinfection. Infected skin was collected from the animals at 24, 72, and 168 h postinfection and processed for H&E staining and microscopic examination. All photomicrographs are at a magnification of ×10. Arrowheads indicate bacterial colonies. Results are representative of 3 independent experiments.
Seventy-two hours postinfection, the R347-treated animals showed an unabated bacterial infection with large ulcers and extensive tissue necrosis as well as deep myonecrosis. Animals treated with vancomycin monotherapy exhibited larger ulcers associated with extensive necrosis of the dermis, subdermal tissue, and subcutaneous fatty tissue, severe muscle necrosis, and bacterial colonization and were lacking evidence of an extensive immune response. Linezolid-treated animals had limited necrosis of the dermis and subdermal tissue with abscess formation but lacked evidence of reepithelialization. In contrast, infected skin from mice treated with the MEDI4893*-antibiotic combination lacked widespread necrosis but exhibited beneficial abscess formation, bacterial containment, and marked mononuclear cell infiltration with evidence of secondary wound healing (e.g., epithelial hyperplasia, granulation tissue formation, and reepithelialization) (Fig. 5). By 168 h postinfection, mice treated with either combination therapy or MEDI4893* alone exhibited evidence of accelerated wound healing, including mononuclear cell expansion resulting in increased thickness of granulation tissue, sequestered bacterial colonies, small abscesses, complete epidermal and myocyte regeneration, and evidence of hair follicle structure development. The newly formed epidermis had regenerative epithelial hyperplasia and no wound gap. All of these features along with the presence of a completely connected and thickened neoepithelial layer are features of skin wound healing (29). Skin from animals that received either vancomycin or linezolid monotherapy exhibited some evidence of secondary healing as noted by epidermal hyperplasia and limited myocyte regeneration; however, it was not as extensive as that observed with the combination therapy, and larger ulcers remained. Taken together, the histopathology results indicate that combination of MEDI4893* with either vancomycin or linezolid not only reduced disease severity and tissue damage but also promoted a robust immune response resulting in abscess formation and accelerated healing of the infected skin.
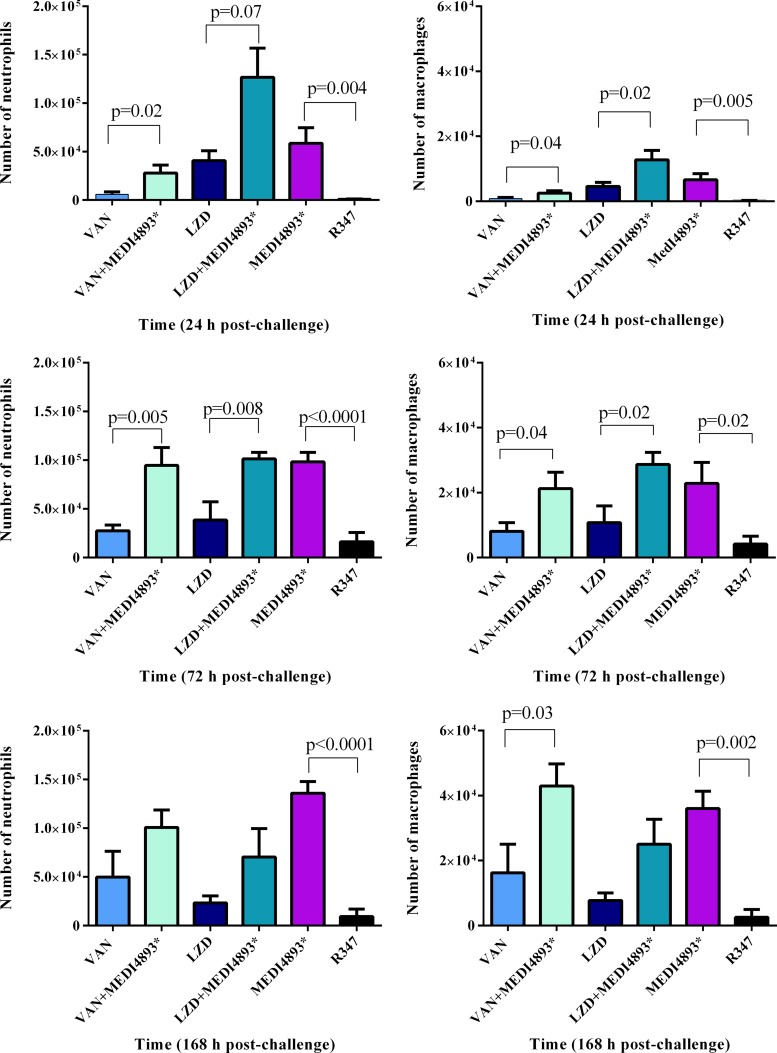
To further characterize the host immune response during infection, neutrophil (PMN) and macrophage infiltration in the infected skin from the animals receiving the various treatments was assessed. In support of the histopathology data, PMN infiltration was significantly increased at 24, 72, and 168 h postinfection in the skin from animals treated with MEDI4893*-vancomycin or MEDI4893*-linezolid versus treatment with the individual antibiotics and was similar to that after treatment with MEDI4893* alone (Fig. 6). Similarly, macrophage levels were greater in the infected skin from animals treated with the MEDI4893*-antibiotic combinations than in that from animals treated with the antibiotics alone, and this difference became more pronounced as time passed (Fig. 6), consistent with an enhanced healing response (27).
FIG 6.
MEDI4893* enhances neutrophil and macrophage infiltration. Skin lesions from S. aureus SF8300-infected mice treated with VAN, LZD, MEDI4893*, R347, or antibiotic-MEDI4893* combinations were collected at 24, 72, or 168 h postinfection, and the neutrophils and macrophages were enumerated by flow cytometry as described in Materials and Methods. Cell number differences were calculated with Student's t test and considered statistically significant if the P value was <0.05 (*). Results are representative of 2 independent experiments.
DISCUSSION
S. aureus SSTI are usually successfully treated with surgical drainage and debridement along with antibiotic therapy (30). However, this is not always successful, and even treatment of infections with susceptible strains can be difficult (4). This is further complicated by the emergence of antibiotic resistance (31). For example, vancomycin-intermediate S. aureus (VISA) and vancomycin-resistant S. aureus (VRSA) have emerged (3, 32, 33), and although not widespread, at least five different linezolid resistance mechanisms have evolved, including the recently identified cfr gene, which also confers resistance to phenicols, lincosamides, pleuromutilins, and streptogramin A (6, 7, 34), further reducing antibiotic treatment options. Given this potential for linezolid resistance, increasing vancomycin MICs, and treatment failures with both antibiotics, we sought to explore the anti-AT antibody MEDI4893* as a complement to existing antibiotic therapy.
S. aureus AT is a cytolytic pore-forming toxin that has been demonstrated to play a key role in many S. aureus disease models, including dermonecrosis, pneumonia, and sepsis (9, 35–37). Secreted AT binds A-disintegrin and metalloprotease 10 (ADAM10), and forms heptameric pores in cell membranes, resulting in cell lysis, tissue damage, and evasion of the protective host immune response (9, 13, 38). The failure of S. aureus mutants defective for AT production to cause dermonecrosis, along with the success of passive and active immunization strategies targeting AT, provides support for a role for AT as a virulence determinant in S. aureus SSTI (9, 10, 18, 35). Consistent with the results obtained following MAb prophylaxis, postinfection therapy with anti-AT MAb MEDI4893* reduced dermonecrotic lesion size in a dose- and time-dependent manner (Fig. 1 and 2). The MEDI4893* treatment effect was similar to that for therapy with linezolid, with an effective treatment initiation window of ∼3 h postinfection, and superior to the ∼2-h therapeutic window for vancomycin. Like in most S. aureus mouse models, the bacterial dose required for consistent infection is relatively high. This results in a rapidly progressive infection with a shortened treatment window than what is seen in humans (5, 39). As a consequence, these data cannot be used to directly translate to a treatment window for MEDI4893* in humans. Its therapeutic activity, however, was compared with those of two antibiotics recommended for human use, and the therapeutic window for MEDI4893* was similar to or better than those for the antibiotics tested. These results indicate that an AT-neutralizing MAb may provide benefit as a treatment for an S. aureus SSTI.
The optimal antibody dose experimentally determined for dermonecrosis in this report (2.5 mg/kg) is clearly lower than the 15 mg/kg reported by Hua et al. for pneumonia (13). This is not surprising based on the 50% effective concentrations (EC50s) (∼0.1 mg/kg) for the MEDI4893* precursor 2A3 in the dermonecrosis model (18). The reason for this is not clear, but they are different models affecting very different organs. The dermonecrosis model involves intradermal bacterial challenge that results in a localized and self-limited skin infection. In contrast, pneumonia is induced by intranasal bacterial challenge, leading to an acute pneumonia accompanied by alveolar damage and loss of lung function and resulting in mortality within 48 h. This is something that should be considered when selecting a dose to prevent or treat different infections in patients.
The mechanisms of action of both vancomycin and linezolid are distinct from that of MEDI4893*. The glycopeptide antibiotic vancomycin disrupts cell wall integrity, resulting in cell lysis (40). This could lead to the release of immunoregulatory factors (e.g., lipoteichoic acid, peptidoglycan, lipoproteins, and toxins), resulting in increased local tissue damage, immune dysregulation, and the potential for disease exacerbation. Linezolid is a bacteriostatic protein synthesis inhibitor reported to reduce S. aureus toxin expression in vivo (41), which would not have an effect on preformed toxin. Therapeutic administration of an AT-neutralizing MAb with an antibiotic could alleviate some of the toxin-mediated damage not affected by either antibiotic, resulting in a more favorable outcome. It was recently reported that addition of MEDI4893* to vancomycin or linezolid treatment regimes in a murine pneumonia model resulted in reduced pulmonary damage, improved lung function, and increased survival relative to that with antibiotic monotherapy (13). In the present study, MEDI4893* adjunctive therapy with either linezolid or vancomycin resulted in improved disease outcome relative to that with treatment with either antibiotic alone. This was evident not only by a reduction in lesion size but also by other biomarkers of a protective immune response, such as increased proinflammatory cytokine expression, immune cell infiltration, beneficial abscess formation, and ultimately accelerated wound healing. Furthermore, even though vancomycin- or linezolid-resistant S. aureus strains are currently rare, adjunctive therapy could reduce the potential for the development of resistance.
Neutrophil infiltration and abscess formation are distinguishing characteristics of an active beneficial host response to S. aureus skin infections (8, 42). Activated neutrophils are attracted to the infection site by perivascular macrophage IL-1β production (10). The infiltrating neutrophils then produce more proinflammatory cytokines and chemokines that recruit additional neutrophils and facilitate abscess formation. The resulting abscess acts to limit bacterial spread and enhance bacterial clearance. AT impairs the host's ability to initiate the proinflammatory cytokine and chemokine response necessary for infection resolution (8, 9). This reduced immune response was recently reported by Abtin et al. to result from AT-mediated lysis of perivascular macrophages at the infection site (10). Suppression of the immune response by AT in the linezolid and vancomycin monotherapy and isotype control treatment groups was apparent, resulting in larger lesion sizes, more severe tissue damage, reduced PMN infiltration, and a delayed healing process relative to that with the combination therapies. Treatment with MEDI4893* or a MEDI4893*-antibiotic combination resulted in increased IL-1β levels at the infection site along with more rapid PMN and macrophage infiltration and abscess formation. This robust immune response then led to a more rapid healing response. The addition of MEDI4893* to antibiotic therapy enables the host to mount a protective immune response that was not observed in mice treated with antibiotics alone.
In addition to protecting against bacterial infection, a robust immune response is necessary for effective healing following skin injury (43). Macrophages have been reported to orchestrate various aspects of both the inflammatory immune response (e.g., inflammatory cytokine expression and clearance of the excess neutrophils) and the wound healing process (e.g., reepithelialization, neovascularization, and granulation tissue formation) in the skin (27, 43, 44). Macrophage numbers increase during inflammation and peak during the granulation or proliferation phase of wound healing, and reduced macrophage numbers correlate with delayed wound healing in mice (29). Consistent with an improved healing response in the MEDI4893*-antibiotic- or MEDI4893*-treated animals, macrophage numbers were increased and there was evidence of epithelial hyperplasia, granulation tissue formation, and reepithelialization at 72 h postinfection which was absent in the animals treated with either vancomycin or linezolid alone (Fig. 5 and 6). An accelerated healing process was still apparent at 168 h postinfection in the MEDI4893*-antibiotic-treated mice as evidenced by increased granulation tissue thickness and complete epidermal and myocyte regeneration. Taken together with the results above, these observations indicate that adding MEDI4893* to an antibiotic treatment regimen against S. aureus provides a benefit at multiple stages of the infection and healing process. Initially, the anti-AT MAb acts to neutralize tissue damage elicited by the toxin and prevent AT-mediated immunosuppression, allowing the host to mount a robust protective immune response leading to more rapid bacterial clearance and improved wound healing (Fig. 6).
Our results demonstrate that therapeutic administration of MEDI4893* in combination with either vancomycin or linezolid results in a more robust immune response leading to reduced disease severity and accelerated healing relative to those with linezolid or vancomycin monotherapy. Consequently, addition of an AT-neutralizing MAb such as clinical candidate MEDI4893 (www.clinicaltrialsregister.eu) to antibiotic monotherapy may provide a benefit over antibiotics alone through its complementary mechanism of action.
ACKNOWLEDGMENTS
We thank José Martinez and Diana Pao for technical assistance.
REFERENCES
- 1.Mera RM, Suaya JA, Amrine-Madsen H, Hogea CS, Miller LA, Lu EP, Sahm DF, O'Hara P, Acosta CJ. 2011. Increasing role of Staphylococcus aureus and community-acquired methicillin-resistant Staphylococcus aureus infections in the United States: a 10-year trend of replacement and expansion. Microb Drug Resist 17:321–328. doi: 10.1089/mdr.2010.0193. [DOI] [PubMed] [Google Scholar]
- 2.Ray GT, Suaya JA, Baxter R. 2013. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a U.S. Population: a retrospective population-based study. BMC Infect Dis 13:252. doi: 10.1186/1471-2334-13-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itani KM, Biswas P, Reisman A, Bhattacharyya H, Baruch AM. 2012. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: a retrospective, propensity score-matched, case-control analysis. Clin Ther 34:1667–1673. doi: 10.1016/j.clinthera.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Labreche MJ, Lee GC, Attridge RT, Mortensen EM, Koeller J, Du LC, Nyren NR, Trevino LB, Trevino SB, Pena J, Mann MW, Munoz A, Marcos Y, Rocha G, Koretsky S, Esparza S, Finnie M, Dallas SD, Parchman ML, Frei CR. 2013. Treatment failure and costs in patients with methicillin-resistant Staphylococcus aureus (MRSA) skin and soft tissue infections: a South Texas Ambulatory Research Network (STARNet) study. J Am Board Fam Med 26:508–517. doi: 10.3122/jabfm.2013.05.120247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilliard JJ, Fernandez J, Melton J, Macielag MJ, Goldschmidt R, Bush K, Abbanat D. 2009. In vivo activity of the pyrrolopyrazolyl-substituted oxazolidinone RWJ-416457. Antimicrob Agents Chemother 53:2028–2033. doi: 10.1128/AAC.00833-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long KS, Vester B. 2012. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob Agents Chemother 56:603–612. doi: 10.1128/AAC.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campanile F, Mongelli G, Bongiorno D, Adembri C, Ballardini M, Falcone M, Menichetti F, Repetto A, Sabia C, Sartor A, Scarparo C, Tascini C, Venditti M, Zoppi F, Stefani S. 2013. Worrisome trend of new multiple mechanisms of linezolid resistance in staphylococcal clones diffused in Italy. J Clin Microbiol 51:1256–1259. doi: 10.1128/JCM.00098-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller LS, Cho JS. 2011. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol 11:505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tkaczyk C, Hamilton MM, Datta V, Yang XP, Hilliard JJ, Stephens GL, Sadowska A, Hua L, O'Day T, Suzich J, Stover CK, Sellman BR. 2013. Alpha toxin suppresses effective innate and adaptive immune responses in a murine dermonecrosis model. PLoS One 8:e75103. doi: 10.1371/journal.pone.0075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abtin A, Jain R, Mitchell AJ, Roediger B, Brzoska AJ, Tikoo S, Cheng Q, Ng LG, Cavanagh LL, von Andrian UH, Hickey MJ, Firth N, Weninger W. 2014. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol 15:45–53. doi: 10.1038/ni.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, Bubeck Wardenburg J, Hunstad DA. 2013. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 56:1554–1561. doi: 10.1093/cid/cit123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oganesyan V, Peng L, Damschroder MM, Cheng L, Sadowska A, Tkaczyk C, Sellman BR, Wu H, Dall'Acqua WF. 10 September 2014. Mechanisms of neutralization of a human anti-alpha toxin antibody. J Biol Chem doi: 10.1074/jbc.M114.601328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Datta V, Ren S, Feng H, Zinsou R, Keller A, O'Day T, Du Q, Cheng L, Damschroder M, Robbie G, Suzich J, Stover CK, Sellman BR. 2014. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 58:1108–1117. doi: 10.1128/AAC.02190-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dall'Acqua WF, Kiener PA, Wu H. 2006. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem 281:23514–23524. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 15.Robbie GJ, Criste R, Dall'acqua WF, Jensen K, Patel NK, Losonsky GA, Griffin MP. 2013. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob Agents Chemother 57:6147–6153. doi: 10.1128/AAC.01285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dall'Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, Wu H, Kiener PA, Langermann S. 2002. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J Immunol 169:5171–5180. doi: 10.4049/jimmunol.169.9.5171. [DOI] [PubMed] [Google Scholar]
- 17.CLSI. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M7-A8. Approved standard, 8th ed. CLSI, Wayne, PA. [Google Scholar]
- 18.Tkaczyk C, Hua L, Varkey R, Shi Y, Dettinger L, Woods R, Barnes A, MacGill RS, Wilson S, Chowdhury P, Stover CK, Sellman BR. 2012. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin Vaccine Immunol 19:377–385. doi: 10.1128/CVI.05589-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein GE, Throckmorton JK, Scharmen AE, Weiss WJ, Prokai L, Smith CL, Havlichek DH. 2013. Tissue penetration and antimicrobial activity of standard- and high-dose trimethoprim/sulfamethoxazole and linezolid in patients with diabetic foot infection. J Antimicrob Chemother 68:2852–2858. doi: 10.1093/jac/dkt267. [DOI] [PubMed] [Google Scholar]
- 20.Estes KS, Derendorf H. 2010. Comparison of the pharmacokinetic properties of vancomycin, linezolid, tigecyclin, and daptomycin. Eur J Med Res 15:533–543. doi: 10.1186/2047-783X-15-12-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sampedro GR, DeDent AC, Becker RE, Berube BJ, Gebhardt MJ, Cao H, Bubeck Wardenburg J. 16 April 2014. Targeting Staphylococcus aureus alpha-toxin as a novel approach to reduce severity of recurrent skin and soft-tissue infections. J Infect Dis doi: 10.1093/infdis/jiu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoshima N, Wang Y, Bubeck Wardenburg J. 2012. Genetic requirement for ADAM10 in severe Staphylococcus aureus skin infection. J Investig Dermatol 132:1513–1516. doi: 10.1038/jid.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montgomery CP, Daniels MD, Zhao F, Spellberg B, Chong AS, Daum RS. 2013. Local inflammation exacerbates the severity of Staphylococcus aureus skin infection. PLoS One 8:e69508. doi: 10.1371/journal.pone.0069508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker RE, Berube BJ, Sampedro GR, Dedent AC, Bubeck Wardenburg J. 2014. Tissue-specific patterning of host innate immune responses by Staphylococcus aureus alpha-toxin. J Innate Immun doi: 10.1159/000360006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MH, Granick JL, Kwok C, Walker NJ, Borjesson DL, Curry FR, Miller LS, Simon SI. 2011. Neutrophil survival and c-kit(+)-progenitor proliferation in Staphylococcus aureus-infected skin wounds promote resolution. Blood 117:3343–3352. doi: 10.1182/blood-2010-07-296970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. 2010. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest 120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. 2011. Macrophages in skin injury and repair. Immunobiology 216:753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Cho JS, Guo Y, Ramos RI, Hebroni F, Plaisier SB, Xuan C, Granick JL, Matsushima H, Takashima A, Iwakura Y, Cheung AL, Cheng G, Lee DJ, Simon SI, Miller LS. 2012. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog 8:e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. 2010. Differential roles of macrophages in diverse phases of skin repair. J Immunol 184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 30.Dryden MS. 2010. Complicated skin and soft tissue infection. J Antimicrob Chemother 65(Suppl 3):35–44. doi: 10.1093/jac/dkq302. [DOI] [PubMed] [Google Scholar]
- 31.Dryden MS. 2014. Developments in skin and soft tissue infection. Curr Opin Infect Dis 27:115. doi: 10.1097/QCO.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 32.Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. 2002. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 34:1481–1490. doi: 10.1086/340353. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi SD, Musser JM, DeLeo FR. 2012. Genomic analysis of the emergence of vancomycin-resistant Staphylococcus aureus. mBio 3(4):e00170-12. doi: 10.1128/mBio.00170-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locke JB, Rahawi S, Lamarre J, Mankin AS, Shaw KJ. 2012. Genetic environment and stability of cfr in methicillin-resistant Staphylococcus aureus CM05. Antimicrob Agents Chemother 56:332–340. doi: 10.1128/AAC.05420-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. 2010. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kernodle DS, Voladri RK, Menzies BE, Hager CC, Edwards KM. 1997. Expression of an antisense hla fragment in Staphylococcus aureus reduces alpha-toxin production in vitro and attenuates lethal activity in a murine model. Infect Immun 65:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bubeck Wardenburg J, Schneewind O. 2008. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berube BJ, Bubeck Wardenburg J. 2013. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tessier PR, Keel RA, Hagihara M, Crandon JL, Nicolau DP. 2012. Comparative in vivo efficacies of epithelial lining fluid exposures of tedizolid, linezolid, and vancomycin for methicillin-resistant Staphylococcus aureus in a mouse pneumonia model. Antimicrob Agents Chemother 56:2342–2346. doi: 10.1128/AAC.06427-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakoulas G, Eliopoulos GM, Fowler VG Jr, Moellering RC Jr, Novick RP, Lucindo N, Yeaman MR, Bayer AS. 2005. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob Agents Chemother 49:2687–2692. doi: 10.1128/AAC.49.7.2687-2692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diep BA, Afasizheva A, Le HN, Kajikawa O, Matute-Bello G, Tkaczyk C, Sellman B, Badiou C, Lina G, Chambers HF. 2013. Effects of linezolid on suppressing in vivo production of staphylococcal toxins and improving survival outcomes in a rabbit model of methicillin-resistant Staphylococcus aureus necrotizing pneumonia. J Infect Dis 208:75–82. doi: 10.1093/infdis/jit129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molne L, Verdrengh M, Tarkowski A. 2000. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect Immun 68:6162–6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koh TJ, DiPietro LA. 2011. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin P, Leibovich SJ. 2005. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Sharma-Kuinkel BK, Wu Y, Tabor DE, Mok H, Sellman BR, Jenkins A, Yu L, Jafri HS, Rude TH, Ruffin F, Schell WA, Park LP, Yan Q, Thaden JT, Messina JA, Fowler VG Jr, Esser MT. 12 November 2014. Characterization of alpha-toxin hla gene variants, alpha-toxin expression levels, and levels of antibody to alpha-toxin in hemodialysis and postsurgical patients with Staphylococcus aureus bacteremia. J Clin Microbiol doi: 10.1128/JCM.02023-14. [DOI] [PMC free article] [PubMed] [Google Scholar]