Abstract
Artemisinin-based combination therapy (ACT) is the recommended first-line treatment for Plasmodium falciparum malaria. It has been suggested that the cytotoxic effect of artemisinin is mediated by free radicals followed by the alkylation of P. falciparum proteins. The endoperoxide bridge, the active moiety of artemisinin derivatives, is cleaved in the presence of ferrous iron, generating reactive oxygen species (ROS) and other free radicals. However, the emergence of resistance to artemisinin in P. falciparum underscores the need for new insights into the molecular mechanisms of antimalarial activity of artemisinin. Here we show that artesunate (ART) induces DNA double-strand breaks in P. falciparum in a physiologically relevant dose- and time-dependent manner. DNA damage induced by ART was accompanied by an increase in the intracellular ROS level in the parasites. Mannitol, a ROS scavenger, reversed the cytotoxic effect of ART and reduced DNA damage, and modulation of glutathione (GSH) levels was found to impact ROS and DNA damage induced by ART. Accumulation of ROS, increased DNA damage, and the resulting antiparasite effect suggest a causal relationship between ROS, DNA damage, and parasite death. Finally, we also show that ART-induced ROS production involves a potential role for NADPH oxidase, an enzyme involved in the production of superoxide anions. Our results with P. falciparum provide novel insights into previously unknown molecular mechanisms underlying the antimalarial activity of artemisinin derivatives and may help in the design of next-generation antimalarial drugs against the most virulent Plasmodium species.
INTRODUCTION
Malaria accounts for hundreds of millions of clinical cases and nearly a million deaths annually (1). Malaria parasites are transmitted by female Anopheles mosquitoes, and infections caused by Plasmodium falciparum and P. vivax are responsible for >90% of infections worldwide. During their life cycle, parasites undergo a complex series of biological and biochemical developments that allow them to grow in their vertebrate hosts and to be successfully transmitted to the invertebrate mosquito vector. In the vertebrate host, actively proliferating intraerythrocytic asexual life cycle stages of the parasite are responsible for all clinical symptoms, including death, and these stages are also the primary targets of antimalarial drugs. Genetic diversity, antigenic variation, and the parasite's ability to adapt to new drugs continue to thwart control efforts. Artesunate (ART) is the semisynthetic derivative of artemisinin, developed from Artemisia annua L., which has been used in China as a traditional medicine for >2,000 years. Currently, artemisinin-based combination therapy (ACT) is recommended by the World Health Organization as the first line of treatment for P. falciparum malaria (2). These drugs act fast, with few side effects, and are also active against P. falciparum strains that are resistant to other traditionally used drugs such as antifolates and quinolones. Artemisinin derivatives have been found to act against various erythrocytic asexual stages as well as developing sexually differentiated immature stages, thus showing promise as antimalarial drugs with transmission-blocking potential (3–5). More recent studies have suggested that intraerythrocytic ring-stage parasites are targets of artemisinin derivatives, and ring stages of resistant parasites display longer survival than sensitive parasites (6, 7).
The decreasing clinical efficacy and emerging resistance to artemisinin derivatives in Thailand and Cambodia have raised serious concerns about future treatment options (8–10). Recent studies have identified putative genes potentially involved in ART resistance mechanisms (11–13) in P. falciparum. Those studies identified several point mutations in the kelch propeller domain (K-13 propeller) showing a strong correlation with slow parasite clearance (9, 11). However, understanding how parasites acquire tolerance to ART has been difficult due to insufficient knowledge of the molecular mechanisms underlying the antimalarial functions of artemisinin. The active moiety of artemisinin is a sesquiterpene lactone containing an endoperoxide bridge whose cleavage in the presence of ferrous iron in a Fenton-type reaction results in the generation of reactive oxygen species (ROS) such as hydroxyl radicals, superoxide anions, and carbon-centered free radicals (14–16). It has been suggested that free radicals are responsible for mediating the cytotoxic action of artemisinin derivatives in the parasites (17–19).
Previous studies have shown that artemisinin derivatives cause alkylation of heme and proteins in the parasite (15–17), bind and inhibit the P. falciparum translationally controlled tumor protein (TCTP) homolog (20), and inhibit sarcoplasmic reticulum Ca2+-transporting ATPase (SERCA) of malaria parasites (identified as PfATP6) (21). Recently, artemisinins have been shown to be distributed to the mitochondrial compartment in the parasite, resulting in impaired mitochondrial functions (22) and ROS-dependent depolarization of plasma and mitochondrial membranes (23). Even though many cellular targets have been identified, the mechanism of action of artemisinin derivatives still remains ambiguous. Free radicals and ROS have been shown to cause DNA damage in cells, providing the premise for our hypothesis that in addition to the above-mentioned effects, the antimalarial action of artemisinin derivatives involves direct DNA damage leading to parasite death. The potential involvement of DNA damage and repair processes in biological effects of artemisinin in P. falciparum is also supported by a recent study identifying several single nucleotide polymorphisms (SNPs) in genes involved in mismatch DNA repair pathways (11).
In this study, we sought to investigate ART-induced DNA damage in P. falciparum. Our studies indicate that ART-induced DNA damage results from oxidative stress via the generation of free radicals and ROS. We also demonstrate that reduced glutathione (GSH) plays an important role in detoxifying ROS and oxidative stress in the parasites. Glutathione is well established for its protective role against any form of oxidative damage. Erythrocytes contain high levels of reduced GSH and other antioxidant enzymes, such as catalase and superoxide dismutase. Thus, mechanisms interfering with the availability of reduced GSH are expected to synergize with drugs such as ART in inducing DNA double-strand breaks (DSBs) and possibly overall cytotoxicity. The fact that erythrocytes carry oxygen bound to hemoglobin makes blood-stage Plasmodium organisms especially vulnerable to oxidative stress under conditions of normal physiological development as well as when exposed to antimalarial drugs such as ART. Our studies suggest that the antiparasite effect of ART is therefore a result of DNA damage, and further studies are needed to characterize the contribution of recombinational DNA repair processes in resistance to artemisinin derivatives.
MATERIALS AND METHODS
Parasite culture.
P. falciparum clone 3D7 was maintained in RPMI 1640 medium supplemented with 25 mM HEPES, 0.37 mM hypoxanthine at 4% hematocrit, and 10% O+ normal human serum (24). Parasites were synchronized by using a sorbitol method, as described previously (25). Briefly, cultured parasites were pelleted and then treated with 5% sorbitol for 10 min at room temperature, followed by three washes with RPMI 1640 medium and further maintenance in culture. Synchronization was repeated two more times until >90% of parasites were in a specific stage. Synchronized intraerythrocytic “ring”-stage parasites were cultured and employed for all studies at between 18 and 20 h of synchronization. Parasitemia was determined after Giemsa staining of thin smears, and trophozoite-stage parasites were used at 4% hematocrit and 1% starting parasitemia. The IC50 (50% inhibitory concentration) of ART used was determined by the SyBR green method (26).
Comet assay of DNA damage and parasite recovery.
An optimized comet assay for P. falciparum (27) was used to assess the extent of DNA damage and recovery from DNA damage. Briefly, parasites at different time points were collected by centrifugation, lysed with 0.01% saponin, washed 3 to 4 times in ice-cold phosphate-buffered saline (PBS), and finally resuspended in PBS for single-cell electrophoresis (comet assay). Diluted cells (30 μl) were mixed with 300 μl 1% low-melting-point agarose (Comet LMAgarose; Trevigen), and the mixture was added to the wells of Trevigen slides. Slides were incubated with detergent lysis solution (Trevigen, Gaithersburg, MD, USA), followed by alkaline lysis treatment and electrophoresis for 30 min at 1 V/cm in 1× Tris-borate-EDTA (TBE). Slides were fixed in 70% ethanol (1 min), rinsed in distilled water, and dried overnight. Slide wells were stained with 1× SyBR green I, photographed at a ×200 magnification by using a Nikon Eclipse 80i instrument (Nikon) with a Sensicam QE High Performance camera (Cooke Corporation), and scored by using Comet Assay IV software. The olive tail moment (OTM) (product of the tail length and the fraction of total DNA in the tail) was calculated to compare the extent of DNA damage in each sample. To study the efficiency of parasite recovery, P. falciparum parasites were treated with ART for different times (30, 45, 60, 90, and 120 min), washed, and recultured in the presence of fresh growth medium and uninfected erythrocytes (“return-to-growth” assay). Cultures were harvested at various time points (24 to 72 h) for preparation of blood smears to check parasite growth and DNA damage by comet assays.
Measurement of intracellular ROS.
ROS levels were measured by using the dichlorodihydrofluorescein diacetate (DCF-DA) method (28), with slight modifications. Synchronized parasites corresponding to 4% hematocrit and 1% infected red blood cells were collected, and DCF-DA was added to a trophozoite-stage P. falciparum culture at a final concentration of 20 μM for 30 min before the addition of various concentrations of ART. Control samples were incubated with PBS instead of ART. The fluorescence (excitation wavelength at 485 nm and emission wavelength at 530 nm) was measured by using an SLM Aminco 8100 fluorescence spectrophotometer.
Glutathione assay.
Total GSH and oxidized forms of glutathione (GSSG) were assayed by using a 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) method (29), with minor modifications of the manufacturer's protocol (Trevigen, MD). Sorbitol-synchronized P. falciparum trophozoite-stage parasites at 1% parasitemia and 4% hematocrit were treated with ART for various times, and cells were lysed in 4 volumes of ice-cold 5% metaphosphoric acid. Cell lysates were centrifuged at 12,000 × g to 14,000 × g for 10 min at 4°C, and supernatants were used for GSH assays. For measurement of GSSG concentrations, 50 μl of the diluted sample (20-fold dilution) in triplicates was treated with 1 μl of 2 M 4-vinyl-pyridine and incubated at room temperature for 60 min. For measurement of total GSH concentrations, 50 μl of the diluted sample (20-fold dilution) was added to 96-well plates in triplicates. One hundred fifty microliters of freshly prepared glutathione reductase in a reaction mixture was added to each sample (GSH and GSSG) and the control, and the absorbance was read immediately at 405 nm by using an enzyme-linked immunosorbent assay (ELISA) plate reader (VersaMax; Molecular Devices) at 1-min intervals over a 10-min time period. Absorbance values at various time points from a set of standards were used to plot standard curves, and the calculated net slope was used to determine the pmol values for total and oxidized glutathione. The reduced GSH concentration was calculated by subtracting the oxidized GSSG concentration from the total glutathione concentration.
Statistical analysis.
All experiments were performed in triplicates, and most experiments were repeated a minimum of three times. Graphpad Prism software (Graphpad Software Inc.) was used for statistical analyses of the data. The statistical significance of various treatments was compared to that of untreated samples by using the Mann-Whitney nonparametric test. A P value of <0.05 was considered significant.
RESULTS
Artesunate causes DNA damage in P. falciparum.
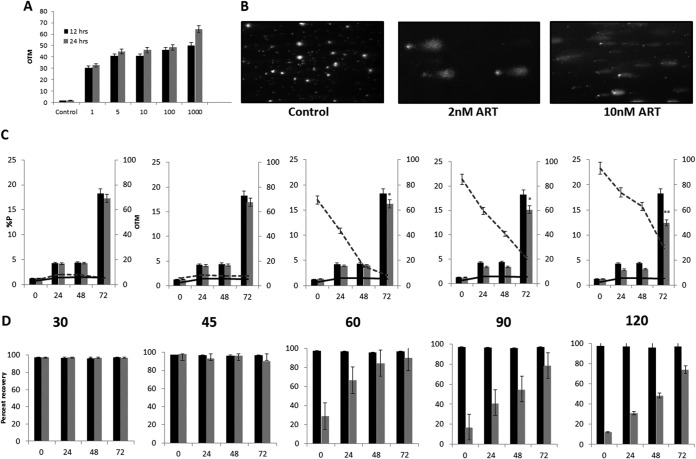
To evaluate if ART causes DNA damage, sorbitol-synchronized trophozoite-stage P. falciparum parasites were treated with increasing concentrations of ART for various times, and parasites were analyzed by a comet assay (27). The results (Fig. 1A and B) demonstrate that ART induced DNA damage in P. falciparum in a dose- and time-dependent manner. Incubation of P. falciparum with as little as 1 IC50 of ART (2 nM) revealed evidence of DNA damage during 12 to 24 h of exposure to the drug. Treatment of parasites with increasing IC50s (1 to 1,000) of ART was accompanied by higher accumulation of DNA damage, shown by an increase in the olive tail moment (OTM). To determine if the damage to DNA was a primary effect of ART or a secondary consequence of parasite death, we performed a “return-to-growth” experiment. Synchronized trophozoite-stage P. falciparum parasites were treated with 2 nM ART (1 IC50 dose) for different times(30, 45, 60, 90, and 120 min), washed three times with RPMI 1640 medium to remove ART, and returned to culture for an additional 72 h to monitor parasite growth, measured as percent parasitemia, and DNA damage by comet assays. Treatment of parasites with 2 nM ART for 30 and 45 min did not reveal any detectable DNA damage and parasite death. On the other hand, ART treatment for 60 min or longer resulted in not only increased DNA damage (increasing OTM values [Fig. 1C] and reduced recovery of damaged nuclei after repair during the parasite regrowth phase [Fig. 1D]) but also diminished parasite survival (Fig. 1C). Exposure of parasites to 2 nM ART for 60, 90, and 120 min resulted in 10%, 21.4%, and 26% reduced recovery of parasites after a 72-h reculture period, respectively (Fig. 1D). These results suggest that it takes a minimum of 60 min of continuous exposure of intraerythrocytic trophozoite-stage parasites for ART-mediated DNA damage to accumulate, resulting in inefficient repair of DNA damage and increasing antiparasite toxicity. It should be noted that the concentration of ART used was just 2 nM, equivalent to 1 IC50 dose, and higher ART concentrations resulted in greater DNA damage (OTM values).
FIG 1.
Artesunate (ART) causes DNA damage in P. falciparum. (A) Comet assay measurement of the olive tail moment (OTM) at 12 h and 24 h after ART treatment (1, 5, 100, and 1,000 IC50s) compared to untreated (Control) parasites. The error bars represent standard errors of the means. (B) Comet assay visualization of DNA damage in P. falciparum parasites upon treatment with 2 nM (1 IC50) and 10 nM (5 IC50s) ART compared to untreated parasites. (C) Parasite growth and OTM values determined by a comet assay during the recovery period compared to those of untreated control parasites maintained in parallel. Parasites were treated with 2 nM ART for 30, 45, 60, 90, and 120 min (left to right), after which they were washed to remove ART, returned to culture, and sampled at 24, 48, and 72 h for determination of percent parasitemia (%P) (black bars, untreated cultures; gray bars, ART-treated cultures) and DNA damage, measured as the OTM, in comet assays (solid line, untreated cultures; dashed line, ART-treated cultures) (*, P < 0.015; **, P < 0.005). (D) Percent recovery of damaged parasite nuclei was analyzed by a comet assay at 24, 48, and 72 h during the reculture phase of parasites after ART treatment. Images from comet assays (minimum n = 10) were randomly scored as damaged (detectable comet) and undamaged (no detectable comet) nuclei for each sample. Shown are results indicating DNA repair as percent recovery of nuclei in control (black bars) and ART-treated (gray bars) cultures. The error bars represent standard errors of the means.
ART affects intracellular ROS levels, leading to DNA damage and parasite death.
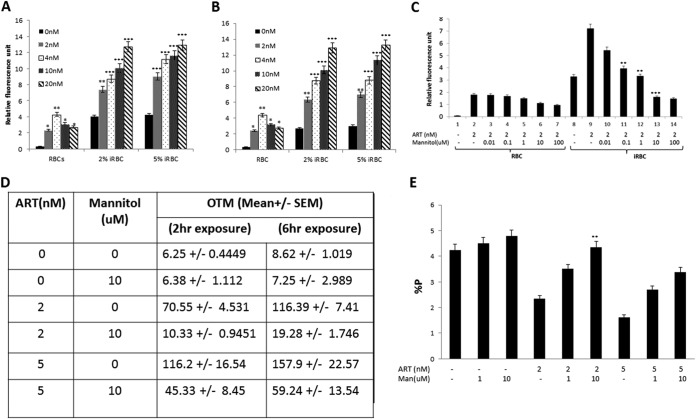
Previous studies suggested increased free radical formation by artemisinin in Plasmodium spp. (30). We set out to determine if DNA damage by ART depends upon prooxidant activity and is mediated by free radicals. We measured the amount of intracellular ROS by the DCF-DA fluorescence method (28). Figure 2A and B show ART dose-dependent increases in ROS levels in the intraerythrocytic trophozoite stages of P. falciparum. Furthermore, we tested whether or not the ART-induced increase in intracellular ROS levels could be attenuated with mannitol, a ROS scavenger. As shown in Fig. 2C, levels of ART (2 nM)-induced ROS in P. falciparum-infected red blood cells were effectively reduced by coincubation with increasing concentrations of mannitol, with nearly complete inhibition at a mannitol concentration of 10 μM. Next, we demonstrated that scavenging of ROS by mannitol significantly prevented DNA damage in parasites resulting from exposure to ART (Fig. 2D). In the presence of mannitol (10 μM), OTM values were 85% and 60% lower in parasites treated with 2 nM (1 IC50 dose) and 5 nM (2.5 IC50 doses) ART, respectively. These results provide direct evidence for a link between ART-mediated DNA damage and increases in intracellular ROS levels. Finally, we established that a decline in the level of ART-induced ROS with mannitol also improves parasite growth. We monitored parasitemia in ART-treated cultures with and without mannitol addition. The antiparasite effect of ART was effectively abrogated by mannitol, with nearly 85% and 52% inhibition of parasite death caused by 2 nM and 5 nM ART, respectively (Fig. 2E). These results further suggest a mechanistic link between ART-induced intracellular ROS production and DNA damage and the accompanying antiparasite effect of ART.
FIG 2.
ART affects intracellular reactive oxygen species (ROS) levels, leading to DNA damage and parasite death. (A and B) Concentration-dependent oxidant activity of ART in elevating ROS levels in P. falciparum. Uninfected red blood cells (RBCs) and infected red blood cells (iRBC) (2% and 5%) were treated with the indicated concentrations of ART (2, 4, 10, and 20 nM) for 2 h (A) and 6 h (B), and ROS levels were measured by using DCF-DA, a fluorescent dye (*, P < 0.015; **, P < 0.005; ***, P < 0.0001). (C) Attenuation of ROS by mannitol. ROS induced by ART were effectively attenuated by coincubation with increasing concentrations of mannitol. Uninfected or infected red blood cells were coincubated with ART and mannitol for 2 h, followed by measurement of ROS levels (*, P < 0.05). (D) Attenuation of ART-induced ROS in the presence of mannitol was accompanied by significantly reduced DNA damage in P. falciparum (**, P <0.005; ***, <0.0001). OTM values were determined by a comet assay in the parasites coincubated with ART (0, 2, and 5 nM) and mannitol (10 μM) for 2 and 6 h. (E) Attenuation of ART-induced ROS by mannitol results in protection from the antimalarial effect of ART. Shown are percentages of infected red blood cells 48 h after treatment with ART (0, 2, and 5 nM) with or without mannitol (0, 1, and 10 μM). The error bars represent standard errors of the means (**, P < 0.005 for 10 μM mannitol).
Time kinetics of the antimalarial effect of ART and role of ROS.
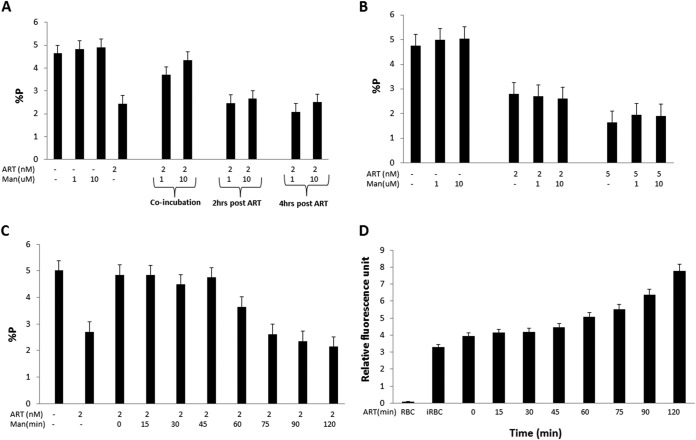
Next, we investigated the kinetic interplay of ROS and parasite killing by ART. The results shown in Fig. 3A demonstrate that mannitol was effective in preventing parasite killing by 2 nM ART when added simultaneously and that a delayed addition of mannitol (2 h and 4 h after ART) did not provide the same benefit. These results established the importance of early effects of ROS in ART-mediated damage in the parasites. We repeated these studies, this time washing (three times with RPMI 1640 medium) the parasites after 2 h of ART treatment to remove extracellular drug prior to the addition of mannitol during 48 h of P. falciparum culture. The results shown in Fig. 3B confirmed our previous conclusion that after 2 h of ART treatment, parasites do not benefit from the presence of mannitol. Since 2 h was already too late, it was of interest to characterize the early kinetics of the mannitol-mediated reversal of the cytotoxic effect of ART. In view of the above-described evidence that significant DNA damage occurs only at 60 min post-ART treatment (Fig. 1C), we reasoned that the presence of mannitol only during the first hour would negate ROS-mediated DNA damage and cytotoxic effects on the parasite. Supporting evidence for this notion is shown in Fig. 3C. Mannitol effectively negated ART-mediated parasite killing when added at up to 45 min post-ART treatment, and any further delay was accompanied by a gradually diminishing protective effect of mannitol. It is also critical to note that treatment of P. falciparum with 2 nM ART led to slow and steady ROS production in the parasite, with a slow phase during the initial 45 min followed by a faster kinetics during further time periods (Fig. 3D), and the production of ROS during the first hour seems to be critical for causing DNA damage and the parasite-killing effect of ART.
FIG 3.
Time kinetics of the antimalarial effect of ART and role of ROS. (A) Mannitol, a scavenger of ROS, was used to probe the early kinetics of the antimalarial effect of ART. Parasites were treated with ART (2 nM) and mannitol (1 and 10 μM), added at either time zero or 2 and 4 h after treatment with ART. Percent parasitemia was determined at the 48-h time point. The error bars represent standard errors of the means. (B) Mechanisms leading to the ART-mediated antiparasite effect manifest during the first 2 h. Parasites were treated with increasing concentrations of ART (0, 2, and 5 nM) for 2 h, washed to remove extracellular ART, and then returned to culture with increasing concentrations of mannitol (0, 1, and 10 μM) for 48 h, and parasitemia was measured. The error bars represent standard errors of the means. (C) A minimum of 45 min is needed before mannitol gradually becomes ineffective in scavenging ROS during the ART-mediated antiparasite effect. Parasites were treated with 2 nM ART for 15 to 120 min, washed to remove extracellular ART, and then returned to culture with 10 μM mannitol for 48 h to assess parasitemia. The error bars represent standard errors of the means. (D) ART-induced ROS levels steadily increase with time. Parasites were treated with 2 nM ART, and the relative fluorescence intensity was measured every 15 min. The error bars represent standard errors of the means.
ART affects total GSH in parasites.
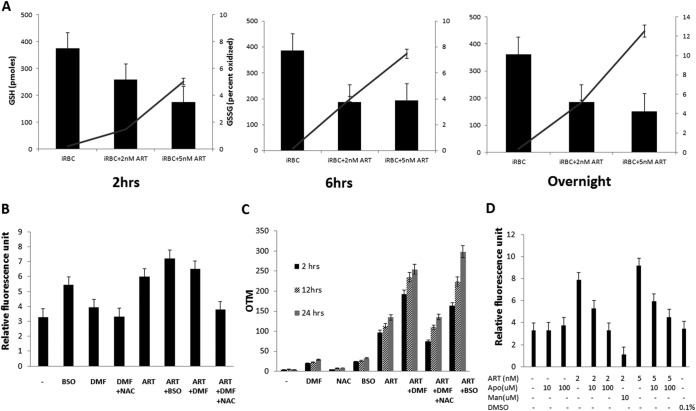
The glutathione (GSH) pathway in Plasmodium spp. has been shown to play a critical role during oxidative stress (31) and in the detoxification of ROS (32–34). Given the evidence for the central role for ROS in the parasite, we reasoned that ART treatment would affect GSH levels in the parasite. Indeed, when infected red blood cells were treated with ART (2 nM and 5 nM) for various time periods (2 h, 6 h, and overnight), there was an overall reduction in the level of total GSH and a parallel increase in the level of the oxidized form of GSH, GSSG (Fig. 4A), indicating mounting oxidative stress on the parasite exerted by increasing concentrations of and time of treatment with ART. Next, we investigated whether perturbations in GSH biosynthesis further accentuate ART-mediated effects on ROS production and DNA damage in the parasites. We employed buthionine sulfoximine (BSO), an inhibitor of γ-glutamyl cysteine synthetase (GCS) involved in GSH biosynthesis (35); dimethyl fumarate (DMF), which efficiently enters cells and forms covalent conjugates with GSH followed by depletion in a dose-dependent manner; and N-acetyl-l-cysteine (NAC), a reducing agent which acts as a free radical scavenger and efficiently negates DMF-induced GSH changes (32). The concentrations of these inhibitors were first optimized for the least impact on parasite viability. BSO and DMF resulted in higher levels of ROS in the parasites, with further additive effects on the parasites in the presence of ART and GSH modulators (Fig. 4B). As expected, NAC negated oxidative damage by minimizing ROS in the parasites. In parallel, we also measured DNA damage in the presence of these GSH inhibitors by a comet assay. BSO and DMF, which increased ROS levels, also resulted in greater DNA damage in the presence of ART, and NAC countered the damaging effect of GSH-modulating agents (Fig. 4C). Finally, to explore a potential role for NADPH oxidase in the production of ROS in the presence of ART, we measured ROS levels in the presence of apocynin (acetovanillone), which is widely used as a specific inhibitor of NADPH oxidase (36). As shown in Fig. 4D, 10 and 100 μM apocynin blocked ART-induced ROS production, with nearly complete blocking in the presence of 100 μM apocynin, thus providing a preliminary suggestion for a potential role for NADPH oxidase in the antimalarial effects of ART.
FIG 4.
ART affects total glutathione (GSH) in parasites. (A) ART treatment decreases total GSH levels and increases levels of the oxidized form of GSH (GSSG). Parasites were treated with increasing concentrations of ART (0, 2, and 5 nM) for the indicated times. Parasite lysates were prepared, and GSH levels were determined by using a DTNB assay. Total GSH concentrations (filled bars) and percentages of the oxidized form (solid lines) were determined. (B) GSH depletion causes an increase in intracellular ROS production in the presence of ART. Parasites were treated with GSH-depleting inhibitors (125 μM BSO, 10 μM DMF, and 1 mM NAC) in the presence or absence of ART for 2 h, and fluorescence intensity was measured. The error bars represent standard errors of the means. (C) GSH depletion causes an increase in DNA damage. Parasites were treated with various GSH-modulating agents in the presence or absence of ART for 2, 12, and 24 h, and OTM values were determined by a comet assay and compared to values for untreated parasites. The error bars represent standard errors of the means. (D) NADPH oxidase-catalyzed ROS are potentially involved in ART-mediated ROS production. Parasites were treated with ART (0, 2, and 5 nM) in the absence or presence of apocynin (0, 10, and 100 μM) for 2 h, and ROS levels were measured. The error bars represent standard errors of the means. DMSO, dimethyl sulfoxide.
DISCUSSION
In this study, we provide the first-ever evidence for an early effect of ART in causing DNA damage in P. falciparum, which manifests in parasite death. The significance of these findings also lies in the fact that this DNA damage response was observed at a physiologically relevant concentration of ART. We first determined the IC50 of ART for P. falciparum parasites (3D7) used in our studies, and we then employed different doses of ART representing multiples of the IC50 dose. As shown by the results presented here, even a dose as low as 2 nM, which represents 1 IC50 dose in vitro, resulted in DNA damage within the first 60 min of the addition of drug to intraerythrocytic asexual-stage parasites. To assess DNA damage, we used a comet assay based on single-cell electrophoresis. This assay quantifies the extent of DNA damage expressed as OTM values, with higher values implying greater DNA damage. We also noticed that depending upon the concentration of ART used, the relative proportions of nuclei with damaged DNA also varied, thus allowing a measure of the prevalence of damaged nuclei (nuclei with damaged DNA expressed as a percentage of undamaged nuclei). It is noteworthy that the concentrations of ART causing DNA damage and oxidative changes (2 and 5 nM used in various experiments) (Fig. 2 to 4) were 2,000 to 4,000 times lower than the peak median plasma concentration of 8,500 nM with an AUC (area under the curve) value of 727 ng · h/ml revealed by pharmacokinetic and pharmacodynamic evaluations of intravenously administered therapeutic doses of ART. In vivo, ART is metabolized into an active derivative, dihydroartemisinin, and the peak median plasma concentration detected was 11,000 nM with an AUC value of 3,492 ng · h/ml. In vivo exposure of parasites to such high concentrations of ART and its metabolites is expected to result in a rapid and extensive DNA damage response leading to antiparasite activity of ART (37, 38).
Our return-to-growth experiments strongly support that damage to DNA by ART was not a secondary effect but a primary event that ultimately was reflected in parasite death. Earlier exposure times (up to 60 min) revealed parasite recovery in culture (90% recovery after a 72-h regrowth period) and also a shortening of the comet tail, indicating repair of DNA damage. DNA damage and parasite growth of ART-treated parasites returned to normal values within 72 h of the recovery phase, further suggesting the significance of functional DNA repair in response to DNA damage. All of the data described above suggest that ART causes early and significant damage to DNA in parasites, leading to parasite death.
Intraparasitic heme iron, which is acquired as a result of hemoglobin digestion by the parasite, reacts with the endoperoxide bridge (active moiety) of artemisinin and mediates the production of free radicals (14). Bioactivated endoperoxides have been shown to generate downstream radical oxygen species, disrupting a number of parasite membranes and enzymes (23). In cancer cells, cytotoxic activity has been shown to arise from ROS-mediated oxidative DNA damage induced by ART (39). Another study showed that genes involved in oxidative stress may also be associated with ART resistance in cancer cells (40). Additionally, a recent study on the prevalence of multiple P. falciparum populations identified a number of SNPs in genes involved in the mismatch DNA repair process (41). The results presented in this study on DNA damage in P. falciparum caused by ART in vitro and the importance of specific DNA damage repair pathways in P. falciparum showing resistance to artemisinin derivatives lead us to hypothesize that DNA damage and repair processes play important roles in P. falciparum's susceptibility and resistance to artemisinin. More recent studies have reported the regulatory cytoprotective role of point mutations in cellular targets such as the kelch propeller domain of kelch-13 in the resistance of P. falciparum to artemisinin (11). Clearly, additional studies are warranted to explore a link between such K-13 propeller polymorphisms and the ROS-mediated DNA damage response (this study) in the intraerythrocytic stages of P. falciparum in response to ART.
We also argued that ROS-mediated DNA damage arising from ART should be antagonized in the presence of ROS scavengers. This was indeed the case when mannitol, a ROS scavenger, was used, and as shown by our results, there was a significant reduction in OTM values, indicating that ART-mediated ROS production leads to oxidative DNA damage, which in turn contributes to the parasite-killing activity of ART. We are aware that the DCF-DA method used for ROS measurement has certain limitations (42), and it measures a broad range of chemically different reactive species. Nevertheless, peroxide-dependent DCF fluorescence has been shown to be dependent on glutathione levels (43), and our studies also suggest a link between the inhibition of GSH synthesis and ROS levels.
The glutathione pathway is crucial during oxidative stress in malaria parasites (31) for detoxifying ROS. Previous studies have also shown that enzymes involved in the GSH redox system are potential antimalarial targets (32–34). Hence, we decided to test if hindering either GSH levels or GSH synthesis in the parasites might impact ART-induced DNA damage. As expected, increased ART concentrations and ART exposure times resulted in increased levels of GSSG. Also, in the presence of BSO, which inhibits the enzyme GCS involved in GSH biosynthesis (44, 45), we saw increases in ROS levels and OTM values, further suggesting the exquisite sensitivity of parasites to the oxidative effect of ART. A similar result for ART-induced oxidative damage was also seen in the presence of DMF, which has been shown to deplete the reduced form of GSH (46), an effect counteracted by NAC. Finally, our studies also provide preliminary evidence that NADPH oxidase may be involved in an oxygen radical-dependent killing mechanism during P. falciparum infection (47). Previous studies have shown that apocynin targets NADPH oxidase and that the antioxidant property of apocynin results from an increase in GSH biosynthesis (48). Even though apocynin has been shown to specifically target NADPH oxidase, the higher concentrations required may also indicate other unknown, “off-target” effects. Further studies using knockouts of genes encoding this enzyme might shed light on ART-induced ROS production and the parasite's ability to cope with the oxidative damage response.
In summary, the results presented in this study establish a role for oxidative stress imposed by ART and a mechanistic link with ROS production, DNA damage, and ART-induced parasite killing, thus providing novel insights into the molecular mechanisms underlying the antimalarial functions of ART. Such insights, along with ongoing studies to identify putative molecular markers associated with resistance to ART (11), will improve our understanding of how parasites are becoming more tolerant to this drug and guide future efforts to develop novel therapeutic strategies for malaria caused by P. falciparum. Finally, our finding that ART-induced cytotoxic effects in P. falciparum are due to DNA damage mediated by increased ROS production in the presence of ART and the role of DNA damage repair processes (such as homologous recombination) opens up new avenues for further investigations focusing on future drug design for malaria treatment.
ACKNOWLEDGMENTS
These studies were partially supported by NIH grant R56 AI68052 and institutional funds.
We thank William Wimley for allowing us to use the spectrofluorometer; Melody C. Baddoo for access to the Comet Assay IV software in the core laboratory, supported by NIH grants from the National Center for Research Resources (5P20RR020152-09) and the National Institute of General Medical Sciences (8 P20 GM103518-09); and Stephanie Chen for initial help with GSH measurement assays. We thank Geetha Bansal for comments on the manuscript and Joni Emmons for editorial assistance.
REFERENCES
- 1.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. 2012. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.WHO. 2012. World malaria report 2012. WHO, Geneva, Switzerland. [Google Scholar]
- 3.Haynes RK, Krishna S. 2004. Artemisinins: activities and actions. Microbes Infect 6:1339–1346. doi: 10.1016/j.micinf.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Price RN, Nosten F, Luxemburger C, ter Kuile FO, Paiphun L, Chongsuphajaisiddhi T, White NJ. 1996. Effects of artemisinin derivatives on malaria transmissibility. Lancet 347:1654–1658. doi: 10.1016/S0140-6736(96)91488-9. [DOI] [PubMed] [Google Scholar]
- 5.Kumar N, Zheng H. 1990. Stage-specific gametocytocidal effect in vitro of the antimalaria drug qinghaosu on Plasmodium falciparum. Parasitol Res 76:214–218. doi: 10.1007/BF00930817. [DOI] [PubMed] [Google Scholar]
- 6.Amaratunga C, Neal AT, Fairhurst RM. 2014. Flow cytometry-based analysis of artemisinin-resistant Plasmodium falciparum in the ring-stage survival assay. Antimicrob Agents Chemother 58:4938–4940. doi: 10.1128/AAC.02902-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, Lim P, Mao S, Sopha C, Sam B, Anderson JM, Duong S, Chuor CM, Taylor WR, Suon S, Mercereau-Puijalon O, Fairhurst RM, Menard D. 2013. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis 13:1043–1049. doi: 10.1016/S1473-3099(13)70252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. 2012. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amaratunga C, Sreng S, Suon S, Phelps ES, Stepniewska K, Lim P, Zhou C, Mao S, Anderson JM, Lindegardh N, Jiang H, Song J, Su XZ, White NJ, Dondorp AM, Anderson TJ, Fay MP, Mu J, Duong S, Fairhurst RM. 2012. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis 12:851–858. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takala-Harrison S, Clark TG, Jacob CG, Cummings MP, Miotto O, Dondorp AM, Fukuda MM, Nosten F, Noedl H, Imwong M, Bethell D, Se Y, Lon C, Tyner SD, Saunders DL, Socheat D, Ariey F, Phyo AP, Starzengruber P, Fuehrer HP, Swoboda P, Stepniewska K, Flegg J, Arze C, Cerqueira GC, Silva JC, Ricklefs SM, Porcella SF, Stephens RM, Adams M, Kenefic LJ, Campino S, Auburn S, MacInnis B, Kwiatkowski DP, Su XZ, White NJ, Ringwald P, Plowe CV. 2013. Genetic loci associated with delayed clearance of Plasmodium falciparum following artemisinin treatment in Southeast Asia. Proc Natl Acad Sci U S A 110:240–245. doi: 10.1073/pnas.1211205110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, et al. . 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meshnick SR, Yang YZ, Lima V, Kuypers F, Kamchonwongpaisan S, Yuthavong Y. 1993. Iron-dependent free radical generation from the antimalarial agent artemisinin (qinghaosu). Antimicrob Agents Chemother 37:1108–1114. doi: 10.1128/AAC.37.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asawamahasakda W, Ittarat I, Pu YM, Ziffer H, Meshnick SR. 1994. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob Agents Chemother 38:1854–1858. doi: 10.1128/AAC.38.8.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berman PA, Adams PA. 1997. Artemisinin enhances heme-catalysed oxidation of lipid membranes. Free Radic Biol Med 22:1283–1288. doi: 10.1016/S0891-5849(96)00508-4. [DOI] [PubMed] [Google Scholar]
- 17.Yang YZ, Asawamahasakda W, Meshnick SR. 1993. Alkylation of human albumin by the antimalarial artemisinin. Biochem Pharmacol 46:336–339. doi: 10.1016/0006-2952(93)90425-V. [DOI] [PubMed] [Google Scholar]
- 18.Meshnick SR, Taylor TE, Kamchonwongpaisan S. 1996. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol Rev 60:301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Efferth T, Kaina B. 2010. Toxicity of the antimalarial artemisinin and its derivatives. Crit Rev Toxicol 40:405–421. doi: 10.3109/10408441003610571. [DOI] [PubMed] [Google Scholar]
- 20.Bhisutthibhan J, Pan XQ, Hossler PA, Walker DJ, Yowell CA, Carlton J, Dame JB, Meshnick SR. 1998. The Plasmodium falciparum translationally controlled tumor protein homolog and its reaction with the antimalarial drug artemisinin. J Biol Chem 273:16192–16198. doi: 10.1074/jbc.273.26.16192. [DOI] [PubMed] [Google Scholar]
- 21.Eckstein-Ludwig U, Webb RJ, Van Goethem ID, East JM, Lee AG, Kimura M, O'Neill PM, Bray PG, Ward SA, Krishna S. 2003. Artemisinins target the SERCA of Plasmodium falciparum. Nature 424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Huang L, Li J, Fan Q, Long Y, Li Y, Zhou B. 2010. Artemisinin directly targets malarial mitochondria through its specific mitochondrial activation. PLoS One 5:e9582. doi: 10.1371/journal.pone.0009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antoine T, Fisher N, Amewu R, O'Neill PM, Ward SA, Biagini GA. 2014. Rapid kill of malaria parasites by artemisinin and semi-synthetic endoperoxides involves ROS-dependent depolarization of the membrane potential. J Antimicrob Chemother 69:1005–1016. doi: 10.1093/jac/dkt486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 25.Walliker D, Beale G. 1993. Synchronization and cloning of malaria parasites. Methods Mol Biol 21:57–66. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. 2007. Assessment and continued validation of the malaria SYBR green I-based fluorescence assay for use in malaria drug screening. Antimicrob Agents Chemother 51:1926–1933. doi: 10.1128/AAC.01607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gopalakrishnan AM, Kumar N. 2013. Opposing roles for two molecular forms of replication protein A in Rad51-Rad54-mediated DNA recombination in Plasmodium falciparum. mBio 4(3):e00252-13. doi: 10.1128/mBio.00252-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Njomnang Soh P, Witkowski B, Gales A, Huyghe E, Berry A, Pipy B, Benoit-Vical F. 2012. Implication of glutathione in the in vitro antiplasmodial mechanism of action of ellagic acid. PLoS One 7:e45906. doi: 10.1371/journal.pone.0045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker K, Gui M, Traxler A, Kirsten C, Schirmer RH. 1994. Redox processes in malaria and other parasitic diseases. Determination of intracellular glutathione. Histochemistry 102:389–395. [DOI] [PubMed] [Google Scholar]
- 30.Cumming JN, Ploypradith P, Posner GH. 1997. Antimalarial activity of artemisinin (qinghaosu) and related trioxanes: mechanism(s) of action. Adv Pharmacol 37:253–297. [DOI] [PubMed] [Google Scholar]
- 31.Vega-Rodriguez J, Franke-Fayard B, Dinglasan RR, Janse CJ, Pastrana-Mena R, Waters AP, Coppens I, Rodriguez-Orengo JF, Srinivasan P, Jacobs-Lorena M, Serrano AE. 2009. The glutathione biosynthetic pathway of Plasmodium is essential for mosquito transmission. PLoS Pathog 5:e1000302. doi: 10.1371/journal.ppat.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghashghaeinia M, Bobbala D, Wieder T, Koka S, Bruck J, Fehrenbacher B, Rocken M, Schaller M, Lang F, Ghoreschi K. 2010. Targeting glutathione by dimethylfumarate protects against experimental malaria by enhancing erythrocyte cell membrane scrambling. Am J Physiol Cell Physiol 299:C791–C804. doi: 10.1152/ajpcell.00014.2010. [DOI] [PubMed] [Google Scholar]
- 33.Pastrana-Mena R, Dinglasan RR, Franke-Fayard B, Vega-Rodriguez J, Fuentes-Caraballo M, Baerga-Ortiz A, Coppens I, Jacobs-Lorena M, Janse CJ, Serrano AE. 2010. Glutathione reductase-null malaria parasites have normal blood stage growth but arrest during development in the mosquito. J Biol Chem 285:27045–27056. doi: 10.1074/jbc.M110.122275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginsburg H, Golenser J. 2003. Glutathione is involved in the antimalarial action of chloroquine and its modulation affects drug sensitivity of human and murine species of Plasmodium. Redox Rep 8:276–279. doi: 10.1179/135100003225002907. [DOI] [PubMed] [Google Scholar]
- 35.Dubois VL, Platel DF, Pauly G, Tribouley-Duret J. 1995. Plasmodium berghei: implication of intracellular glutathione and its related enzyme in chloroquine resistance in vivo. Exp Parasitol 81:117–124. doi: 10.1006/expr.1995.1099. [DOI] [PubMed] [Google Scholar]
- 36.'t Hart BA, Simons JM, Knaan-Shanzer S, Bakker NP, Labadie RP. 1990. Antiarthritic activity of the newly developed neutrophil oxidative burst antagonist apocynin. Free Radic Biol Med 9:127–131. [DOI] [PubMed] [Google Scholar]
- 37.Witkowski B, Khim N, Chim P, Kim S, Ke S, Kloeung N, Chy S, Duong S, Leang R, Ringwald P, Dondorp AM, Tripura R, Benoit-Vical F, Berry A, Gorgette O, Ariey F, Barale JC, Mercereau-Puijalon O, Menard D. 2013. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrob Agents Chemother 57:914–923. doi: 10.1128/AAC.01868-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byakika-Kibwika P, Lamorde M, Mayito J, Nabukeera L, Mayanja-Kizza H, Katabira E, Hanpithakpong W, Obua C, Pakker N, Lindegardh N, Tarning J, de Vries PJ, Merry C. 2012. Pharmacokinetics and pharmacodynamics of intravenous artesunate during severe malaria treatment in Ugandan adults. Malar J 11:132. doi: 10.1186/1475-2875-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berdelle N, Nikolova T, Quiros S, Efferth T, Kaina B. 2011. Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Mol Cancer Ther 10:2224–2233. doi: 10.1158/1535-7163.MCT-11-0534. [DOI] [PubMed] [Google Scholar]
- 40.Kelter G, Steinbach D, Konkimalla VB, Tahara T, Taketani S, Fiebig HH, Efferth T. 2007. Role of transferrin receptor and the ABC transporters ABCB6 and ABCB7 for resistance and differentiation of tumor cells towards artesunate. PLoS One 2:e798. doi: 10.1371/journal.pone.0000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miotto O, Almagro-Garcia J, Manske M, Macinnis B, Campino S, Rockett KA, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Duong S, Nguon C, Chuor CM, Saunders D, Se Y, Lon C, Fukuda MM, Amenga-Etego L, Hodgson AV, Asoala V, Imwong M, Takala-Harrison S, Nosten F, Su XZ, Ringwald P, Ariey F, Dolecek C, Hien TT, Boni MF, Thai CQ, Amambua-Ngwa A, Conway DJ, Djimde AA, Doumbo OK, Zongo I, Ouedraogo JB, Alcock D, Drury E, Auburn S, Koch O, Sanders M, Hubbart C, Maslen G, Ruano-Rubio V, Jyothi D, Miles A, O'Brien J, Gamble C, Oyola SO, et al. . 2013. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet 45:648–655. doi: 10.1038/ng.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ II, Ischiropoulos H. 2012. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tampo Y, Kotamraju S, Chitambar CR, Kalivendi SV, Keszler A, Joseph J, Kalyanaraman B. 2003. Oxidative stress-induced iron signaling is responsible for peroxide-dependent oxidation of dichlorodihydrofluorescein in endothelial cells: role of transferrin receptor-dependent iron uptake in apoptosis. Circ Res 92:56–63. doi: 10.1161/01.RES.0000048195.15637.AC. [DOI] [PubMed] [Google Scholar]
- 44.Reliene R, Schiestl RH. 2006. Glutathione depletion by buthionine sulfoximine induces DNA deletions in mice. Carcinogenesis 27:240–244. doi: 10.1093/carcin/bgi222. [DOI] [PubMed] [Google Scholar]
- 45.Griffith OW. 1982. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem 257:13704–13712. [PubMed] [Google Scholar]
- 46.Spencer SR, Wilczak CA, Talalay P. 1990. Induction of glutathione transferases and NAD(P)H:quinone reductase by fumaric acid derivatives in rodent cells and tissues. Cancer Res 50:7871–7875. [PubMed] [Google Scholar]
- 47.Bozdech Z, Ginsburg H. 2004. Antioxidant defense in Plasmodium falciparum—data mining of the transcriptome. Malar J 3:23. doi: 10.1186/1475-2875-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lapperre TS, Jimenez LA, Antonicelli F, Drost EM, Hiemstra PS, Stolk J, MacNee W, Rahman I. 1999. Apocynin increases glutathione synthesis and activates AP-1 in alveolar epithelial cells. FEBS Lett 443:235–239. doi: 10.1016/S0014-5793(98)01723-2. [DOI] [PubMed] [Google Scholar]