Abstract
Imported malaria has been a great challenge for public health in China due to decreased locally transmitted cases and frequent exchange worldwide. Plasmodium falciparum has been mainly responsible for the increasing impact. Currently, artesunate plus amodiaquine, one of the artemisinin combination therapies recommended by the World Health Organization, has been mainly used against uncomplicated P. falciparum malaria in China. However, drug resistance marker polymorphism in returning migrant workers has not been demonstrated. Here, we have evaluated the prevalence of pfmdr1 and pfcrt polymorphisms, as well as the K13 propeller gene, a molecular marker of artemisinin resistance, in migrant workers returned from Ghana to Shanglin County, Guangxi Province, China, in 2013. A total of 118 blood samples were randomly selected and used for the assay. Mutations of the pfmdr1 gene that covered codons 86, 184, 1034, and 1246 were found in 11 isolates. Mutations at codon N86Y (9.7%) were more frequent than at others, and Y86Y184S1034D1246 was the most prevalent (63.6%) of the four haplotypes. Mutations of the pfcrt gene that covered codons 74, 75, and 76 were observed in 17 isolates, and M74N75T76 was common (70.6%) in three haplotypes. Eight different genotypes of the K13 propeller were first observed in 10 samples in China, 2 synonymous mutations (V487V and A627A) and 6 nonsynonymous mutations. C580Y was the most prevalent (2.7%) in all the samples. The data presented might be helpful for enrichment of molecular surveillance of antimalarial resistance and will be useful for developing and updating antimalarial guidance in China.
INTRODUCTION
Malaria is the most important parasitic protozoan infection that poses serious threats to human health globally (1). China has had success in reducing the morbidity and mortality of malaria to low levels through continuous and large-scale interventions (2). Despite the large decreases in local cases, imported malaria has been a great challenge to public health due to frequent exchanges worldwide. Plasmodium falciparum, the deadly species, has been mainly responsible for the increasing impact (3). Imported malaria may pose high risks to malaria-free localities in which Anopheles mosquitoes are prevalent during the transmission season, and severe malaria is mainly caused by P. falciparum without timely diagnosis and effective treatment. In China, malaria cases significantly increased in 2013 (n = 4,128), mainly due to the large number of migrant workers who had returned from Ghana with P. falciparum infection (n = 1,046; 25.3%) reported in Shanglin County, Guangxi Province, China. In response to such an emergency, artesunate (AS) plus amodiaquine (AQ), one of the artemisinin combination therapies (ACTs) recommended by the World Health Organization (WHO) and China, was mainly used against uncomplicated P. falciparum infection (4).
AS plus AQ is one of the WHO-recommended ACTs to treat uncomplicated P. falciparum malaria worldwide (5). It was adopted as the first-line treatment in 2006 by WHO (6), and it was found that the combination of AS plus AQ is therapeutically superior to a combination of chloroquine (CQ) plus pyrimethamine-sulfadoxine (SP) and significantly reduced gametocyte carriage following treatment (7). The efficacy and tolerability of AS plus AQ has been tested formally in several clinical trials in different epidemiological settings in Africa (8–12). In China, AQ has been widely used as a monotherapy for nearly 30 years, and drug tolerance/resistance was first observed in Yunnan Province, China; the evidence showed that it also elicits cross-resistance against CQ and piperaquine (PQ) (13, 14). On the other hand, AQ was shown to have synergism in combination with AS in some regions of China (15). Because of this, AS plus AQ was adopted as one of the four ACTs against uncomplicated P. falciparum malaria in China. At present, no artemisinin resistance has been observed in China, but emergent resistance in the Greater Mekong Subregion (GMS) (Cambodia, Laos, Myanmar, Thailand, Vietnam, and Yunnan Province, China) poses a great challenge for the control and elimination of malaria in China (16, 17). In Africa, the emergence and spread of CQ, AQ, and antifolate antimalarial resistance has long been observed (18, 19), and a decreased response to AS plus AQ was also observed (12). Although it has yet to be established whether artemisinin resistance has spread westward, the spread of resistant parasites to sub-Saharan Africa would be disastrous (20). Due to the increasing importation of P. falciparum malaria from Africa recently and the fact that little was known about the current drug resistance, particularly among Chinese migrant workers, a study was urgently needed to provide some useful suggestions for rational administration. The aim of this study was to evaluate the drug resistance polymorphism of migrant workers in Shanglin County, Guangxi Province, which will be used as evidence for further molecular surveillance of drug resistance and also will be useful for developing and updating antimalarial guidance.
MATERIALS AND METHODS
Study design.
Imported P. falciparum malaria has sharply increased in Shanglin County, mainly due to migrant workers who had returned from Ghana. To estimate the incidence of malaria and the prevalence of polymorphisms of drug resistance-related molecular markers in migrant workers due to be administered AS plus AQ (Guilin Pharmaceutical Co. Ltd., Guilin, Guangxi Autonomous Region, China), and in order to provide useful suggestions for future treatment of such clustered imported P. falciparum malaria, all individual case information was carefully reviewed and analyzed, and bioassays were performed by a random-sampling method.
Study sites.
We conducted this study in Shanglin County, Guangxi Province, located in south China. Reported malaria cases significantly increased in 2013 in the county due to the clustered migrant workers who had returned from Ghana, with a sharp peak observed in June.
Samples for study.
Blood samples from migrant workers with fever were screened for malaria, and 1,251 samples with malaria confirmed at enrollment in Shanglin County were labeled with study numbers, names, and dates and stored in sealed frozen tubes at −80°C until use. Since migrant workers who had returned from Ghana accounted for most (n = 1,046) of the total P. falciparum cases (n = 1,104), we randomly selected for PCR 118 samples of P. falciparum from the migrant workers who had returned from Ghana to evaluate the polymorphism.
Laboratory methods.
DNA was isolated from selected blood samples with a QIAamp DNA minikit (Qiagen, Valencia, CA). Known P. falciparum polymorphisms were assessed at the following alleles: pfmdr1 N86Y, Y184F, S1034C, N1042D, and D1246Y and pfcrt M74I, N75E, and K76T. Also, we investigated the mutation of the PF3D7_1343700 kelch propeller domain (PF13_0238, also called the K13 propeller), a molecular marker of artemisinin resistance. Polymorphisms were evaluated using nested PCR, followed by restriction fragment length polymorphism (RFLP) analysis, as described previously (21, 22). Sequencing was carried out by Shanghai DNA BioTechnologies Co., Ltd. (Shanghai, China). Sequences were analyzed with the BLAST program (http://blast.ncbi.nlm.nih.gov/). Multiple nucleotide sequence alignments and analysis were performed using the BioEdit Sequence Alignment Editor (http://www.mbio.ncsu.edu/BioEdit/bioedit.html).
Data analysis.
Data were analyzed with Microsoft Excel and SAS version 9.2. The chi-square (χ2) test or Fisher's exact test was used to assess differences. P values were calculated and were considered statistically significant at <0.05.
Ethical considerations.
The study was reviewed and approved by the ethical committee of the Chinese Center for Disease Control and Prevention (China CDC).
RESULTS
Enrollment.
A total of 1,251 migrant workers were diagnosed with malaria in 2013: 1,104 with P. falciparum infections, 107 with Plasmodium vivax infections, 21 with Plasmodium ovale infections, 9 with Plasmodium malariae infections, and 10 with mixed infections. Among the 1,046 migrant workers with P. falciparum infections who had returned from Ghana, 118 subjects were enrolled in the study, and blood samples were collected from them and analyzed (Fig. 1).
FIG 1.
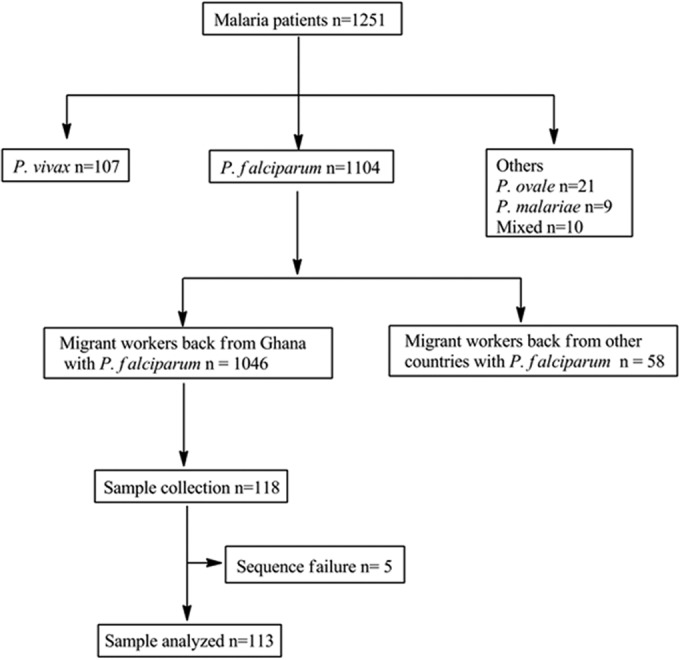
Screening, enrollment, and follow-up of subject patients.
Epidemiologic profile of malaria in Guangxi, 2013.
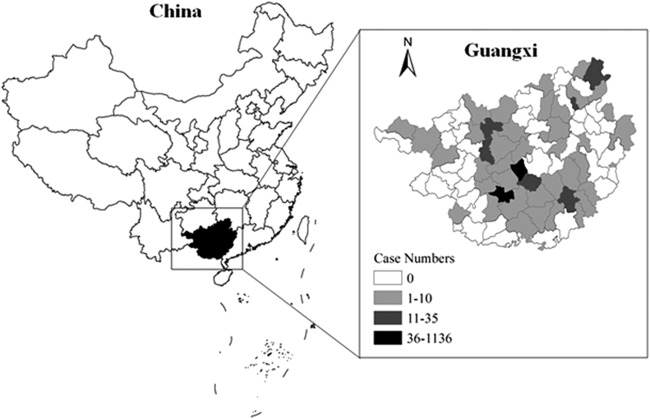
Reported cases increased sharply in 2013, particularly in June and July, due to the clustered migrant workers who had returned from Ghana. In total, 1,251 malaria cases were recorded in the province during the whole year in a Web-based reporting system, all of which were considered imported malaria, and this corresponded to a 468.6% increase from the 220 cases reported in 2012 (P < 0.05) (Fig. 2). A proportion (84.1%) of the total malaria cases and 87.0% of the total P. falciparum malaria cases were reported in Shanglin County, and most of them (98.2%; n = 1,033) were in workers who had returned from Ghana.
FIG 2.
Malaria in Guangxi Province, China, in 2013. The map was created using ArcGIS 10.1 (Environmental Systems Research Institute, Inc.).
Mutational analysis of pfcrt and pfmdr1.
Of the 8 mutation types, no N1042D pfmdr1 mutation was found, and it is not discussed further here. Mutations of the pfmdr1 gene that covered codons 86, 184, 1034, and 1246 were sequenced successfully in 11 isolates. Mutations at codon N86Y (9.7%) was more frequent than the others (Table 1), and Y86Y184S1034D1246 was the most prevalent (63.6%) of the four reported haplotypes (Table 2). Mutations of the pfcrt gene that covered codons 74, 75, and 76 were found in 17 isolates. Three different pfcrt genotypes were found, among which M74N75T76 was common (70.6%) (Table 2).
TABLE 1.
Selection of P. falciparum polymorphisms treated with artesunate plus amodiaquine
| Gene | SNPa | Occurrence of mutation |
||
|---|---|---|---|---|
| n | % | 95% CIb | ||
| pfmdr1 (n = 11) | N86Y | 11 | 9.7 | 4.31–15.12 |
| Y184F | 2 | 1.8 | 0.55–3.01 | |
| S1034C | 1 | 0.9 | 0.34–1.54 | |
| N1042D | 0 | 0 | 0 | |
| D1246Y | 2 | 1.8 | 0.49–3.10 | |
| pfcrt (n = 17) | M74I | 1 | 0.9 | 0.27–1.51 |
| N75E | 4 | 4.4 | 2.31–6.52 | |
| K76T | 13 | 11.5 | 6.05–16.87 | |
SNP, single-nucleotide polymorphism.
CI, confidence interval.
TABLE 2.
Prevalences of genotypes of candidate genes pfmdr1 and pfcrt
| Candidate gene | Genotypea | Prevalence (%) of mutation in selected samples |
|---|---|---|
| pfmdr1 (n = 11) | Y86F184S1034D1246 (n = 2) | 18.2 |
| Y86Y184S1034D1246 (n = 7) | 63.6 | |
| Y86Y184C1034Y1246 (n = 1) | 9.1 | |
| Y86Y184S1034Y1246 (n = 1) | 9.1 | |
| pfcrt (n = 17) | I74N75T76 (n = 1) | 5.9 |
| M74N75T76 (n = 12) | 70.6 | |
| M74E75K76 (n = 4) | 23.5 |
The mutated amino acids are in boldface.
Gene polymorphism of the K13 propeller.
Eight different genotypes were observed in 10 samples, including 2 synonymous mutations and 6 nonsynonymous mutations (Table 3). The synonymous mutations were V487V and A627A, with prevalences of 0.9% (n = 1) and 0.9% (n = 1), respectively. Of the 6 nonsynonymous mutations, C580Y was the most prevalent (2.7%; n = 3) and was found in all the samples. Furthermore, one patient was found to have a mixed V487L692 genotype.
TABLE 3.
Polymorphisms observed in the K13 propeller in P. falciparum isolatesa
| Codon position | Amino acid reference | Nucleotide reference | Amino acid mutation | Nucleotide mutationd | Prevalence of mutation (%) |
|---|---|---|---|---|---|
| 487b,c | V | GTA | V | gtG | 0.9 (n = 1) |
| 539 | R | AGA | T | aCa | 0.9 (n = 1) |
| 575c | R | AGA | T | aCa | 1.8 (n = 2) |
| 580 | C | TGT | Y | tTt | 2.7 (n = 3) |
| 580c | C | TGT | F | tAt | 0.9 (n = 1) |
| 584 | D | GAT | V | gTt | 0.9 (n = 1) |
| 627b,c | A | GCT | A | gcA | 0.9 (n = 1) |
| 692c | V | GTT | L | Ctt | 0.9 (n = 1) |
Cases were all imported from Ghana.
Synonymous mutation.
Mutated site not previously reported.
Mutations are in boldface.
DISCUSSION
ACT has been recommended as the first-line therapy to address the resistance of P. falciparum to monotherapies and to improve treatment outcomes. AS plus AQ, dihydroartemisinin (DHA) plus PQ, and the other two ACTs were the preferred antimalarials used against uncomplicated P. falciparum in China. However, widespread artemisinin resistance was observed in the GMS and resistance to CQ, AQ, and other antimalarials was observed in Africa (16, 23–25), which may represent a great challenge from resistant parasites imported into China with the migrant population, immediately affecting therapy efficacy. Therefore, we designed this study to evaluate the drug resistance marker polymorphism and the prevalence of an artemisinin resistance marker (K13 propeller) in migrant workers in order to provide useful suggestions for rational administration in China.
Guangxi Province is located in south China and is classified as a “malaria unstable” region (26). Malaria incidence decreased to 0.47/100,000 in 2012, and no local P. falciparum malaria has been observed since 2003 (27, 28). However, due to the large number of migrant workers returning from Ghana, malaria cases increased sharply in 2013. This was because most Chinese migrant workers in Ghana were deported by the Ghana government, and most of them were from Shanglin County.
Both pfmdr1 and pfcrt were selected for chloroquine resistance, and they also have been reported to be associated with in vitro responses to AS and AQ (12, 29). The results showed a high prevalence of pfmdr1 N86Y, which was not frequently found along the China-Myanmar border (30). This could be explained by the fact that a high prevalence of the pfmdr1 N86Y allele was seen in Ghana due to excess use of CQ (31), and it was also observed in other regions of Africa after extensive use of AS plus AQ (32, 33); also, most of the pfmdr1 gene copy number amplifications in field samples harbor an asparagine at amino acid position 86 of the pfmdr1 gene (34). Codons 184, 1034, and 1246 were also found in the studied samples, and this was similar to the results of Danquah et al. in Burkina Faso, which may also be due to differences in transmission intensity and drug usage (29). The Y86Y184S1034Y1246 haplotype was detected more frequently than others, also suggesting the widespread occurrence of the N86Y allele in migrant workers.
pfcrt K76T has been widely used as a reliable marker for CQ resistance, and it was found at a high prevalence in China (35). This was consistent with our study results showing that pfcrt K76T played the predominant role in the pfcrt genotypes. Molecular studies have shown that parasites transfected with pfcrt of the S72V73M74N75T76 haplotype have decreased sensitivity to AQ and its active metabolite (36, 37), and this haplotype was detected in 19% of infected individuals in Tanzania after the increasing use of AQ in the region (38). However, two other haplotypes, I74N75T76 and M74E75K76, were also found in our study, suggesting that selection of other pfcrt haplotypes is still needed. Another factor that may contribute to the high prevalence of pfcrt K76T is the continued use of chloroquine as a first-line drug for P. vivax infection over several decades in Ghana, which indicates that the chloroquine pressure for the maintenance of the pfcrt mutation in P. falciparum is still present in the country (31).
Artemisinin and its derivatives have been used for falciparum malaria treatment in China since the late 1970s (39). In vitro assays have shown that the susceptibility of P. falciparum to artemisinins is declining in China, but no evidence has been detected for artemisinin resistance (40). Here, we have also investigated the prevalence of K13 propeller gene polymorphism in migrant workers to understand the status of artemisinin resistance. Two synonymous mutations, V487V and A627A, were observed, with prevalences of 0.9% (n = 1), and neither had been reported before. Six nonsynonymous mutations were also found, and three of them were unreported. Also, the results showed that C580Y was the predominant allele (2.7%; n = 3); it was identified in slow-clearing parasites in malaria patients treated with artemisinin, which was supported by the work of Ariey et al., who frequently detected it from 2001 and 2002 to 2011 and 2012 in Pailin and Battambang in Cambodia (41). The M476I allele, which can significantly increase resistance to artemisinin, was not found in this study. This indicated that the mutated K13 propeller gene alleles exist in migrant workers returning from Ghana, which may raise concerns about the emergence of artemisinin resistance in Africa, and the findings support further clinical trials associated with K13 propeller mutations, which will be useful to identify additional genetic loci involved in monitoring the emergence of artemisinin resistance.
According to the antimalarial strategy of China, piperaquine phosphate is the recommended antimalarial chemoprophylaxis to be used in the area of P. falciparum endemicity. However, Chinese travelers often take ACTs to treat malaria in Africa. Despite the fact that AS plus AQ was introduced in Ghana as the first-line drug for treatment of uncomplicated malaria in 2004 (42), no evidence has shown that antimalarial resistance has occurred due to drug abuse. Therefore, more related research should be carried out to determine whether mutation of the K13 propeller has possibly occurred in the infecting Plasmodium species or if it has appeared in the general population in the GMS.
The prevalence of the K13 propeller gene polymorphism detected in migrant workers in China revealed that the use of antimalarials should be based on the resistance status in the importing country and that rational use of antimalarials against the Plasmodium species imported from Africa and Southeast Asia should be adopted. In addition, routine monitoring and surveillance, as recommended by the WHO global plan for artemisinin resistance containment, should be continuously strengthened. It is also necessary to carry out additional clinical investigations to complement sentinel surveillance, including analysis either of drug markers or risk factors or of new approaches to monitor resistance.
In conclusion, high prevalences of pfmdr1N86Y and D1246Y and pfcrt K76T alleles were observed in migrant workers who had returned from Ghana to Shanglin County, Guangxi Province. In addition, we have reported for the first time 8 K13 propeller gene polymorphisms, 2 synonymous mutations and 6 nonsynonymous mutations, among which the C580Y allele has the predominant role. The present data might be helpful for enrichment of molecular surveillance of antimalarial resistance and will be useful for developing and updating the antimalarial guidance in China.
ACKNOWLEDGMENTS
This work was supported by the Special Fund for Health Research in the Public Interest (grant no. 201202019).
We declare that we have no competing interests.
REFERENCES
- 1.World Health Organization. 2013. World malaria report: 2013. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Cao J, Sturrock HJ, Cotter C, Zhou S, Zhou H, Liu Y, Tang L, Gosling RD, Feachem RG, Gao Q. 2014. Communicating and monitoring surveillance and response activities for malaria elimination: China's “1-3-7” strategy. PLoS Med 11:e1001642. doi: 10.1371/journal.pmed.1001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng J, Xia ZG. 2014. Analysis of trends in cases of malaria reported from 2004 to July 2013 in the People's Republic of China. Zhongguo Bing Yuan Sheng Wu Xue Za Zhi 9:442–446. (In Chinese.) [Google Scholar]
- 4.Chinese Center for Disease Control and Prevention. 2011. China's technical scheme of malaria elimination. Chinese Center for Disease Control and Prevention, Beijing, China. [Google Scholar]
- 5.World Health Organization. 2010. Guidelines for the treatment of malaria, 2nd ed. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 6.World Health Organization. 2006. Guidelines for the treatment of malaria. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 7.Sowunmi A, Fehintola FA, Adedeji AA, Gbotosho GO, Tambo E, Fateye BA, Happi TC, Oduola AM. 2005. Open randomized study of artesunate-amodiaquine vs. chloroquine-pyrimethamine-sulfadoxine for the treatment of uncomplicated Plasmodium falciparum malaria in Nigerian children. Trop Med Int Health 10:1161–1170. doi: 10.1111/j.1365-3156.2005.01503.x. [DOI] [PubMed] [Google Scholar]
- 8.Ndiaye JL, Randrianarivelojosia M, Sagara I, Brasseur P, Ndiaye I, Faye B, Randrianasolo L, Ratsimbasoa A, Forlemu D, Moor VA, Traore A, Dicko Y, Dara N, Lameyre V, Diallo M, Djimde A, Same-Ekobo A, Gaye O. 2009. Randomized, multicentre assessment of the efficacy and safety of ASAQ—a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria. Malar J 8:125. doi: 10.1186/1475-2875-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirima SB, Tiono AB, Gansane A, Diarra A, Ouedraogo A, Konate AT, Kiechel JR, Morgan CC, Olliaro PL, Taylor WR. 2009. The efficacy and safety of a new fixed-dose combination of amodiaquine and artesunate in young African children with acute uncomplicated Plasmodium falciparum. Malar J 8:48. doi: 10.1186/1475-2875-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adjuik M, Agnamey P, Babiker A, Borrmann S, Brasseur P, Cisse M, Cobelens F, Diallo S, Faucher JF, Garner P, Gikunda S, Kremsner PG, Krishna S, Lell B, Loolpapit M, Matsiegui PB, Missinou MA, Mwanza J, Ntoumi F, Olliaro P, Osimbo P, Rezbach P, Some E, Taylor WR. 2002. Amodiaquine-artesunate versus amodiaquine for uncomplicated Plasmodium falciparum malaria in African children: a randomised, multicentre trial. Lancet 359:1365–1372. doi: 10.1016/S0140-6736(02)08348-4. [DOI] [PubMed] [Google Scholar]
- 11.Sowunmi A, Balogun T, Gbotosho GO, Happi CT, Adedeji AA, Fehintola FA. 2007. Activities of amodiaquine, artesunate, and artesunate-amodiaquine against asexual- and sexual-stage parasites in falciparum malaria in children. Antimicrob Agents Chemother 51:1694–1699. doi: 10.1128/AAC.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nsobya SL, Dokomajilar C, Joloba M, Dorsey G, Rosenthal PJ. 2007. Resistance-mediating Plasmodium falciparum pfcrt and pfmdr1 alleles after treatment with artesunate-amodiaquine in Uganda. Antimicrob Agents Chemother 51:3023–3025. doi: 10.1128/AAC.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H, Liu D, Huang K, Yang Y, Yang P, Liao M, Zhang C. 1999. Assay of sensitivity of Plasmodium falciparum to chloroquine, amodiaquine, piperaquine, mefloquine and quinine in Yunnan province. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 17:43–45. (In Chinese.) [PubMed] [Google Scholar]
- 14.Chen C. 2014. Development of antimalarial drugs and their application in China: a historical review. Infect Dis Poverty 3:9. doi: 10.1186/2049-9957-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Gao B, Huang K. 1999. Comparison of sensitivity of artesunate-sensitive and artesunate-resistant Plasmodium falciparum to chloroquine and amodiaquine. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 17:353–355. (In Chinese.) [PubMed] [Google Scholar]
- 16.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller O, Sie A, Meissner P, Schirmer RH, Kouyate B. 2009. Artemisinin resistance on the Thai-Cambodian border. Lancet 374:1419. doi: 10.1016/S0140-6736(09)61857-2. [DOI] [PubMed] [Google Scholar]
- 18.Trape JF, Pison G, Spiegel A, Enel C, Rogier C. 2002. Combating malaria in Africa. Trends Parasitol 18:224–230. doi: 10.1016/S1471-4922(02)02249-3. [DOI] [PubMed] [Google Scholar]
- 19.Roper C, Pearce R, Bredenkamp B, Gumede J, Drakeley C, Mosha F, Chandramohan D, Sharp B. 2003. Antifolate antimalarial resistance in southeast Africa: a population-based analysis. Lancet 361:1174–1181. doi: 10.1016/S0140-6736(03)12951-0. [DOI] [PubMed] [Google Scholar]
- 20.Mok S, Imwong M, Mackinnon MJ, Sim J, Ramadoss R, Yi P, Mayxay M, Chotivanich K, Liong KY, Russell B, Socheat D, Newton PN, Day NP, White NJ, Preiser PR, Nosten F, Dondorp AM, Bozdech Z. 2011. Artemisinin resistance in Plasmodium falciparum is associated with an altered temporal pattern of transcription. BMC Genomics 12:391. doi: 10.1186/1471-2164-12-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duraisingh MT, Jones P, Sambou I, von Seidlein L, Pinder M, Warhurst DC. 2000. The tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarials mefloquine and artemisinin. Mol Biochem Parasitol 108:13–23. doi: 10.1016/S0166-6851(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 22.Djimde A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourte Y, Coulibaly D, Dicko A, Su XZ, Nomura T, Fidock DA, Wellems TE, Plowe CV. 2001. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med 344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 23.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ. 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mutabingwa TK, Muze K, Ord R, Briceno M, Greenwood BM, Drakeley C, Whitty CJ. 2009. Randomized trial of artesunate+amodiaquine, sulfadoxine-pyrimethamine+amodiaquine, chlorproguanal-dapsone and SP for malaria in pregnancy in Tanzania. PLoS One 4:e5138. doi: 10.1371/journal.pone.0005138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charmot G, Le Bras J, Dupont B, Sansonetti P, Lapresle C. 1986. Resistance of Plasmodium falciparum to amodiaquine and quinine in eastern Africa. Presse Med 15:889. (In French.). [PubMed] [Google Scholar]
- 26.Chinese Center for Disease Control and Prevention. 2005. National malaria surveillance program. Chinese Center for Disease Control and Prevention, Beijing, China. [Google Scholar]
- 27.Xia ZG, Feng J, Zhou SS. 2013. Malaria situation in the People's Republic of China in 2012. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 31:413–418. (In Chinese.) [PubMed] [Google Scholar]
- 28.Li JH, Li J, Qin YX, Guo CK, Huang YM, Lin Z, Jiang ZH, Wei HY, Lin KM, Du JF, Mao W. 2014. Appraisal of the effect and measures on control malaria for sixty years in Guangxi. Re Dai Yi Xue Za Zhi 14:361–364. (In Chinese.) [Google Scholar]
- 29.Danquah I, Coulibaly B, Meissner P, Petruschke I, Muller O, Mockenhaupt FP. 2010. Selection of pfmdr1 and pfcrt alleles in amodiaquine treatment failure in north-western Burkina Faso. Acta Trop 114:63–66. doi: 10.1016/j.actatropica.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Huang F, Tang L, Yang H, Zhou S, Liu H, Li J, Guo S. 2012. Molecular epidemiology of drug resistance markers of Plasmodium falciparum in Yunnan Province, China. Malar J 11:243. doi: 10.1186/1475-2875-11-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mockenhaupt FP, Ehrhardt S, Eggelte TA, Agana-Nsiire P, Stollberg K, Mathieu A, Markert M, Otchwemah RN, Bienzle U. 2005. Chloroquine-treatment failure in northern Ghana: roles of pfcrt T76 and pfmdr1 Y86. Ann Trop Med Parasitol 99:723–732. doi: 10.1179/136485905X75395. [DOI] [PubMed] [Google Scholar]
- 32.Dokomajilar C, Lankoande ZM, Dorsey G, Zongo I, Ouedraogo JB, Rosenthal PJ. 2006. Roles of specific Plasmodium falciparum mutations in resistance to amodiaquine and sulfadoxine-pyrimethamine in Burkina Faso. Am J Trop Med Hyg 75:162–165. [PubMed] [Google Scholar]
- 33.Froberg G, Jornhagen L, Morris U, Shakely D, Msellem MI, Gil JP, Bjorkman A, Martensson A. 2012. Decreased prevalence of Plasmodium falciparum resistance markers to amodiaquine despite its wide scale use as ACT partner drug in Zanzibar. Malar J 11:321. doi: 10.1186/1475-2875-11-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veiga MI, Ferreira PE, Jornhagen L, Malmberg M, Kone A, Schmidt BA, Petzold M, Bjorkman A, Nosten F, Gil JP. 2011. Novel polymorphisms in Plasmodium falciparum ABC transporter genes are associated with major ACT antimalarial drug resistance. PLoS One 6:e20212. doi: 10.1371/journal.pone.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang GQ, Guan YY, Zheng B, Wu S, Tang LH. 2009. Molecular assessment of Plasmodium falciparum resistance to antimalarial drugs in China. Trop Med Int Health 14:1266–1271. doi: 10.1111/j.1365-3156.2009.02342.x. [DOI] [PubMed] [Google Scholar]
- 36.Sidhu AB, Verdier-Pinard D, Fidock DA. 2002. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298:210–213. doi: 10.1126/science.1074045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beshir K, Sutherland CJ, Merinopoulos I, Durrani N, Leslie T, Rowland M, Hallett RL. 2010. Amodiaquine resistance in Plasmodium falciparum malaria in Afghanistan is associated with the pfcrt SVMNT allele at codons 72 to 76. Antimicrob Agents Chemother 54:3714–3716. doi: 10.1128/AAC.00358-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alifrangis M, Dalgaard MB, Lusingu JP, Vestergaard LS, Staalsoe T, Jensen AT, Enevold A, Ronn AM, Khalil IF, Warhurst DC, Lemnge MM, Theander TG, Bygbjerg IC. 2006. Occurrence of the Southeast Asian/South American SVMNT haplotype of the chloroquine-resistance transporter gene in Plasmodium falciparum in Tanzania. J Infect Dis 193:1738–1741. doi: 10.1086/504269. [DOI] [PubMed] [Google Scholar]
- 39.Li GQ, Guo XB, Fu LC, Jian HX, Wang XH. 1994. Clinical trials of artemisinin and its derivatives in the treatment of malaria in China. Trans R Soc Trop Med Hyg 88(Suppl 1):S5–S6. [DOI] [PubMed] [Google Scholar]
- 40.Zhang G, Guan Y, Zheng B, Wu S, Tang L. 2008. No PfATPase6 S769N mutation found in Plasmodium falciparum isolates from China. Malar J 7:122. doi: 10.1186/1475-2875-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asante KP, Abokyi L, Zandoh C, Owusu R, Awini E, Sulemana A, Amenga-Etego S, Adda R, Boahen O, Segbaya S, Mahama E, Bart-Plange C, Chandramohan D, Owusu-Agyei S. 2010. Community perceptions of malaria and malaria treatment behaviour in a rural district of Ghana: implications for artemisinin combination therapy. BMC Public Health 10:409. doi: 10.1186/1471-2458-10-409. [DOI] [PMC free article] [PubMed] [Google Scholar]