Abstract
Pentavalent antimonials have been the first-line treatment for leishmaniasis for decades. However, the development of resistance to sodium stibogluconate (SSG) has limited its use, especially for treating visceral leishmaniasis (VL). The present work aims to optimize a cationic liposomal formulation of SSG for the treatment of both SSG-sensitive (AG83) and SSG-resistant (GE1F8R and CK1R) Leishmania donovani infections. Parasite killing was determined by the 3-(4,5-dimethylthiazol-2)-2,5-diphenyltetrazolium bromide (MTT) assay and microscopic counting of Giemsa-stained macrophages. Macrophage uptake studies were carried out by confocal microscopic imaging. Parasite-liposome interactions were visualized through transmission electron microscopy. Toxicity tests were performed using assay kits. Organ parasite burdens were determined by microscopic counting and limiting dilution assays. Cytokines were measured by enzyme-linked immunosorbent assays (ELISAs) and flow cytometry. Although all cationic liposomes studied demonstrated leishmanicidal activity, phosphatidylcholine (PC)-dimethyldioctadecylammonium bromide (DDAB) vesicles were most effective, followed by PC-stearylamine (SA) liposomes. Since entrapment of SSG in PC-DDAB liposomes demonstrated enhanced ultrastructural alterations in promastigotes, PC-DDAB-SSG vesicles were further investigated in vitro and in vivo. PC-DDAB-SSG could effectively alleviate SSG-sensitive and SSG-resistant L. donovani infections in the liver, spleen, and bone marrow of BALB/c mice at a dose of SSG (3 mg/kg body weight) not reported previously. The parasiticidal activity of these vesicles was attributed to better interactions with the parasite membranes, resulting in direct killing, and generation of a strong host-protective environment, necessitating a very low dose of SSG for effective cures.
INTRODUCTION
Visceral leishmaniasis (VL), caused by the protozoan parasite Leishmania donovani, results in 0.2 to 0.4 million cases reported annually and poses a threat to about 200 million people worldwide. With the advent of the human immunodeficiency viruses, VL has surged as an opportunistic infection among AIDS patients (1). Pentavalent antimonial drugs are the first-line drugs against all forms of leishmaniasis, although their use for the treatment of VL has been discontinued in India due to the emergence of resistance. Extended treatment regimens, parenteral administration, and toxic side effects limit patient compliance with treatment. Frequent therapeutic failures due to resistance to the antimonial drug sodium stibogluconate (SSG) necessitate the use of second-line drugs such as amphotericin B (AMB), which are more toxic. Alternate therapies for VL have resulted in the development of several lipid- and liposome-based formulations of conventional drugs, with enhanced efficacies and reduced toxicities (2–7). The advantages of the use of liposomes as drug delivery vehicles for treating macrophage-resident parasites such as Leishmania involve their ease of preparation, low toxicity, and biodegradability and the natural liposome homing sites in macrophages of the reticuloendothelium system (8). Their efficacies, however, depend on several physical parameters, including size, phospholipid composition, vesicle stability, and most importantly, surface charge (9–13). Various studies with differentially charged liposomes indicate increased uptake and thus enhanced homing of cationic liposomes, in comparison to neutral or anionic liposomes, to dendritic cells and macrophages (14–17). In addition, we reported earlier that cationic liposomes bearing egg phosphatidylcholine (PC) and stearylamine (SA) (i.e., PC-SA) had direct killing activity against various strains of Leishmania, an activity not observed with anionic or neutral liposomes (18, 19). Encapsulation of a suboptimal dose of SSG within these cytolytic vesicles (i.e., PC-SA-SSG) further enhanced the therapeutic potential of either of the monotherapies, as single-dose treatments against both SSG-responsive and non-SSG-responsive established murine VL (20, 21). The aim of antileishmanial therapy is to achieve maximum efficacy with minimum doses of the drug and carrier for a successful cure. Therefore, we investigated the potential leishmanicidal activity of cationic liposomes, composed of various cationic lipids, against both SSG-sensitive and SSG-resistant L. donovani parasites, in the search for an optimal formulation of SSG to treat a nonhealing murine model of VL.
MATERIALS AND METHODS
Materials.
Cetyltrimethylammonium bromide (CTAB), dodecyltrimethylammonium bromide (DTAB), dimethyldioctadecylammonium bromide (DDAB), dioleoyloxytrimethylammonium propane (DOTAP), and egg PC were purchased from Sigma-Aldrich (St. Louis, MO). SA was purchased from Fluka (Buchs, Switzerland) (Table 1). SSG was purchased from Gluconate Health Ltd. (Kolkata, India). Medium 199 (M199) and RPMI 1640 medium used in the experiments were purchased from Sigma-Aldrich. Both fetal bovine serum (FBS) and antibiotics (penicillin-streptomycin) were Gibco products from Invitrogen Corp. Assay kits for performing toxicity tests (serum urea, creatinine, serum glutamate pyruvate transaminase [SGPT], and serum alkaline phosphatase [ALP] assays) were from Dr. Reddy's Laboratories Ltd. (Hyderabad, India). 3-(4,5-Dimethylthiazol-2)-2,5-diphenyltetrazolium bromide (MTT) was from MP Biomedicals, LLC (Solon, OH). All other chemicals were of analytical grade.
TABLE 1.
Chemical formulae of cationic lipids
| Lipid | IUPAC name | Chemical formula | Molecular wt |
|---|---|---|---|
| CTAB | Hexadecyltrimethylammonium bromide | C19H42NBr | 364.46 |
| DTAB | Dodecyltrimethylammonium bromide | C15H34NBr | 308.35 |
| DDAB | Didodecyldimethylammonium bromide | C38H80NBr | 631 |
| SA | Octadecan-1-amine | C18H39N | 269.5 |
| DOTAP | N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride | C43H80NO4Cl | 698.54 |
Animals and parasites.
BALB/c mice (approximately 25 g), 4 to 6 weeks of age, were used for in vivo studies, in accordance with standard procedures approved by the Animal Ethics Committee of the Indian Institute of Chemical Biology (protocol 147/1999/CPCSEA) under the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India. The Leishmania donovani isolates AG83 (MHOM/IN/83/AG83), GE1F8R (derived from GE1; MHOM/IN/89/GE1), and CK1R (clinical isolate, isolated as MHOM/IN/1995/CK from a non-SSG-responsive patient) (21) were maintained by serial passage in hamsters. Amastigotes were isolated from infected spleens and allowed to transform to promastigotes by cultivation at 22°C in complete medium, i.e., M199 (pH 7.4) supplemented with 20% heat-inactivated FBS, 2 mM glutamine, 25 mM HEPES, 100 IU/ml penicillin, and 100 μg/ml streptomycin.
Preparation of liposomes.
Cationic liposomes were prepared with 20 mg of egg PC with CTAB, DTAB, DDAB, DOTAP, or SA (Table 1) at a molar ratio of 7:2, as reported previously (21), and were desiccated overnight. The thin dry films were dispersed in 1 ml of 20 mM phosphate-buffered saline (PBS). For drug entrapment, lipid films containing PC-SA or PC-DDAB were dispersed in 20 mM PBS containing 1 mg/ml of SSG. For fluorescent labeling of liposomes, rhodamine B was dissolved at 0.1 mg/ml with the lipids, and the following day the films were dispersed in PBS containing 5-carboxyfluorescein (0.1 mg/ml). The mixtures were vortex mixed, and the suspensions were sonicated for 30 s in an ultrasound probe sonicator, followed by incubation for 2 h at 4°C. Unencapsulated SSG or dye was separated from the liposomes by three successive centrifugations at 30,000 × g for 30 min each at 4°C (20). The encapsulation efficiency values for SSG within PC-SA-SSG and PC-DDAB-SSG liposomes, as measured by atomic absorption spectroscopy (AAS), were 60% and 65%, respectively (22). The PC/SA/SSG and PC/DDAB/SSG molar ratios were 7:2:0.4 and 7:2:0.6, respectively.
Physicochemical characterization of liposomes.
The average diameters of liposomes were determined in appropriately diluted liposome suspensions using photon correlation spectroscopy with a Nano Zs Zeta-Sizer system (Malvern Instruments, Worcestershire, United Kingdom), as described earlier (23). The electrophoretic mobilities and zeta potentials of the complexes were measured using a Zetaplus zeta potential analyzer (Brookhaven Instruments Corp., Holtsville, NY). Small aliquots from the different cationic liposomal preparations, prepared as described above but in 20 mM phosphate buffer without saline, were diluted to an appropriate volume with the same buffer, and the zeta potentials and mobilities of the particles were measured at 25°C (23).
Effects on L. donovani promastigotes.
To investigate the effects of cationic liposomes on promastigotes freshly transformed from amastigotes of L. donovani AG83, GE1F8R, or CK1R, 2 × 106 parasites/ml suspended in complete medium were incubated with graded concentrations (8.85 to 212 μM) of cationic liposomes for 1 h at 37°C (18). The control parasites were incubated with 0.01% Triton X-100. Following treatment, the tubes were washed with PBS. The pellets were resuspended in 100 μl of a solution of 2 mg/ml MTT in PBS, and the mixtures were incubated for 4 h at 22°C. The reduced formazan, which is a measure of cell viability, was dissolved in dimethyl sulfoxide. The absorbance at 550 nm was measured in a spectrophotometer (Hitachi Electronics, Japan) (24).
Effects on parasite-infected macrophages.
Peritoneal macrophages from naive BALB/c mice were isolated and cultured at 37°C with 5% CO2 in RPMI 1640 medium supplemented with 10% FBS, penicillin (100 IU/ml), and streptomycin (100 μg/ml). Resident macrophages (106 cells) were infected for 3 h at 37°C with freshly transformed promastigotes, at a macrophage/promastigote ratio of 1:10. The unphagocytosed parasites were removed by three washes with medium. Infected macrophages were then incubated at 37°C with medium containing graded concentrations of free cationic liposomes. In another experiment, infected macrophages were incubated with SSG-loaded PC-SA and PC-DDAB liposomes. After 1 h, excess liposomes were removed and the cells were placed in fresh complete RPMI 1640 medium for an additional 72 h. Cells were then air dried, fixed in methanol, and stained with Giemsa stain. The numbers of amastigotes in 200 macrophages in drug-treated and control cultures were counted microscopically (20).
Isolation of splenic amastigotes.
To isolate amastigotes (25), spleens of infected hamsters were rinsed in ice-cold 20 mM PBS with glucose (1.8%) and lightly homogenized. Macroscopic particles were allowed to settle, and the turbid suspension was decanted. This suspension was centrifuged at 100 × g for 10 min. The amastigote-enriched suspension was centrifuged at 800 × g for 10 min. The pellet was suspended in 45% Percoll (4.0 ml), 25% Percoll (2.0 ml) was layered over the amastigote suspension, and the preparation was centrifuged at 10,000 × g for 45 min. The band containing amastigotes was taken, washed with PBS (three times), and resuspended in RPMI 1640 medium. The freshly isolated amastigotes (2 × 105) were incubated with or without recombinant annexin V (2 μg/100 μl) for 30 min at 26°C and subsequently were treated with PC-SA or PC-DDAB liposomes (213 μM) for 1 h at 37°C. The killing activities were determined with the MTT assay, as described above.
Confocal microscopy.
Peritoneal macrophages were isolated and charged with parasites as described above. To assess the difference in the uptake of liposomes, infected macrophages were incubated with PC-SA or PC-DDAB liposomes, loaded with fluorescent dyes for 1 h, and washed three times with PBS. The cells were then fixed with 4% formaldehyde and mounted in ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes). The cells were observed with an Andor spinning-disk live-cell confocal/total internal reflection fluorescence (TIRF) microscope (Andor Technology, Belfast, United Kingdom). The lasers used had wavelengths of 405 nm, 488 nm, and 561 nm.
Electron microscopy.
Transmission electron microscopy (TEM) was carried out with PBS-treated, PC-DDAB-treated, and PC-DDAB-SSG-treated promastigotes (24). Briefly, cells were fixed with 3% glutaraldehyde in PBS, postfixed with 1% OsO4 for 1 to 2 h, gradually dehydrated in ethanol, and finally embedded in Spurr's resin. Ultrathin sections cut with a DuPont diamond knife in an ultramicrotome were stained with uranyl acetate and lead acetate and were observed with a FEI Tecnai G2 Spirit Bio Twin FP 5018/40 transmission electron microscope (FEI Co., Hillsboro, OR).
In vitro and in vivo cytotoxicity assays.
Hemolytic assays were carried out with different concentrations of cationic liposomes incubated with packed human erythrocytes, at a ratio of 1:10 (vol/vol), for 1 h at 37°C (26). The samples were then centrifuged at 800 × g for 10 min. By measuring the optical density at 540 nm in a spectrophotometer, the release of hemoglobin from lysed erythrocytes was monitored. Controls used for 0% hemolysis (blank) and 100% hemolysis consisted of erythrocytes suspended in PBS and 1% Triton X-100, respectively.
The cytotoxicity of different cationic liposomes for peritoneal macrophages was assessed with a lactate dehydrogenase (LDH) assay (27). Adherent macrophages (106 cells) were incubated with different concentrations of cationic liposomes in RPMI 1640 medium for 3 h at 37°C. LDH activity was determined by measuring the decrease in the absorbance of NADH at 340 nm in the macrophage supernatants.
Specific enzyme levels related to normal kidney and liver functions were chosen to determine the in vivo toxic effects of cationic liposomal formulations. PC-DDAB and PC-DDAB-SSG were injected intravenously (i.v.) into healthy mice at 5, 8, and 10 mg of PC/mouse in 200 μl PBS. Analyses of serum levels of creatinine, urea, and enzymes such as serum glutamate pyruvate transaminase (SGPT) and serum alkaline phosphatase (ALP) were carried out 15 days after drug administration, using the respective assay kits.
Infection and treatment.
For experimental infections, BALB/c mice were injected with 2.5 × 107 hamster spleen-derived L. donovani amastigotes via the tail vein. Eight-week-infected mice (6 or 7 mice per group) received a single i.v. dose of either free SSG (3 mg/kg body weight), drug-free PC-DDAB liposomes (5 mg of PC/mouse), or PC-DDAB-SSG (75 μg SSG, equivalent to 3 mg/kg, associated with 5 mg of PC/mouse). For comparative evaluation of efficacy, groups of BALB/c mice were treated with a single injection of 75 μg of SSG in 5 mg of PC either as PC-DDAB-SSG or as PC-SA-SSG. At 90 days postinfection, animals were sacrificed, and liver and spleen parasite burdens were determined by examining methanol-fixed, Giemsa-stained imprints of the cut organs; results were expressed as Leishman-Donovan units (LDU), calculated as the number of amastigotes per 1,000 nucleated cells × organ weight (mg) (21). In the case of bone marrow, 100 μl of PBS-flushed bone marrow suspension was smeared over glass slides, fixed with methanol, and then stained with Giemsa stain to estimate the number of parasites (21). In selected experiments, limiting dilution assays were performed, in which homogenates of liver and spleen were serially diluted and cultured for 2 weeks. Parasite burdens were expressed as the total number of parasites per organ, and the mean values for parasite burdens in three mice were calculated (21).
In vivo immunomodulatory activity.
Healthy mice were injected with PC-SA-SSG or PC-DDAB-SSG at the indicated doses, via the tail vein. At 10 days postinjection, splenocytes were isolated and incubated as described earlier (28). Concentrations of gamma interferon (IFN-γ) and interleukin 10 (IL-10) were measured in the culture supernatants in response to stimulation with concanavalin A (ConA) and lipopolysaccharide (LPS) (25), respectively, by sandwich enzyme-linked immunosorbent assay (ELISA) (BD Pharmingen kit), as recommended by the manufacturer. In another set of experiments, splenocytes from differently treated mice were cultured overnight and stained with fluorochrome-labeled antibodies against CD4, CD8, CD19, IFN-γ, and IL-10 (28). Data were acquired in a FACSCanto cell analyzer (Becton Dickinson) and analyzed with FACSDiva software.
Statistical analysis.
Data are expressed as means ± standard errors of the mean; 50% inhibitory concentration (IC50) values were calculated as 50% inhibitory concentrations for suppression of parasite growth by sigmoidal regression analysis, using Microsoft Excel 2007. Statistical differences between two groups were determined with Student's t test, and differences between multiple groups were determined using one-way analysis of variance (ANOVA) followed by Tukey's multiple-comparison test. P values of ≤0.05 were considered significant.
RESULTS
Liposome formulations and characterization.
Size measurements of the diluted liposomes formed with various cationic lipids, performed on the day of preparation, indicated average diameters of 252.0 ± 39.77 nm, 299.7 ± 23.76 nm, 197.6 ± 10.25 nm, 303.8 ± 35.22 nm, and 240.3 ± 79.25 nm for PC-CTAB, PC-DTAB, PC-DDAB, PC-SA, and PC-DOTAP, respectively, with no significant differences between the vesicles (P > 0.05) (Table 2). All liposomes were of heterogeneous size, and their polydispersity indices varied between 0.2 and 0.4. The sizes remained consistent for 4 weeks for all liposomes except for PC-DTAB, which showed an increase, probably due to aggregation. The zeta potentials of PC-CTAB, PC-DTAB, PC-DDAB, PC-SA, and PC-DOTAP liposomes were 23.2 ± 3.9 mV, 20.3 ± 4.4 mV, 33.6 ± 8.4 mV, 23.5 ± 6 mV, and 18.5 ± 2.1 mV, respectively (Table 2). The greatest electrophoretic mobility of PC-DDAB correlated with its highest zeta potential.
TABLE 2.
Physicochemical properties of cationic liposomes
| Formulation | Particle diameter (nm) | Zeta potential (mV) | Electrophoretic mobility (μV/cm) |
|---|---|---|---|
| PC-CTAB | 252.0 ± 39.77 | 23.2 ± 3.9 | 5.5 |
| PC-DTAB | 299.7 ± 23.76 | 20.3 ± 4.4 | 4.5 |
| PC-DDAB | 197.6 ± 10.25 | 33.6 ± 8.4 | 16.7 |
| PC-SA | 303.8 ± 35.22 | 23.5 ± 6 | 15 |
| PC-DOTAP | 240.3 ± 79.25 | 18.5 ± 2.1 | 2.5 |
Antileishmanial activity of cationic liposomes against L. donovani promastigotes.
We reported previously that SA-bearing liposomes (PC-SA) had potent antileishmanial activity (18, 19). To determine whether liposomes prepared with other cationic lipids also had antiparasitic effects, cationic liposomes at graded concentrations were incubated for 1 h with both SSG-sensitive AG83 and SSG-resistant GE1F8R and CK1R strains of promastigote cultures. Of the five cationic liposome types evaluated, PC-CTAB and PC-DOTAP had no appreciable antiparasitic effects, even at concentrations as high as 213 μM, for both AG83 and GE1F8R (Fig. 1). In contrast, PC-DTAB and PC-DDAB were found to have appreciable leishmanicidal activity. PC-SA and PC-DDAB were nearly equipotent at higher doses, tested against both strains of L. donovani. At concentrations of 213 μM, these liposomes induced >85% killing of L. donovani promastigotes, compared to PBS treatment. The IC50 values of PC-SA and PC-DDAB for AG83 promastigotes were 20 and 24 μM and those for GE1F8R were 52 and 70 μM, respectively (Table 3). For CK1R, all of the liposomes had high IC50s (>80 μM), with PC-DDAB being most effective at 81.21 μM.
FIG 1.
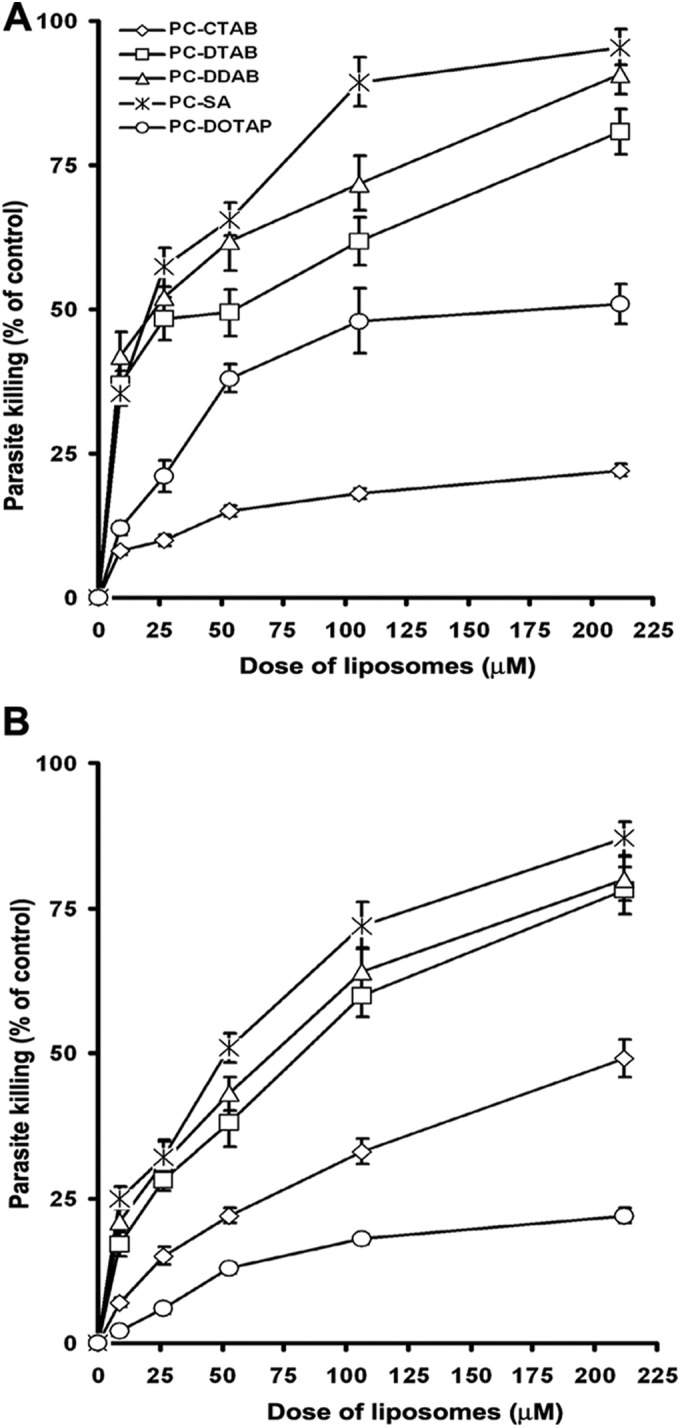
Leishmanicidal activity of graded concentrations of various cationic liposomes against promastigotes of the SSG-responsive strain AG83 (A) and the SSG-resistant strain GE1F8R (B), at 22°C. Values are means ± standard errors of three independent experiments performed in duplicate.
TABLE 3.
In vitro antileishmanial activity of cationic liposomes
| Formulation | IC50 (μM) |
|||
|---|---|---|---|---|
| Promastigotes |
Amastigotes |
|||
| AG83 | GE1F8R | AG83 | GE1F8R | |
| PC-CTAB | 510 ± 4 | 220 ± 9 | 62 ± 0.5 | 230 ± 0.5 |
| PC-DTAB | 53 ± 12.8 | 83 ± 8.2 | 92 ± 3.2 | 43 ± 1.3 |
| PC-DDAB | 24 ± 17.4 | 70 ± 1.6 | 27 ± 0.5a | 40 ± 0.8a |
| PC-SA | 20 ± 33.9 | 52 ± 5 | 53 ± 2.4 | 70 ± 1.6 |
| PC-DOTAP | 180 ± 2.2 | 456 ± 6 | 216 ± 0.1 | 436 ± 0.7 |
P < 0.05, compared to PC-SA.
Cationic liposomes elicit antileishmanial activity against intracellular amastigotes.
Treatment of infected macrophages with various cationic liposomes resulted in dose-dependent killing of L. donovani amastigotes of both SSG-sensitive and SSG-resistant strains by all of the liposomes (Fig. 2). PC-SA, PC-DDAB, and PC-DTAB induced effective inhibition of parasite growth. Results demonstrated that infected macrophages treated with PC-DDAB showed remarkable reductions in parasite burdens (96% and 91% for AG83 and GE1F8R, respectively), compared to macrophages treated with PC-SA (89% and 81% for AG83 and GE1F8R, respectively), after 72 h of incubation at a dose of 213 μM (P < 0.05). Based on the IC50s, PC-DDAB had 2- and 1.7-fold greater efficacy than PC-SA liposomes against AG83 and GE1F8R amastigotes, respectively (Table 3). Amastigotes of the clinical isolate CK1R were much more susceptible to cationic liposomes, with PC-DDAB, PC-DTAB, and PC-CTAB being more effective than PC-SA and PC-DOTAP. PC-DDAB showed an IC50 of 16.10 μM, a value 5-fold greater than the IC50 for PC-SA. In an MTT-based viability study using freshly isolated splenic amastigotes (AG83), we showed that the parasites were more susceptible to PC-DDAB than PC-SA and the activities were reversed by annexin V treatment to almost the same extents (see Fig. S1 in the supplemental material).
FIG 2.
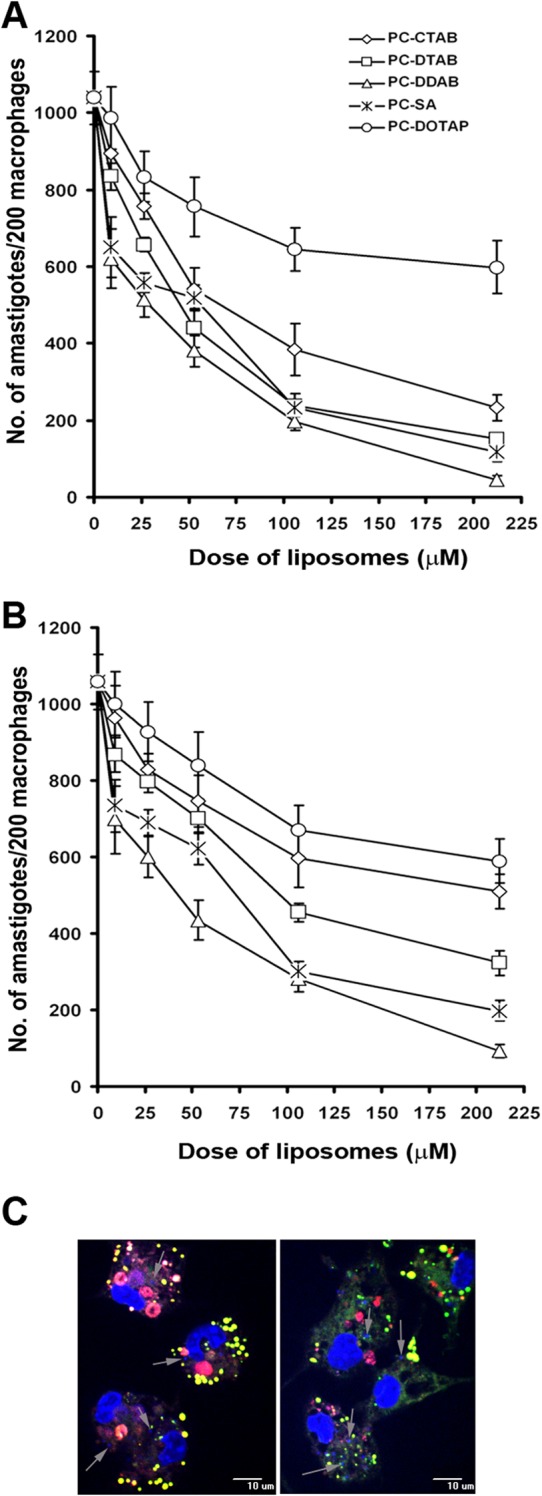
(A and B) Effects of graded concentrations of various cationic liposomes on L. donovani amastigote proliferation within murine peritoneal macrophages, using the AG83 (A) and GE1F8R (B) strains. Values are means ± standard errors of three independent experiments. (C) Uptake of PC-SA (left) and PC-DDAB (right) liposomes inside infected macrophages. Peritoneal macrophages isolated from BALB/c mice were infected with L. donovani promastigotes for 3 h and subsequently treated with fluorescent PC-SA or PC-DDAB liposomes. Macrophage nuclei are stained with DAPI (large blue areas). Intact liposomes are visible close to the amastigote nuclei, colocalizing green and red fluorescence and appearing yellow. Arrows, L. donovani amastigotes (small blue areas) inside macrophages. Magnification, ×40. Data are representative of two independent experiments.
Uptake of liposomes into infected macrophages.
Fig. 2C shows PC-SA (Fig. 2C, left) and PC-DDAB (Fig. 2C, right) liposomes inside infected macrophages. We microscopically visualized the intracellular fates of PC-SA and PC-DDAB liposomes by labeling the membrane with rhodamine B (red) and encapsulating the green dye 5-carboxyfluorescein (green). Intact liposomes (yellow) were seen inside the infected macrophages (nuclei stained blue), close to the amastigotes (small dots stained blue). Although we did not observe any significant differences in the uptake of liposomes at the initial time points (data not shown), liposomes bearing DDAB showed a more diffuse appearance at 2 h after treatment, whereas PC-SA appeared more punctate (Fig. 2C). It appears from the images that PC-DDAB liposomes interact better with the amastigotes, resulting in faster disintegration and release of their contents, as we could see the diffused green dye in the cytoplasm. In the case of PC-SA, most of the liposomes appeared intact, with the dye entrapped, at the same time point. Another interesting observation was that liposomes were preferentially taken up by infected macrophages versus uninfected macrophages (data not shown), probably due to changes in phagocytic behavior after infection (29). From these observations, it can be inferred that PC-DDAB could serve as an effective carrier of SSG and might deliver the drug to intracellular amastigotes better than PC-SA liposomes.
Ultrastructural alterations induced by PC-DDAB-SSG.
Electron microscopic analysis demonstrated many alterations in the ultrastructure of promastigotes treated with PC-DDAB and PC-DDAB-SSG formulations. In contrast to untreated parasites (Fig. 3A), promastigotes treated with a 75% inhibitory concentration dose of PC-DDAB appeared with increased intracellular vacuolization (Fig. 3B). However, parasites treated with an equivalent dose of PC-DDAB with incorporated SSG became completely permeated, as manifested by a loss of electron-dense intracellular space and a loss of the shapes of cytoplasmic organelles (Fig. 3C). This effect could be caused by alterations in the physicochemical properties of the membrane system of the parasites, making them more susceptible to disruption. The ultrastructural alterations did not pinpoint any specific primary site of action of PC-DDAB-SSG on promastigotes, although it is likely that the parasite membranes are affected.
FIG 3.
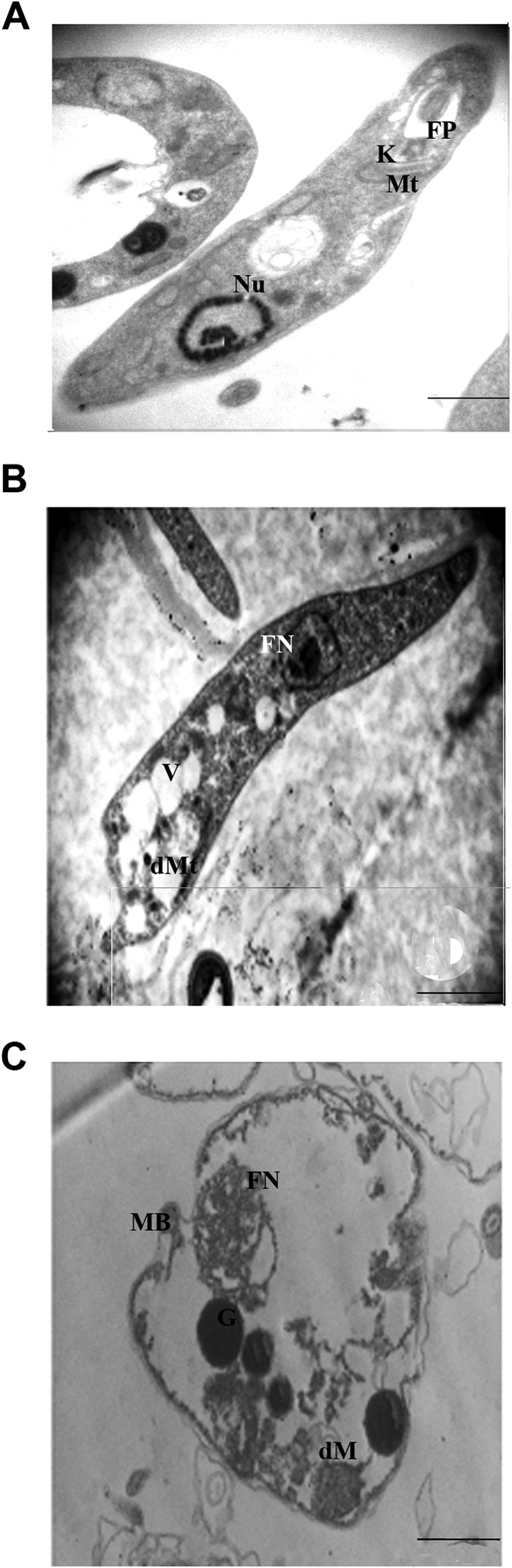
Electron microscopic assessment of L. donovani promastigotes treated with free and SSG-encapsulated PC-DDAB. (A) Control promastigotes demonstrate an elongated body and a normal aspect of the intracellular organelles. (B) The 75% effective dose (ED75) of PC-DDAB induced vacuolization and alterations at the plasma membrane. (C) Parasites treated with the ED75 of PC-DDAB incorporating SSG were spherical, with a complete absence of electron-dense materials. Bars, 2 μm (magnification, ×30,000) (A and B) or 1 μm (magnification, ×60,000) (C). Nu, nucleus; Mt, mitochondria; K, kinetoplast; FP, flagellar pocket; DMt, dilated mitochondria; FN, fragmented nucleus; V, vacuoles; dM, dilated matrix of mitochondria; G, granules; MB, membrane blebbing.
Toxicity assays.
Considering the profound antileishmanial activity of PC-DDAB, which was even better than that of PC-SA, we proceeded to perform toxicity screening tests that are routinely used in drug development programs to determine the safety of the drug. The cytotoxic effects toward murine macrophages and human erythrocytes were measured with LDH and red blood cell (RBC) lysis assays. All of these liposomes were nontoxic in the therapeutic range of doses (see Table S1 in the supplemental material). Further in vivo toxicity studies were carried out with the PC-DDAB liposomes by estimating serum ALP and SGPT levels (specific enzymes related to liver dysfunction) and serum urea and creatinine levels (related to kidney dysfunction) at 15 days after injection of therapeutic as well as higher doses of liposomes, which demonstrated levels within the normal ranges (data not shown), indicating no toxicity under the given conditions.
Combination therapy with suboptimal SSG doses in PC-DDAB liposomes showed enhanced antileishmanial efficacy.
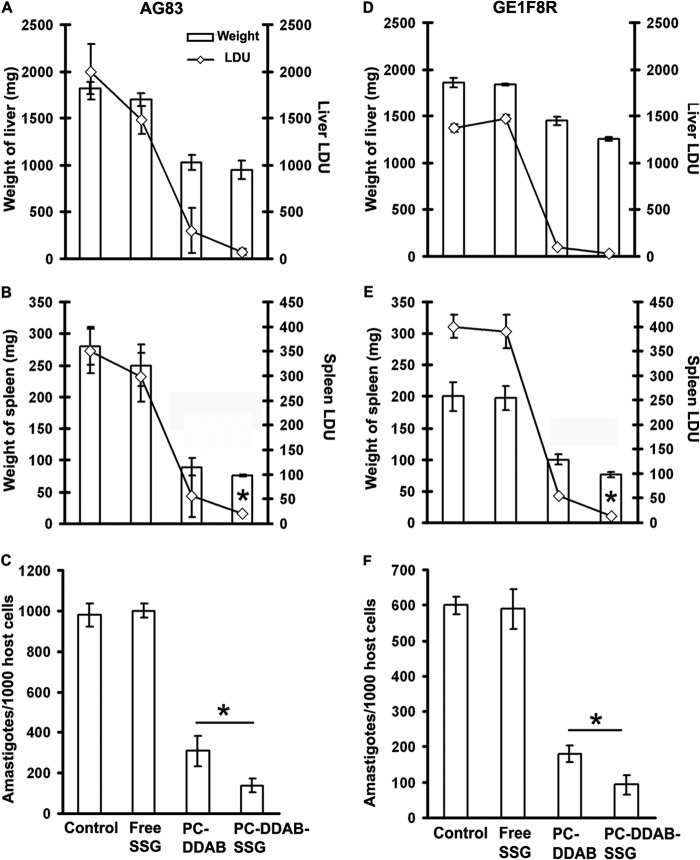
A single suboptimal dose of SSG (3 mg/kg), both in free form and entrapped in PC-DDAB liposomes (5 mg PC/mouse), and empty PC-DDAB (5 mg PC/mouse) were administered intravenously to 60-day-infected BALB/c mice. The animals were sacrificed 30 days posttreatment to analyze the therapeutic potentiality against both SSG-sensitive AG83 and SSG-resistant GE1F8R L. donovani infections (Fig. 4) (20). Free SSG could suppress AG83 infection by only 26% in the liver and 10% in the spleen, with almost no activity against parasites in the bone marrow. Lower levels of parasites were observed in the group treated with only PC-DDAB vesicles (85%, 82%, and 68.4% suppression of liver, spleen, and bone marrow parasite burdens, respectively; P < 0.05), in comparison to untreated infected controls. Of the three therapies, combined therapy with SSG within PC-DDAB resulted in maximal inhibition, with 96.4%, 99.6%, and 86% suppression of AG83 parasite burdens in liver, spleen, and bone marrow, respectively (Fig. 4A to C). More importantly, PC-DDAB-SSG formulations could also mount successful activity against SSG-resistant GE1F8R L. donovani parasites, suppressing parasite burdens in liver, spleen, and bone marrow by 97.9%, 96.5%, and 84.4%, respectively, whereas free SSG was completely unsuccessful (Fig. 4D to F). The decreases in spleen and bone marrow parasite burdens induced by the combination therapy were significantly greater (P < 0.05) than those seen with only vesicles against both AG83 and GE1F8R strains. In addition, PC-DDAB-SSG-cured mice exhibited significant decreases (P < 0.05) in the liver and spleen weights, compared with untreated infected mice (Fig. 4A to D). These data indicate that therapy with SSG in PC-DDAB vesicles could overcome SSG resistance and also the natural failure of BALB/c mice to contain parasites in the visceral organs, particularly in the spleen and bone marrow, organs that are generally refractory to a broad range of chemotherapeutic interventions.
FIG 4.
In vivo antileishmanial activity of free and SSG-encapsulated PC-DDAB liposomes. (A, B, D, and E) Therapeutic effects of drugs were analyzed as reductions in weight and Leishman-Donovan units (LDU) in liver (A and D) and spleen (B and E) of AG83-infected (A and B) and GE1F8R-infected (D and E) mice. (C and F) Bone marrow parasite loads of AG83 (C) and GE1F8R (F) were expressed as amastigotes per 1,000 bone marrow nuclei. Values are means ± standard errors (n = 5) of two independent experiments. *, P < 0.05 versus control for organ weight and versus PC-DDAB for LDU, by ANOVA.
Comparative antileishmanial activities of liposomal SSG formulations.
Recently we showed that PC-SA-SSG could cure established VL irrespective of SSG sensitivity (21). To assess better therapeutic options against established VL, a comparative study between PC-DDAB-SSG and previously reported PC-SA-SSG liposomal formulations was performed with an SSG-sensitive strain. The in vitro antileishmanial activities of graded doses of both cationic liposomal formulations were analyzed. We observed that PC-DDAB-SSG therapy was significantly more efficient than PC-SA-SSG treatment, with 8.8 μM lipids in PC-DDAB-SSG formulations suppressing 70% of the parasite burden, in contrast to 33% observed with PC-SA-SSG (P < 0.001) (Fig. 5A). The IC50 calculated for PC-DDAB-SSG was 6.3 μM lipid. A 2-fold higher dose of PC-SA-SSG could induce an equivalent effect.
FIG 5.
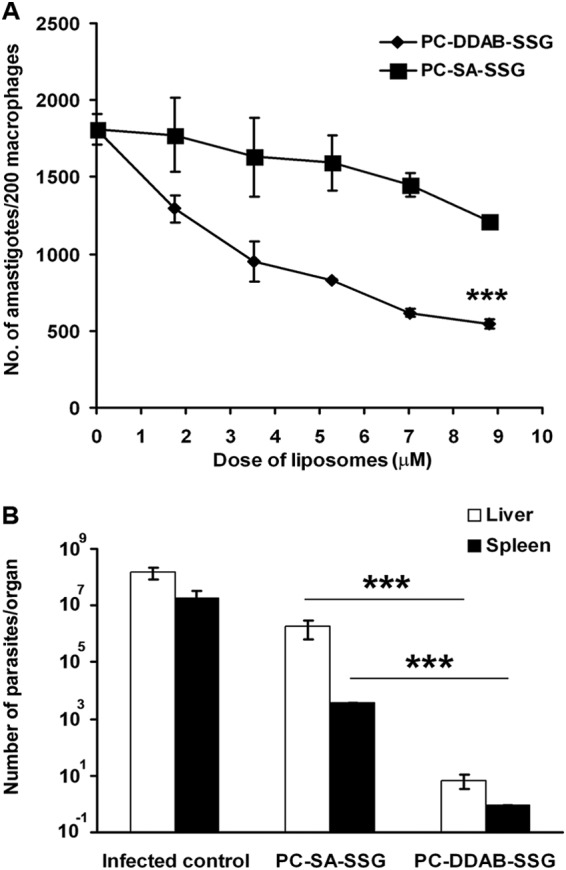
Comparative analysis of therapeutic efficacies of PC-SA-SSG versus PC-DDAB-SSG formulations against L. donovani AG83 infections. (A) In vitro leishmanicidal activity was documented as the number of amastigotes in 200 macrophages after 72 h of treatment. Data represent means of three independent experiments performed in triplicate. (B) Suppression of liver and spleen parasite burdens in 3-month-infected and treated BALB/c mice. Data represent means ± standard errors (n = 5) of total parasites per organ. ***, P < 0.001 by Student's t test (A) or P < 0.0001 by ANOVA (B).
To determine which cationic vesicles synergized better with SSG against in vivo infections with L. donovani, 60-day-infected BALB/c mice were subjected to treatment with PC-SA-SSG and PC-DDAB-SSG liposomal formulations and the levels of protection were evaluated 30 days posttreatment (Fig. 5B). Seventy-five micrograms of SSG within PC-DDAB vesicles at 5 mg PC/mouse significantly induced 105-fold and 104-fold greater suppression of liver and spleen parasitic burdens, respectively, compared to treatment with an equivalent dose of PC-SA-SSG (P < 0.0001), demonstrating that SSG encapsulated in PC-DDAB vesicles is more efficacious than PC-SA liposomes in suppressing liver and spleen infections with AG83 parasites.
Immunomodulatory activity of PC-DDAB-SSG.
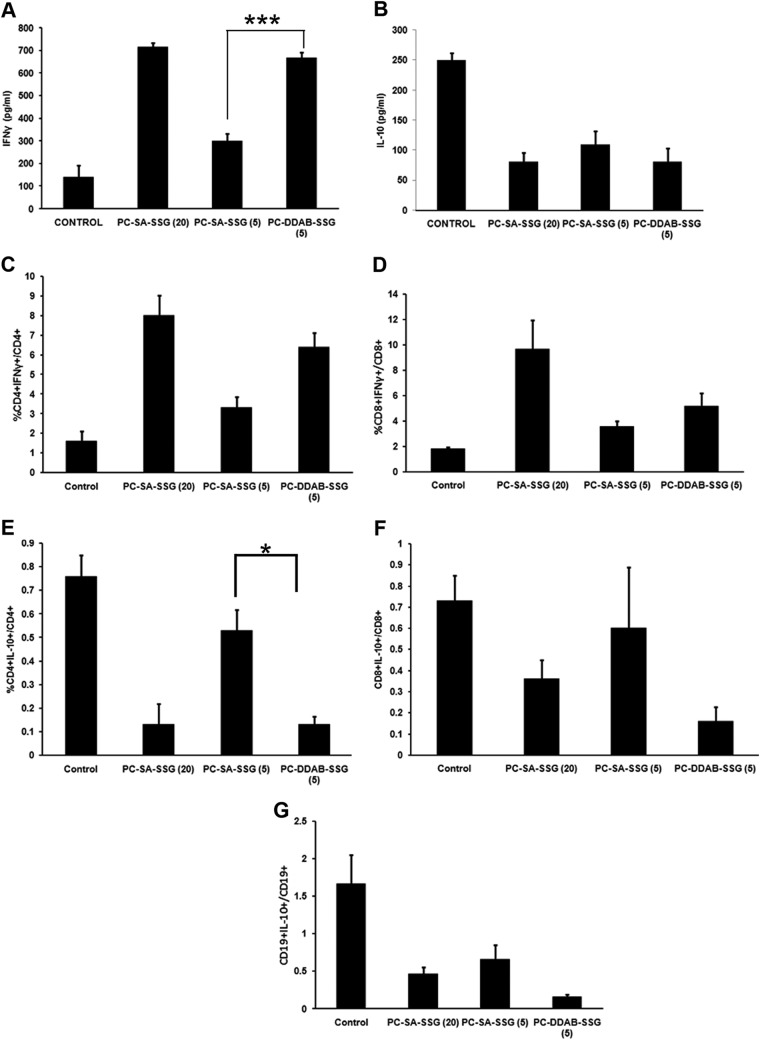
We reported previously that, in addition to direct killing of the parasites, PC-SA-SSG at a dose of 20 mg PC/mouse could effectively downregulate proparasitic IL-10 levels and induce IFN-γ production from splenocytes, providing a host immune status favorable for cure (21). To evaluate whether PC-DDAB-SSG at 5 mg PC/mouse could be as effective as PC-SA-SSG at a dose of 20 mg PC/mouse to bring about positive immunomodulation, we injected healthy BALB/c mice with SSG entrapped in PC-SA or PC-DDAB (5 mg PC/mouse) and compared the results with those obtained with our previously reported therapeutic dose, as mentioned above. All of the liposomal treatments showed significant decreases in IL-10 levels and upregulation of IFN-γ levels (P < 0.001, one-way ANOVA) (Fig. 6A and B). Interestingly, healthy mice treated with PC-DDAB-SSG at 5 mg PC/mouse demonstrated effective downregulation of IL-10 levels and concomitant upregulation of IFN-γ levels, which were comparable to the responses observed with a 4 times higher dose of PC-SA-SSG (P > 0.05). The IFN-γ response observed with PC-SA-SSG at 5 mg PC/mouse was comparatively weaker, and although IL-10 production was suppressed to an appreciable extent, the IFN-γ upsurge was significantly greater in the PC-DDAB-SSG-treated animals (P < 0.0001). Flow cytometric analysis revealed that IFN-γ production from CD4+ and CD8+ cells increased significantly (P < 0.05, one-way ANOVA) in all treated groups, with comparable IFN-γ production with PC-SA-SSG at 20 mg PC/mouse and PC-DDAB-SSG at 5 mg PC/mouse (Fig. 6C and D). Interleukin 10 production from CD4+ and CD8+ T cells and CD19+ B cells decreased in all treated mice, compared to untreated controls (Fig. 6E to G), with significant decreases in CD4+ and CD19+ cells (P < 0.05, one-way ANOVA). The decrease in IL-10 levels from CD4+ cells observed with PC-DDAB-SSG at 5 mg PC/mouse was again comparable to that observed with PC-SA-SSG at 20 mg PC/mouse. Additionally, the decreases in intracellular IL-10 levels from CD4+ T cells were significant for both PC-DDAB-SSG-treated and high-dose PC-SA-SSG-treated healthy mice, compared with low-dose PC-SA-SSG-treated mice (P < 0.05) (Fig. 6E). We also checked the ability of our liposomal therapy to induce delayed-type hypersensitivity (DTH) reactions in infected hosts posttreatment. PC-DDAB and PC-DDAB-SSG showed strong DTH reactions in response to leishmanial antigen, with PC-DDAB being the major contributor. The difference in footpad swelling between PC-DDAB and PC-DDAB-SSG was insignificant, however (data not shown).
FIG 6.
ELISA (A and B) and fluorescence-activated cell sorting analysis (C to G) of splenocytes from normal BALB/c mice treated with PC-SA-SSG or PC-DDAB-SSG. (A and B) Splenocytes were isolated from mice treated with PC-SA-SSG or PC-DDAB-SSG liposomes and were cultured for determination of IFN-γ (A) and IL-10 (B) levels. (C to G) Splenocytes treated with PC-SA-SSG or PC-DDAB-SSG were gated for CD4+ (C and E), CD8+ (D and F), and CD19+ (G) cells and permeabilized, and intracellular IFN-γ (C and D) and IL-10 (E, F, and G) levels were determined. Data represent means ± standard errors (n = 4). ***, P < 0.0001; *, P < 0.05.
DISCUSSION
We first compared the physicochemical properties of the liposomes. The tissue distributions and immune-potentiating properties of liposomes depend on their size (9, 30–32). Since all of the liposomes were of similar sizes, we could not draw any correlations between their sizes and efficacies. Therefore, we next checked whether any major differences existed in the charges of the liposomes, which may account for differences in antiparasitic activities. Inhibition of parasite growth was previously shown to be associated with the positive charge of the liposomes (18). Our attempt to correlate physical properties of the cationic liposomes with potent antileishmanial activity indicated that the presence of positive charge paralleled the optimal leishmanicidal efficiency. PC-DDAB liposomes showed the highest zeta potential and electrophoretic mobility, which correlated best with its antiparasitic activity, followed by PC-SA. We had previously studied the antileishmanial activity of PC-SA liposomes (21). Therefore, all of the liposomes were compared with respect to PC-SA for equivalent or better activity.
Among all of the vesicles tested, liposomes composed of double alkyl chains, i.e., DTAB and DDAB, showed significant antileishmanial activity like PC-SA against both promastigotes and intracellular amastigotes of L. donovani strains AG83 and GE1F8R. Promastigotes of the clinically isolated strain CK1R were almost equally susceptible, but interestingly the intracellular amastigotes displayed much higher levels of susceptibility to the liposomes, compared to GE1F8R. All bromide-containing cationic liposomes demonstrated potent activity against intracellular amastigotes, with PC-DDAB being the most effective. Unsaturated lipid DOTAP-containing liposomes failed to show effective killing of Leishmania promastigotes as well as amastigotes. Earlier reports showed CTAB to be a better antibacterial agent than DDAB, which may be due to the more bulky nature of DDAB (33). As Leishmania parasites are larger than bacteria and have a membrane structure that exposes negatively charged lipids on their surfaces, DDAB may be better suited for interactions with their membranes for subsequent killing. Cationic liposomes probably interact with macrophage surface receptors (34) and subsequently are endocytosed by them (35). Inside the macrophages, these cationic liposomes probably exert dual effects by interacting directly with the intracellular parasites and also activating macrophage microbicidal activity. Both PC-SA and PC-DDAB localized close to the amastigotes when observed microscopically, but PC-DDAB could more efficiently interact and elicit responses. The bulk of cationic charge on DDAB may contribute to this activity. Leishmania membranes consist of multiple polar and nonpolar lipids (36), which contribute to the surface charges. The difference in the susceptibility of promastigotes and amastigotes may be due to differences in the compositions of their plasma membranes (37). Freshly isolated splenic amastigotes were more susceptible to PC-DDAB treatment than PC-SA treatment, and treatment with annexin V reversed this activity, implicating a role of negatively charged membrane phosphatidylserine (PS), although contributions of other negatively charged components cannot be ruled out. PS is expressed on the surface of infective Leishmania, and PS exposure has been reported to correlate with infectivity (38, 39). Thus, anti-PS killing activity through cationic liposomes can provide an effective strategy to combat the disease.
In our earlier studies, both L. donovani strain GE1F8R, selected in the laboratory for SSG resistance, and strain CK1R, a resistant clinical isolate (IC50 values of >100 μg/ml for both strains), showed equivalent rates of death in response to PC-SA-SSG treatment (21). The strain-independent activity was due to increased retention of the drug inside the parasites. Although multiple mechanisms are involved in rendering the parasites unresponsive to pentavalent antimonials both in vitro and in clinical settings (40–43), the ultimate reason for nonaction of drugs is decreased uptake or poor retention in the parasite. Although we observed some differences in susceptibility of the two strains to cationic liposomes, PC-DDAB was found to be consistently efficacious toward the isolates. The resistance mechanism in the laboratory-developed GE1F8R strain was studied by Mookerjee Basu et al. (42). Infection with GE1F8R induces the upregulation of multidrug resistance protein 1 (MRP1) and permeability glycoprotein in host cells, resulting in nonaccumulation of intracellular SSG following treatment with SSG, favoring parasite replication (42). A role of the aquaglyceroporin (AQP) family of genes in the uptake of antimonials has also been reported for some L. donovani isolates (43). The studies indicate that, while downregulation of AQP1 may be one of the mechanisms of antimony resistance, it is not an invariable feature of such resistance, particularly in the case of GE1F8R (44). Although the resistance mechanisms of CK1R have not been studied in detail, the differences in expression levels of proteins responsible for influx and/or efflux of the drug may account for the difference in susceptibility observed by us (44). Interestingly, our liposomal formulation bypasses the strain-to-strain variations in susceptibility to SSG. The cationic liposomes cause depletion of cytosolic ATP (45), rendering the efflux pumps ineffective. Moreover, liposomes containing DDAB form a more rigid structure than SA in association with helper lipid PC, which allows longer retention and slow release of the drug for sustained activity (46).
Cationic liposomes have often been associated with cytotoxicity. The liposomes used here were potentially nontoxic at therapeutic doses, suggesting their suitability for further studies. Therefore, we proceeded to perform in vivo efficacy studies with free and drug-entrapped liposomes. The in vivo data indicated that therapy with 3 mg/kg body weight SSG in PC-DDAB vesicles could overcome the natural failure of BALB/c mice to contain parasites not only in the liver but also in the spleen and bone marrow, organs generally refractory to a broad range of chemotherapeutic interventions (4, 5). The antileishmanial activity results suggested synergistic activity of SSG in combination with PC-DDAB. To our knowledge, there has been no report of such profound antileishmanial activity with such a low dose of the drug encapsulated in liposomes, against SSG-sensitive and -resistant parasites. Successful therapy of VL is accompanied by antiparasitic immunity and prevention of relapse and reinfection. We report here that 75 μg of SSG in merely 5 mg PC/mouse of PC-DDAB could effectively downregulate proparasitic IL-10 levels, providing a favorable immune status in the host. Recent studies by Mukherjee et al. (47) show increased IL-10 secretion and resultant MDR1 upregulation in SSG-resistant clinical isolates. In light of this finding, we may propose that liposomal SSG reverses this situation in favor of the host. Antiparasitic IFN-γ secretion is the key to clearance of infection and establishment of host protection (48). Liposomes containing saturated lipids preferably induce Th1-type immune responses (49) and DDAB is a known IFN-γ inducer (50). High concentrations of this cytokine in mice treated with PC-DDAB-SSG strengthen our claim for its profound leishmanicidal activity. Previously we observed synergism between PC-SA and SSG for IL-10 suppression in vitro (21). In the present study, however, we compared the therapeutically active dose of PC-DDAB-SSG with an equivalent dose of PC-SA-SSG.
The antiparasitic activity of positively charged SA-bearing liposomes was well observed against Toxoplasma, Trypanosoma, and Leishmania parasites (18, 19, 51, 52), and this might be attributed to charge-based interactions of cationic liposomes with negatively charged membranes of parasites (45, 53, 54), followed by direct cytotoxic action. Vesicles formed of DDAB exhibited antibacterial activity against different species of bacteria (55, 56) and fungistatic activity against Candida albicans, which were attributed to surface charge interactions (56, 57). Charge-based interactions were reported for antimicrobial cationic peptides such as magainin 2, temporins, and CA(1-8)M(1-18) (58, 59), which interact with pathogen membranes, leading to degeneration of intracellular organelles, blurred definition of the boundaries of intracellular organelles, increases in electron-translucent intracellular space, and apparent expansion and disruption of membrane structures. Here, among other vesicles, we have screened DDAB vesicles for maximal efficacy against Leishmania promastigotes and amastigotes. Experiments with DDAB-containing liposomes have pointed toward nonionic and hydrophobic interactions with membrane lipids or the hydrophobic portions of membrane proteins (60), although some degree of ionic interactions with phosphate groups of phospholipids and other negatively charged components of the membrane cannot be ruled out. It is possible that the stable binding of DDAB liposomes to parasite membranes allows the drug to enter inside, adding to the cellular disruption observed in the electron microscopic experiments. Although there are reports that promastigotes are insensitive to pentavalent antimony, studies show that high concentrations of the drug make promastigotes sensitive (40, 61). Furthermore, DDAB has been established as a potent adjuvant, mounting strong cell-mediated immune responses and proinflammatory reactions (30, 50). The immunoadjuvant properties of DDAB have been attributed to longer availability of DDAB-bearing liposomes at the site of injection and recruitment of monocytes for the generation of effective immune responses (62). Herein, a strong Th1 immune response synergizes with the parasite killing to confer better therapeutic protection to the host. Although the immune reactions that follow PC-DDAB-SSG therapy remain to be studied, triggering a strong antiparasitic immune environment in healthy mice injected with the formulation resulted in favorable modulation of the host immune responses.
Various liposomal formulations of SSG have been reported to elicit significant leishmanicidal activity in vivo in chronically infected BALB/c mice. Although these formulations demonstrated spectacular success against liver parasites (4–6, 63), they failed to perform in spleen (6, 64). Our previous liposomal SSG formulation with cationic SA-bearing liposomes could successfully eliminate SSG-sensitive and SSG-resistant parasites from liver, spleen, and bone marrow. However, these activities were obtained with the dose of 20 mg PC/mouse. In the present study, we demonstrated that 75 μg of SSG in 5 mg PC/mouse of PC-DDAB was enough for eradication of parasites from the liver, spleen, and bone marrow of the animals. The dose of SSG that could elicit such potent curative responses in a single administration was only 3 mg/kg body weight, 1/100th of the dose of free drug that is optimal for treatment (64). Such reductions in liposome and drug dosages may be favorable for the host cells, preventing unnecessary toxicity and attenuating the chances of refractoriness to antimonial therapy, making PC-DDAB-SSG a prospective antileishmanial agent.
The results obtained strongly demonstrate the advantages of using PC-DDAB encapsulating a very low dose of SSG against L. donovani infections, irrespective of SSG sensitivity. The use of SSG has been discontinued in India due to the emergence of clinical resistance. Since our liposomal formulation works by bypassing the resistance-inducing mechanisms of the parasite, we propose that this kind of approach can be developed further to increase the clinical life of SSG and other drugs that are prone to resistance development.
The liposomal formulation of SSG prepared in PC-DDAB confers cures against SSG-sensitive and SSG-resistant L. donovani infections in BALB/c mice at a suboptimal dose of the drug not reported earlier. This therapeutic strategy involving efficient targeting and immunomodulation can be utilized for the development of safe, single-shot, therapeutic agents not only against Leishmania infections but also against other parasitic diseases and cancer, where drug resistance limits the use of various potent therapeutic agents.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by research grants from the Council of Scientific and Industrial Research (grant BSC0114), the Department of Science and Technology (grant SR/SO/BB-75/2007), and the Indian Council of Medical Research (grant 45/36/2006/PHA/BMS to P.P.), Government of India. R.S. is a Council of Scientific and Industrial Research Fellow.
We are grateful to Md Asad and Anushila Gangopadhyay for the flow cytometry work and to Diptadeep Sarkar for confocal microscopy studies. We thank Anirban Bhattacharya for help with manuscript preparation. The assistance of Janmenjoy Midya in the animal experiments is duly acknowledged.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03305-14.
REFERENCES
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M. 2012. The WHO Leishmaniasis Control Team, leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bern C, Adler-Moore J, Berenguer J, Boelaert M, den Boer M, Davidson RN, Figueras C, Gradoni L, Kafetzis DA, Ritmeijer K, Rosenthal E, Royce C, Russo R, Sundar S, Alvar J. 2006. Liposomal amphotericin B for the treatment of visceral leishmaniasis. Clin Infect Dis 43:917–924. doi: 10.1086/507530. [DOI] [PubMed] [Google Scholar]
- 3.Mishra J, Saxena A, Singh S. 2007. Chemotherapy of leishmaniasis: past, present and future. Curr Med Chem 14:1153–1169. doi: 10.2174/092986707780362862. [DOI] [PubMed] [Google Scholar]
- 4.New RR, Chance ML, Thomas SC, Peters W. 1978. Antileishmanial activity of antimonials entrapped in liposomes. Nature 272:55–56. doi: 10.1038/272055a0. [DOI] [PubMed] [Google Scholar]
- 5.Alving CR, Steck EA, Chapman WL Jr, Waits VB, Hendricks LD, Swartz GM Jr, Hanson WL. 1978. Therapy of leishmaniasis: superior efficacies of liposome-encapsulated drugs. Proc Natl Acad Sci U S A 75:2959–2963. doi: 10.1073/pnas.75.6.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter KC, Dolan TF, Alexander J, Baillie AJ, McColgan C. 1989. Visceral leishmaniasis: drug carrier system characteristics and the ability to clear parasites from the liver, spleen and bone marrow in Leishmania donovani infected BALB/c mice. J Pharm Pharmacol 41:87–91. doi: 10.1111/j.2042-7158.1989.tb06399.x. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Pal A, Vyas S. 2010. Drug delivery strategies for therapy of visceral leishmaniasis. Expert Opin Drug Deliv 7:371–402. doi: 10.1517/17425240903548232. [DOI] [PubMed] [Google Scholar]
- 8.Gregoriadis G. 1993. Liposome technology. CRC Press, Boca Raton, FL. [Google Scholar]
- 9.Brewer JM, Pollock KG, Tetley L, Russell DG. 2004. Vesicle size influences the trafficking, processing, and presentation of antigens in lipid vesicles. J Immunol 173:6143–6150. doi: 10.4049/jimmunol.173.10.6143. [DOI] [PubMed] [Google Scholar]
- 10.Nakanishi T, Kunisawa J, Hayashi A, Tsutsumi Y, Kubo K, Nakagawa S, Nakanishi M, Tnaka K, Mayumi T. 1999. Positively charged liposome functions as an efficient immunoadjuvant in inducing cell-mediated immune response to soluble proteins. J Control Release 61:233–240. doi: 10.1016/S0168-3659(99)00097-8. [DOI] [PubMed] [Google Scholar]
- 11.Frezard F. 1999. Liposomes: from biophysics to the design of peptide vaccines. Braz J Med Biol Res 32:181–189. [DOI] [PubMed] [Google Scholar]
- 12.Ignatius R, Mahnke K, Rivera M, Hong K, Isdell F, Steinman RM, Pope M, Stamatatos L. 2000. Presentation of proteins encapsulated in sterically stabilized liposomes by dendritic cells initiates CD8+ T-cell responses in vivo. Blood 96:3505–3513. [PubMed] [Google Scholar]
- 13.Henriksen-Lacey M, Christensen D, Bramwell VW, Lindenstrom T, Agger EM, Andersen P, Perrie Y. 2010. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J Control Release 145:102–108. doi: 10.1016/j.jconrel.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Schwendener RA, Lagocki PA, Rahman YE. 1984. The effects of charge and size on the interaction of unilamellar liposomes with macrophages. Biochim Biophys Acta 772:93–101. doi: 10.1016/0005-2736(84)90521-2. [DOI] [PubMed] [Google Scholar]
- 15.Mutsaers SE, Papadimitriou JM. 1988. Surface charge of macrophages and their interaction with charged particles. J Leukoc Biol 44:17–26. [DOI] [PubMed] [Google Scholar]
- 16.Aramaki Y, Akiyama K, Hara T, Tsuchiya S. 1995. Recognition of charged liposomes by rat peritoneal and splenic macrophages: effects of fibronectin on the uptake of charged liposomes. Eur J Pharm Sci 3:63–70. doi: 10.1016/0928-0987(94)00075-B. [DOI] [Google Scholar]
- 17.Moghimi SM, Patel HM. 2002. Modulation of murine liver macrophage clearance of liposomes by diethylstilbestrol: the effect of vesicle surface charge and a role for the complement receptor Mac-1 (CD11b/CD18) of newly recruited macrophages in liposome recognition. J Control Release 78:55–65. doi: 10.1016/S0168-3659(01)00481-3. [DOI] [PubMed] [Google Scholar]
- 18.Afrin F, Dey T, Anam K, Ali N. 2001. Leishmanicidal activity of stearylamine-bearing liposomes in vitro. J Parasitol 87:188–193. doi: 10.2307/3285199, doi:. [DOI] [PubMed] [Google Scholar]
- 19.Dey T, Anam K, Afrin F, Ali N. 2000. Antileishmanial activities of stearylamine-bearing liposomes. Antimicrob Agents Chemother 44:1739–1742. doi: 10.1128/AAC.44.6.1739-1742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal S, Ravindran R, Ali N. 2004. Combination therapy using sodium antimony gluconate in stearylamine-bearing liposomes against established and chronic Leishmania donovani infection in BALB/c mice. Antimicrob Agents Chemother 48:3591–3593. doi: 10.1128/AAC.48.9.3591-3593.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roychoudhury J, Sinha R, Ali N. 2011. Therapy with sodium stibogluconate in stearylamine-bearing liposomes confers cure against SSG-resistant Leishmania donovani in BALB/c mice. PLoS One 6:e17376. doi: 10.1371/journal.pone.0017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts WL, Rainey PM. 1993. Antimony quantification in Leishmania by electrothermal atomic absorption spectroscopy. Anal Biochem 211:1–6. doi: 10.1006/abio.1993.1223. [DOI] [PubMed] [Google Scholar]
- 23.Bhowmick S, Mazumdar T, Sinha R, Ali N. 2010. Comparison of liposome based antigen delivery systems for protection against Leishmania donovani. J Control Release 141:199–207. doi: 10.1016/j.jconrel.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Palit P, Hazra A, Maity A, Vijayan RS, Manoharan P, Banerjee S, Mondal NB, Ghoshal N, Ali N. 2012. Discovery of safe and orally effective 4-aminoquinaldine analogues as apoptotic inducers with activity against experimental visceral leishmaniasis. Antimicrob Agents Chemother 56:432–445. doi: 10.1128/AAC.00700-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakrabarti G, Basu A, Manna PP, Mahato SB, Mandal NB, Bandyopadhyay S. 1999. Indolylquinoline derivatives are cytotoxic to Leishmania donovani promastigotes and amastigotes in vitro and are effective in treating murine visceral leishmaniasis. J Antimicrob Chemother 43:359–366. doi: 10.1093/jac/43.3.359. [DOI] [PubMed] [Google Scholar]
- 26.Papo N, Shai Y. 2005. A molecular mechanism for lipopolysaccharide protection of Gram-negative bacteria from antimicrobial peptides. J Biol Chem 280:10378–10387. doi: 10.1074/jbc.M412865200. [DOI] [PubMed] [Google Scholar]
- 27.Bergmeyer HU, Bernt E. 1974. Methods of enzymatic analysis. Academic Press, New York, NY. [Google Scholar]
- 28.Banerjee A, De M, Ali N. 2008. Complete cure of experimental visceral leishmaniasis with amphotericin B in stearylamine-bearing cationic liposomes involves down-regulation of IL-10 and favorable T cell responses. J Immunol 181:1386–1398. doi: 10.4049/jimmunol.181.2.1386. [DOI] [PubMed] [Google Scholar]
- 29.Borborema SE, Schwendener RA, Osso JA Jr, de Andrade HF Jr, do Nascimento N. 2011. Uptake and antileishmanial activity of meglumine antimoniate-containing liposomes in Leishmania (Leishmania) major-infected macrophages. Int J Antimicrob Agents 38:341–347. doi: 10.1016/j.ijantimicag.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Henriksen-Lacey M, Devitt A, Perrie Y. 2011. The vesicle size of DDA:TDB liposomal adjuvants plays a role in the cell-mediated immune response but has no significant effect on antibody production. J Control Release 154:131–137. doi: 10.1016/j.jconrel.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Hsu MJ, Juliano RL. 1982. Interactions of liposomes with the reticuloendothelial system. II. Nonspecific and receptor-mediated uptake of liposomes by mouse peritoneal macrophages. Biochim Biophys Acta 720:411–419. [DOI] [PubMed] [Google Scholar]
- 32.Abra RM, Hunt CA. 1981. Liposome disposition in vivo. III. Dose and vesicle-size effects. Biochim Biophys Acta 666:493–503. [DOI] [PubMed] [Google Scholar]
- 33.Melo LD, Palombo RR, Petri DF, Bruns M, Pereira EM, Carmona-Ribeiro AM. 2011. Structure-activity relationship for quaternary ammonium compounds hybridized with poly(methyl methacrylate). ACS Appl Mater Interfaces 3:1933–1939. doi: 10.1021/am200150t. [DOI] [PubMed] [Google Scholar]
- 34.Altin JG. 2012. Liposomes and other nanoparticles as cancer vaccines and immunotherapeutics, p 135–178. In Baschieri S. (ed), Innovation in vaccinology: from design through to delivery and testing. Springer, New York, NY. [Google Scholar]
- 35.Das A, Ali N. 2014. Combining cationic liposomal delivery with MPL-TDM for cysteine protease cocktail vaccination against Leishmania donovani: evidence for antigen synergy and protection. PLoS Negl Trop Dis 8:e3091. doi: 10.1371/journal.pntd.0003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dueñas-Romero AM, Loiseau PM, Saint-Pierre-Chazalet M. 2007. Interaction of sitamaquine with membrane lipids of Leishmania donovani promastigotes. Biochim Biophys Acta 1768:246–252. doi: 10.1016/j.bbamem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Sadick MD, Raff HV. 1985. Differences in expression and exposure of promastigote and amastigote membrane molecules in Leishmania tropica. Infect Immun 47:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wanderley JL, Moreira ME, Benjamin A, Bonomo AC, Barcinski MA. 2006. Mimicry of apoptotic cells by exposing phosphatidylserine participates in the establishment of amastigotes of Leishmania (L) amazonensis in mammalian hosts. J Immunol 176:1834–1839. doi: 10.4049/jimmunol.176.3.1834. [DOI] [PubMed] [Google Scholar]
- 39.França-Costa J, Wanderley JL, Deolindo P, Zarattini JB, Costa J, Soong L, Barcinski MA, Barral A, Borges VM. 2012. Exposure of phosphatidylserine on Leishmania amazonensis isolates is associated with diffuse cutaneous leishmaniasis and parasite infectivity. PLoS One 7:e36595. doi: 10.1371/journal.pone.0036595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukherjee A, Padmanabhan PK, Singh S, Roy G, Girard I, Chatterjee M, Ouellette M, Madhubala R. 2007. Role of ABC transporter MRPA, γ-glutamylcysteine synthetase and ornithine decarboxylase in natural antimony-resistant isolates of Leishmania donovani. J Antimicrob Chemother 59:204–211. doi: 10.1093/jac/dkl494. [DOI] [PubMed] [Google Scholar]
- 41.Mandal G, Sarkar A, Saha P, Singh N, Sundar S, Chatterjee M. 2009. Functionality of drug efflux pumps in antimonial resistant Leishmania donovani field isolates. Indian J Biochem Biophys 46:86–92. [PubMed] [Google Scholar]
- 42.Mookerjee Basu J, Mookerjee A, Banerjee R, Saha M, Singh S, Naskar K, Tripathy G, Sinha PK, Pandey K, Sundar S, Bimal S, Das PK, Choudhuri SK, Roy S. 2008. Inhibition of ABC transporters abolishes antimony resistance in Leishmania infection. Antimicrob Agents Chemother 52:1080–1093. doi: 10.1128/AAC.01196-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandal S, Maharjan M, Singh S, Chatterjee M, Madhubala R. 2010. Assessing aquaglyceroporin gene status and expression profile in antimony-susceptible and -resistant clinical isolates of Leishmania donovani from India. J Antimicrob Chemother 65:496–507. doi: 10.1093/jac/dkp468. [DOI] [PubMed] [Google Scholar]
- 44.Maharjan M, Singh S, Chatterjee M, Madhubala R. 2008. Role of aquaglyceroporin (AQP1) gene and drug uptake in antimony-resistant clinical isolates of Leishmania donovani. Am J Trop Med Hyg 79:69–75. [PubMed] [Google Scholar]
- 45.Banerjee A, Roychoudhury J, Ali N. 2008. Stearylamine-bearing cationic liposomes kill Leishmania parasites through surface exposed negatively charged phosphatidylserine. J Antimicrob Chemother 61:103–110. doi: 10.1093/jac/dkm396. [DOI] [PubMed] [Google Scholar]
- 46.Angelini G, Chiarini M, De Maria P, Fontana A, Gasbarri C, Siani G, Velluto D. 2011. Characterization of cationic liposomes: influence of the bilayer composition on the kinetics of the liposome breakdown. Chem Phys Lipids 164:680–687. doi: 10.1016/j.chemphyslip.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee B, Mukhopadhyay R, Bannerjee B, Chowdhury S, Mukherjee S, Naskar K, Allam US, Chakravortty D, Sundar S, Dujardin JC, Roy S. 2013. Antimony-resistant but not antimony-sensitive Leishmania donovani up-regulates host IL-10 to overexpress multidrug-resistant protein 1. Proc Natl Acad Sci U S A 110:E575–E582. doi: 10.1073/pnas.1213839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selvapandiyan A, Dey R, Nylen S, Duncan R, Sacks D, Nakhasi HL. 2009. Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J Immunol 183:1813–1820. doi: 10.4049/jimmunol.0900276. [DOI] [PubMed] [Google Scholar]
- 49.Christensen D, Henriksen-Lacey M, Kamath AT, Lindenstrøm T, Korsholm KS, Christensen JP, Rochat AF, Lambert PH, Andersen P, Siegrist CA, Perrie Y, Agger EM. 2012. A cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct CD4 T cell-inducing capacity compared to its unsaturated analog. J Control Release 160:468–476. doi: 10.1016/j.jconrel.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Korsholm KS, Agger EM, Foged C, Christensen D, Dietrich J, Andersen CS, Geisler C, Andersen P. 2007. The adjuvant mechanism of cationic dimethyldioctadecylammonium liposomes. Immunology 121:216–226. doi: 10.1111/j.1365-2567.2007.02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tachibana H, Yoshihara E, Kaneda Y, Nakae T. 1988. In vitro lysis of the bloodstream forms of Trypanosoma brucei gambiense by stearylamine-bearing liposomes. Antimicrob Agents Chemother 32:966–970. doi: 10.1128/AAC.32.7.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tachibana H, Yoshihara E, Kaneda Y, Nakae T. 1990. Protection of Toxoplasma gondii-infected mice by stearylamine-bearing liposomes. J Parasitol 76:352–355. doi: 10.2307/3282665. [DOI] [PubMed] [Google Scholar]
- 53.Dwyer DM. 1977. Leishmania donovani: surface membrane carbohydrates of promastigotes. Exp Parasitol 41:341–358. [DOI] [PubMed] [Google Scholar]
- 54.McConville MJ, Ferguson MA. 1993. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J 294:305–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sicchierolli SM, Mamizuka EM, Carmona-Ribeiro AM. 1995. Bacteria flocculation and death by cationic vesicles. Langmuir 11:2991–2995. doi: 10.1021/la00008a024. [DOI] [Google Scholar]
- 56.Mamizuka EM, Carmona-Ribeiro AM. 2007. Cationic liposomes as antimicrobial agents, p 636–647. In Méndez-Vilas A. (ed), Communicating current research and educational topics and trends in applied microbiology. Formatex, Badajoz, Spain. [Google Scholar]
- 57.Vieira DB, Carmona-Ribeiro AM. 2008. Cationic nanoparticles for delivery of amphotericin B: preparation, characterization and activity in vitro. J Nanobiotechnology 6:6–18. doi: 10.1186/1477-3155-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diaz-Achirica P, Ubach J, Guinea A, Andreu D, Rivas L. 1998. The plasma membrane of Leishmania donovani promastigotes is the main target for CA(1-8)M(1-18), a synthetic cecropin A-melittin hybrid peptide. Biochem J 330:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mangoni ML, Saugar JM, Dellisanti M, Barra D, Simmaco M, Rivas L. 2005. Temporins, small antimicrobial peptides with leishmanicidal activity. J Biol Chem 280:984–990. doi: 10.1074/jbc.M410795200. [DOI] [PubMed] [Google Scholar]
- 60.Baechtel FS, Prager MD. 1982. Interaction of antigens with dimethyldioctadecylammonium bromide, a chemically defined biological response modifier. Cancer Res 42:4959–4963. [PubMed] [Google Scholar]
- 61.Croft SL, Sundar S, Fairlamb AH. 2006. Drug resistance in leishmaniasis. Clin Microbiol Rev 19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henriksen-Lacey M, Christensen D, Bramwell VW, Lindenstrøm T, Agger EM, Andersen P, Perrie Y. 2011. Comparison of the depot effect and immunogenicity of liposomes based on dimethyldioctadecylammonium (DDA), 3β-[N-(N′,N′-dimethylaminoethane)carbomyl]cholesterol (DC-Chol), and 1,2-dioleoyl-3-trimethylammonium propane (DOTAP): prolonged liposome retention mediates stronger Th1 responses. Mol Pharmacol 8:153–161. doi: 10.1021/mp100208f. [DOI] [PubMed] [Google Scholar]
- 63.Carter KC, Sundar S, Spickett C, Pereira OC, Mullen AB. 2003. The in vivo susceptibility of Leishmania donovani to sodium stibogluconate is drug specific and can be reversed by inhibiting glutathione biosynthesis. Antimicrob Agents Chemother 47:1529–1535. doi: 10.1128/AAC.47.5.1529-1535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carter KC, Baillie AJ, Alexander J, Dolan TF. 1988. The therapeutic effect of sodium stibogluconate in BALB/c mice infected with Leishmania donovani is organ-dependent. J Pharm Pharmacol 40:370–373. doi: 10.1111/j.2042-7158.1988.tb05271.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.