Abstract
The resistance of Plasmodium falciparum to some antimalarial drugs is linked to single-nucleotide polymorphisms (SNPs). Currently, there are no methods for the identification of resistant parasites that are sufficiently simple, cheap, and fast enough to be performed at point-of-care, i.e., in local hospitals where drugs are prescribed. Primer extension methods (PEXT) were developed to identify 4 SNPs in P. falciparum positioned at amino acids 86, 184, and 1246 of the P. falciparum multidrug resistance 1 gene (pfmdr1) and amino acid 76 of the chloroquine resistance transporter gene (pfcrt). The PEXT products were visualized by a nucleic acid lateral flow immunoassay (NALFIA) with carbon nanoparticles as the detection labels. PCR-PEXT-NALFIAs showed good correlation to the reference methods, quantitative PCR (qPCR) or direct amplicon sequence analysis, in an initial open-label evaluation with 17 field samples. The tests were further evaluated in a blind study design in a set of 150 patient isolates. High specificities of 98 to 100% were found for all 4 PCR-PEXT genotyping assays. The sensitivities ranged from 75% to 100% when all PEXT-positive tests were considered. A number of samples with a low parasite density were successfully characterized by the reference methods but failed to generate a result in the PCR-PEXT-NALFIA, particularly those samples with microscopy-negative subpatent infections. This proof-of principle study validates the use of PCR-PEXT-NALFIA for the detection of resistance-associated mutations in P. falciparum, particularly for microscopy-positive infections. Although it requires a standard thermal cycler, the procedure is cheap and rapid and thus a potentially valuable tool for point-of-care detection in developing countries.
INTRODUCTION
Malaria is a life-threatening disease caused by parasites that are transmitted to people through the bites of infected mosquitoes. According to the latest estimates released in December 2013, there were about 207 million cases of malaria in 2012 and an estimated 627,000 deaths worldwide (1). Of the six malaria species that naturally infect humans (Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale curtisi, P. ovale wallikeri, Plasmodium malariae, and Plasmodium knowlesi), infection by P. falciparum is seen most often in fatal cases (2, 3). Malaria is endemic in many tropical and subtropical areas in developing countries and continues to be a threat to travelers from regions that are not endemic for the disease (4). Furthermore, the emergence of drug-resistant parasites hampers the control of malaria worldwide. The P. falciparum multidrug resistance 1 gene (pfmdr1) and the P. falciparum chloroquine resistance transporter gene (pfcrt) played an important role in the development of chloroquine-resistant P. falciparum strains during the 20th century (5). Single-nucleotide polymorphisms (SNPs) in these genes are also now implicated in the reduced sensitivity of P. falciparum to artemisinin combination therapy (ACT) in Africa, as we recently showed in Kenya (6).
Fully effective drug administration would require accurate point-of-care diagnosis of P. falciparum resistant strains, as is common for bacterial infections, but this is not current practice. The genotyping of SNPs in P. falciparum has routinely been performed by PCR-restriction fragment length polymorphism (PCR-RFLP), PCR-sequence-specific oligonucleotide probe (PCR-SSOP), quantitative PCR (qPCR), and direct sequencing of PCR products, but none of these are currently appropriate for bedside use (6–9). Genotyping based on sequence-specific detection can be performed in 4 different allele-specific ways: hybridization, nucleotide incorporation, oligonucleotide ligation, and invasive cleavage (10, 11). Allele-specific nucleotide incorporation by means of primer extension (PEXT) is a robust approach based on the high accuracy of DNA polymerase to extend a primer only when its 3′ end perfectly matches the template. The addition of both the wild-type and the mutant primers in the PEXT reaction mixture enables the detection of wild-type and/or drug-resistant P. falciparum strains in a duplex PEXT reaction. PEXT reactions showed promising results in studies to detect SNPs in mammalian drug-metabolizing enzymes (12–15).
In most cases, the detection of various genotypes is done by linking light emission by luminescence, (time-resolved) fluorescence, fluorescence resonance energy transfer, or fluorescence polarization to specific product amplification (10, 11, 16). However, such detection systems are difficult to implement in developing countries, because they require expensive equipment and expertise. An alternative simple and cheap detection system is the nucleic acid lateral flow immunoassay (NALFIA) (17). The use of carbon nanoparticles to visualize amplification products in this lateral flow assay is particularly important for the sensitivity of the method (18–21). A combined multiplex PCR-NALFIA system was successfully tested in a field trial for the detection of different Plasmodium species (22).
This article describes the development and evaluation of PCR-PEXT-NALFIA protocols for the detection of the substitutions N86Y, Y184F, and D1246Y in the pfmdr1 gene and in the K76T substitution in the pfcrt gene. The approach described here utilized distinct 5′ tags for the wild-type and mutant PEXT products, which were captured on the corresponding immobilized antibody on the membrane of the NALFIA. To investigate and optimize the performances of the assays, a small set of 17 field samples was tested, and the results were compared to the results of qPCR. Finally, a blinded comparison between the PCR-PEXT-NALFIA and direct sequencing of conventional PCR products was carried out with a large set of 150 P. falciparum isolates from malaria patients.
MATERIALS AND METHODS
Reagents.
Washing buffer consisted of 5 mM borate buffer (pH 8.8) diluted from a mixture of 100 mM disodium tetraborate decahydrate (VWR, Leuven, Belgium), 100 mM boric acid (Merck, Darmstadt, Germany), and bovine serum albumin (BSA) (Sigma-Aldrich Chemie BV, Zwijndrecht, The Netherlands) to a final concentration of 1% (wt/vol). Storage buffer was prepared by adding BSA to 100 mM borate buffer to a final concentration of 1% (wt/vol). NaN3 (0.02% [wt/vol]) (Merck) was added to washing buffer and storage buffer. Running buffer was prepared by adding BSA and Tween 20 (Merck) to 100 mM borate buffer to final concentrations of 1% (wt/vol) and 0.05% (vol/vol), respectively.
Polyclonal antibodies against digoxigenin (Dig) from goat (Abcam, Cambridge, United Kingdom), dinitrophenyl-KLH (DNP) from rabbit (Molecular Probes, Inc., Eugene, OR, USA), Texas Red from rabbit (Molecular Probes, Inc.), fluorescein isothiocyanate (FITC) from rabbit (AbD Serotec, Düsseldorf, Germany), and Alexa Fluor 488 (Molecular Probes, Inc.) were used. A monoclonal antibody against Cy5 from mouse (Sigma-Aldrich Chemie BV) was used.
P. falciparum strains and DNA isolation.
The malaria patients contributing blood samples to this study were selected from participants in a clinical trial conducted by the Multi-Drug Resistance in Malaria under Combination Therapy (MALACTRES) project in Mbita, Kenya, in 2009, all of whom provided written informed consent (6), or from malaria-infected travelers diagnosed in the United Kingdom for whom an anonymized DNA sample was available in the United Kingdom Malaria Reference Laboratory (MRL) archive. In both cases, P. falciparum genomic DNA was isolated from peripheral blood samples of malaria patients using the Qiagen DNA kit (Hilden, Germany), according to the manufacturer's instructions.
Genotyping reference methods.
The genotyping of pfcrt by qPCR and pfmdr1 by direct sequencing of PCR products was performed according to standard operating procedures in the United Kingdom MRL, based on previously published research protocols (6, 9, 23, 24).
PCR.
For the PCR-PEXT-NALFIAs, DNA fragments of pfcrt and pfmdr1 containing the SNPs of interest were amplified by PCR. The PCR mixture consisted of 1× PCR buffer [50 mM Tris-HCl, 10 mM KCl, 5 mM (NH4)2SO4 (pH 8.3); Roche Diagnostics GmbH, Mannheim, Germany], 4 mM MgCl2 (Roche Diagnostics GmbH), 100 μM dinucleoside triphosphates (dNTPs) (Thermo Fisher Scientific, Vantaa, Finland), 200 nM forward primer, 200 nM reverse primer, 1 U of FastStart Taq DNA polymerase (Roche Diagnostics GmbH), and 2 μl of purified DNA template, filled to a total volume of 25 μl with mQ nuclease-free water (Invitrogen Ltd., Paisley, United Kingdom). The sequences of the forward and the reverse primers (Eurogentec, Seraing, Belgium) are described in Table 1. The PCR program, comprising 1 cycle of 10 min at 95°C, 40 cycles of 30 s at 95°C, 30 s at 56°C, and 1 min at 72°C, and 1 cycle of 7 min at 72°C, was applied in the Veriti 96-well thermal cycler (Applied Biosystems, Foster City, CA, USA).
TABLE 1.
Sequences of forward and reverse primers as used in the PCR step and the expected fragment sizes
| Primera | Sequence (5′ to 3′) | Amplicon size (bp) |
|---|---|---|
| Pfmdr1-FW1 | ACA-AAA-AGA-GTA-CCG-CTG-AAT | 534 |
| Pfmdr1-RV1 | AAA-CGC-AAG-TAA-TAC-ATA-AAG-TC | |
| Pfmdr1-FW4 | TCG-TTG-GAG-AAA-CAG-GTA-GTG | 359 |
| Pfmdr1-RV4 | CAA-TGT-TGC-ATC-TTC-TCT-TCC | |
| Pfcrt-FW | ACG-AGC-GTT-ATA-GAG-AAT-TAG | 422 |
| Pfcrt-RV | GTT-GTG-AGT-TTC-GGA-TGT-TAC |
FW, forward primer; RV, reverse primer.
Primer extension reaction.
The mutation-specific primers for the primer extension (PEXT) reaction are listed in Table 2. The wild-type and mutant primers differ by the 3′-terminal nucleotide, which anneals to the polymorphic residue within each of the amplicons, as the polymerase in the PEXT reaction is unable to continue DNA strand synthesis beyond a single 3′ mismatch. The wild-type and mutant PEXT primers were each 5′-end labeled to discriminate the two products.
TABLE 2.
Labeled primer sequences in the PEXT reactionsa
| Primer | Sequence (5′ to 3′) | 5′ tagb |
|---|---|---|
| Pfmdr1-86N | GGA-TTA-ATA-TCA-TCA-CCT-AAA-TT | Digoxigenin |
| Pfmdr1-86Y | GGA-TTA-ATA-TCA-TCA-CCT-AAA-TA | TEG-dinitrophenol |
| Pfmdr1-184Y | AAA-AAA-AAA-AGC-ATT-TTT-TAT-TAA-TGA-CCA-AAT-AT | Texas Red |
| Pfmdr1-184F | AAA-AAA-AAA-AGC-ATT-TTT-TAT-TAA-TGA-CCA-AAT-AA | 6-FAM |
| Pfmdr1-1246D | AAA-AAA-AAA-AAA-AAA-AAC-TAT-TGA-AAA-TAA-GTT-TCT-AAG-ATC | Cy5 |
| Pfmdr1-1246Y | AAA-AAA-AAA-AAA-AAA-AAC-TAT-TGA-AAA-TAA-GTT-TCT-AAG-ATA | Alexa Fluor 488 |
| Pfcrt-76K | AAA-AAA-AAA-AAA-AAA-AAT-TTT-GTT-TAA-AGT-TCT-TTT-AGC-AAA-AAT-TT | Digoxigenin |
| Pfcrt-76T | AAA-AAA-AAA-AAA-AAA-AAT-TTT-GTT-TAA-AGT-TCT-TTT-AGC-AAA-AAT-TG | TEG-dinitrophenol |
Different 5′ tags were used for the detection of each genotype in a codon to discriminate the wild-type and the mutant genotype on the NALFIA strip.
TEG, triethylene glycol; FAM, 6-carboxyfluorescein.
For the genotyping of codons 86 and 1246 in the pfmdr1 gene, the PEXT reaction mixture consisted of 1× ThermoPol reaction buffer [20 mM Tris-HCl, 10 mM (NH4)2SO4, 10 mM KCl, 2 mM MgSO4, 0.1% Triton X-100 (pH 8.8); New England BioLabs, Inc., Ipswich, MA], 1 μM PEXT wild-type primer, 1 μM PEXT mutant primer, 12.5 μM dATP (Fermentas, St. Leon-Rot, Germany), 12.5 μM dCTP (Fermentas), 12.5 μM dGTP (Fermentas), 6.25 μM dTTP (Fermentas), 6.25 μM biotin-16-dUTP (Roche Diagnostics GmbH), 0.5 U of Vent (exo-) DNA polymerase (New England BioLabs), and 2 μl of amplicons of the first PCR, filled to 10 μl with mQ water (Invitrogen Ltd.). For genotyping of codon 76 in the pfcrt gene, the PEXT reaction mixture was the same as described above, but 1 μM Pfcrt-FW primer was added to the reaction mixture. The genotyping of codon 184 in the pfmdr1 gene was performed as described above, but the concentrations of both PEXT wild-type primer and PEXT mutant primer were 0.25 μM. The sequences of the PEXT wild-type primers and the PEXT mutant primers (Eurogentec) are described in Table 2. For optimal signal intensities, a 5′ spacer of 10 adenosine nucleotides was added to the PEXT primers amplifying codon 184 in the pfmdr1 gene, and a 5′ spacer of 15 adenosine nucleotides was added to the PEXT primers amplifying codon 1246 in the pfmdr1 gene and codon 76 in the pfcrt gene.
The PEXT reactions were applied in the Veriti 96-well thermal cycler (Applied Biosystems). The PEXT program included the following steps: 1 cycle of 5 min at 95°C and 10 (part of the small sample set) or 15 (the other part of the small and the whole of the large sample set) cycles comprising 15 s at 95°C, 15 s at 58°C, and 15 s at 72°C.
Carbon conjugate.
A 1% (wt/vol) Spezial Schwarz 4 carbon solution (Degussa, Frankfurt, Germany) was prepared in mQ water. This solution was sonicated for 5 min in a Bioruptor sonicator (Diagenode SA, Liège, Belgium). The carbon solution was diluted 5× in coupling buffer. The resulting 0.2% (wt/vol) carbon solution was sonicated again for 5 min. Next, 350 μg of NeutrAvidin (Pierce Biotechnology, Rockford, IL, USA) was added to 1 ml of a 0.2% (wt/vol) carbon solution. The solution was stirred overnight at 4°C. After coupling, the carbon solution was divided in two Eppendorf tubes. In each tube, 500 μl of washing buffer was added. The tubes were centrifuged at 13,636 × g for 15 min at 4°C. Each pellet in the tube was resuspended in 0.5 ml of washing buffer, and the resuspended pellets were each washed 3 times with 0.5 ml of washing buffer. After the last washing step, each pellet was resuspended in 0.5 ml of storage buffer. The two conjugate solutions were pooled and stored at 4°C until use.
Preparation of NALFIA strips.
HiFlow Plus HFB13502 nitrocellulose membrane (Millipore Ireland, Tullagreen, Carrigtwohill, Co. Cork, Ireland) was adhered to a backing (G & L, San Jose, CA, USA), and absorbent pads (Schleicher & Schuell, Dassel, Germany) were adhered to the top of the membranes, overlapping the membranes by 2 mm.
The membranes were sprayed using a Linomat 4 (Camag, Berlin, Germany) sprayer. The amount of antibody sprayed on a membrane was 0.8 μl/cm. For the genotyping of each SNP, a two-line NALFIA strip was prepared by spraying the first antibody at 1.2 cm from the bottom of the membrane and the second antibody at 1.5 cm from the bottom. The first antibody detected the mutant PEXT products, and the second antibody detected the wild-type PEXT products. In Table 3, the antibodies sprayed on the membranes are depicted. The antibodies were diluted in spraying buffer, and in the case of the Cy5 antibody, sucrose (Merck, Darmstadt, Germany) was added to a final concentration of 2% (wt/vol).
TABLE 3.
Overview of the antibodies sprayed on the membranes and the concentrations used
| PEXT product | Antibody againsta: | Concentration (ng/μl) |
|---|---|---|
| pfmdr1-86Asn 200 | Dig | 200 |
| pfmdr1-86Tyr 400 | DNP | 400 |
| pfmdr1-184Tyr 400 | Texas Red | 400 |
| pfmdr1-184Phe 50 | FITC | 50 |
| pfmdr1-1246Asp 750 | Cy5 | 750 |
| pfmdr1-1246Tyr 200 | Alexa Fluor 488 | 200 |
| pfcrt-76Lys 200 | Dig | 200 |
| pfcrt-76Thr 400 | DNP | 400 |
Dig, digoxigenin; DNP, 2,4-dinitrophenol; FITC, fluorescein isothiocyanate.
The sprayed membranes were incubated overnight at 37°C, and then the membranes were cut into 5-mm wide NALFIA strips using a BioDot CM4000 cutter (Irvine, CA, USA). The strips were packaged in an aluminum pouch (Nefab, Barneveld, The Netherlands) that contained a silica desiccant (Multisorb Technologies, Inc., NY, USA), and they were sealed and stored at room temperature until use.
NALFIA.
The NALFIA reactions for genotyping were performed by adding 70 μl of running buffer, 1 (small sample set) or 2 μl (large sample set) of carbon NeutrAvidin conjugate, and 2 μl of PEXT sample in a well of a Costar low-binding microtiter plate (Corning Incorporated, NY, USA). The NALFIA strips were placed in the wells at room temperature (RT) and scanned by a GS-800 calibrated densitometer (Bio-Rad, Veenendaal, The Netherlands) after 30 min.
RESULTS
Optimization of the PEXT reaction mixture and PEXT program for the individual detection of each SNP.
The influence of the length of A nucleotide spacers was investigated in the pfmdr1 codon 1246 detection. The lengths of 0, 3, 8, and 15 A nucleotides between the 5′ tag and the primer sequence were compared. The highest intensities of the signals were found with a 15-A nucleotide spacer (data not shown). This 15-A nucleotide spacer was also used in the PEXT primers, which detected codon 76 in the pfcrt gene. However, the use of a 15-A nucleotide spacer containing PEXT primers in the genotyping of codons 86 and 184 in the pfmdr1 gene resulted in some background signals. Optimal results were obtained without an A spacer for the genotyping of codon 86 and with a 10-A nucleotide spacer for the genotyping of codon 184.
Following the first PCR, 2 μl of amplified product was added directly to the PEXT reaction mixture. In the PEXT reaction, a primer extension product was formed by annealing of the PEXT primer, followed by amplification. However, agarose gel electrophoresis showed that in addition to this PEXT product, a seminested-PCR product was also formed due to the PCR forward primer from the first PCR and the PEXT primer of the second PEXT reaction (data not shown). The influence of adding an additional PCR forward primer to the PEXT reaction mixture was investigated. In the case of genotyping codon 76 in the pfcrt gene, improved NALFIA signals were found when 1 μM additional PCR forward primer (Pfcrt-FW) was added in the PEXT reaction. However, an elevated PCR forward primer concentration in the genotyping of codons 86, 184, and 1246 in pfmdr1 did not result in increased NALFIA signals and, in some tests, resulted in higher background signals.
The concentrations of the PEXT primers and the number of PEXT cycles were also optimized. The best concentrations of the PEXT primers for codon 184 in the pfmdr1 gene were 0.25 μM, and 1 μM was best for the other codons. The optimal number of PEXT cycles was empirically determined to be 10 for the genotyping of codon 86 in the pfmdr1 gene and codon 76 in the pfcrt gene and 15 for the genotyping of the other SNPs. These conditions were used for the small sample set. To harmonize the procedure, 15 PEXT cycles were used for genotyping the samples in the large sample set.
Genotyping of codons 86, 184, and 1246 in the pfmdr1 gene and codon 76 in the pfcrt gene in a small sample set of 17 field samples.
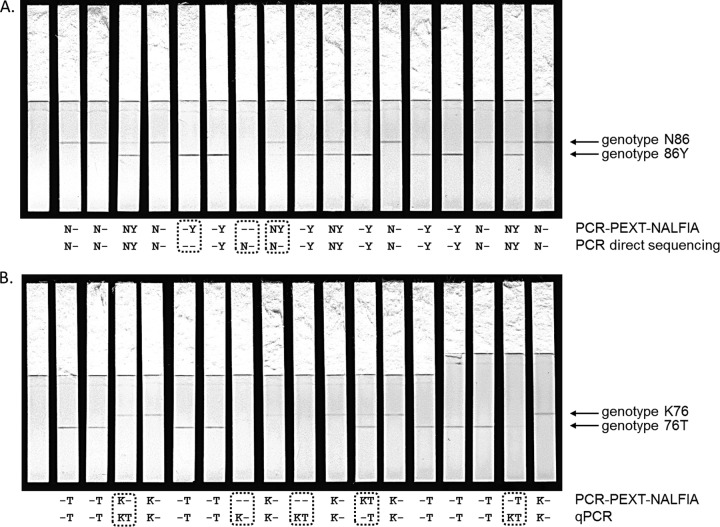
Pilot experiments indicated that the performance of the PCR-PEXT-NALFIA was substantially better with Qiagen-purified DNA than with Chelex-extracted DNA from dried filter paper blood spots (data not shown). Therefore, Qiagen-purified DNA from 17 field samples collected as part of the MALACTRES study in Mbita, Kenya, was used for the initial testing of the PCR-PEXT-NALFIA protocols for the genotyping of codons 86, 184, and 1246 in the pfmdr1 gene and codon 76 in the pfcrt gene (6). The samples were tested in a blind fashion, and the results were compared to those of the reference methods qPCR (pfcrt) and direct sequencing (pfmdr1). After running the NALFIA strips for 30 min, the presence or absence of the signals was read for each. As examples, Fig. 1 presents images of the NALFIA strips for the pfmdr1 codon 86 (pfmdr1-86) (Fig. 1A) and pfcrt-76 (Fig. 1B) PCR-PEXT reactions, taken after 30 min of flow time. Although some bands appear faint in the images presented, the contrast was good in the physical test, and the discrimination of faint signals was easily achieved. Good agreement was found between the NALFIA results and those from previous genotyping efforts. The scores of the two methods were identical in the genotyping of pfmdr1-1246D. The genotyping of pfmdr1-86N resulted in the same scores for 16 samples, and a false-negative score was found for 1 sample. Two samples resulted in different scores for pfmdr1-86Y, pfmdr1-184Y, pfmdr1-184F, and pfcrt-76T. The scores differed for 3 samples in the genotyping of pfmdr1-1246Y and for 4 samples in the genotyping of pfcrt-76K. In each case, these discrepancies occurred in isolates for which a mixed genotype was scored by one of the two methods.
FIG 1.
Genotyping of codon 86 in the pfmdr1 gene (A) and of codon 76 in the pfcrt gene (B) from 17 DNA samples. One blank containing no DNA template in the first PCR was included (first strip). The upper test line detected genotype N86, and the lower test line genotype 86Y, or K76 and 76T, respectively. The strips were scored after a 30-min run of the PEXT samples and compared to PCR direct sequencing (A) and qPCR (B) (below each line). Discordant results are indicated by a dashed box.
Genotyping of codons 86, 184, and 1246 in the pfmdr1 gene and codon 76 in the pfcrt gene in a large sample set of 150 clinical samples.
The optimized SNP detection protocols were further evaluated in a blinded test with a set of 150 DNA samples prepared at the United Kingdom MRL. These comprised four water-only no-template negative-control samples and 146 DNA extracts from peripheral blood samples from malaria patients in the United Kingdom. Of these, 48 had high parasite densities, with >2% of peripheral erythrocytes infected, 92 were described as having low parasitemia, and the remaining six were confirmed to be parasite negative by nested PCR. Blood film results were available for 140 patients, of which 87 had visible P. falciparum parasitemia on examination of Giemsa-stained fixed slides. Thus, a substantial number of the samples used were low-density subpatent infections undetected by microscopy, which had been confirmed to be parasite positive by PCR. These reflected the role of the MRL in providing an expert diagnostic service for routine and difficult cases of suspected malaria in the United Kingdom, and they fell into 4 categories. Six blood samples were confirmed to be parasite negative by qPCR, 47 were confirmed P. falciparum positive by qPCR but negative by microscopy (i.e., submicroscopic parasitemia), and 87 had visible P. falciparum parasitemia on examination of Giemsa-stained fixed slides. Of these, 48 blood samples harbored high parasite densities, such that ≥2% of the microscopically observed erythrocytes were infected by P. falciparum. The project staff members in Wageningen were blinded as to the parasite infection status and parasite densities of all samples.
The NALFIA strips were scored after 30 min, and the results were compared to the results achieved with qPCR (pfcrt) or direct sequencing of PCR products (pfmdr1). Table 4 presents estimates of the diagnostic accuracy of the PEXT method for each marker. In general, the newly developed PCR-PEXT-NALFIAs show more negative scores than those from qPCR or sequencing, suggesting the two gold standard methods have a lower limit of detection. As shown in Table 4, the sensitivities for three allele-specific tests (pfcrt-76T, pfmdr1-184F, and pfmdr1-1246Y) fell to <90%. Nevertheless, high specificity (0.98 to 1.00) was achieved for all genotyping assays, suggesting that all tests were highly accurate but did not identify all alleles present when the parasite densities were low.
TABLE 4.
Diagnostic accuracy of PEXT genotyping assays at four polymorphic positions in two genes associated with antimalarial drug resistance in P. falciparum
| Gene | Codon | Allele | Sensitivity |
Specificity |
||
|---|---|---|---|---|---|---|
| % (95% CI)a | Proportionb | % (95% CI) | Proportionc | |||
| pfcrt | 76 | Lys (K) | 100 (91.8–100.0) | 43/43 | 100 (88.4–100.0) | 30/30 |
| Thr (T) | 82.0 (66.5–92.5) | 32/39 | 100 (89.7–100.0) | 34/34 | ||
| pfmdr1 | 86 | Asn (N) | 97.9 (92.6–99.7) | 93/95 | 100 (79.4–100.0) | 16/16 |
| Tyr (Y) | 92.9 (76.5–99.1) | 26/28 | 100 (95.7–100.0) | 83/83 | ||
| 184 | Tyr (Y) | 100 (94.1–100.0) | 61/61 | 97.9 (88.9–99.9) | 47/48 | |
| Phe (F) | 86.6 (76.0–93.7) | 58/67 | 100 (91.6–100.0) | 42/42 | ||
| 1246 | Asp (D) | 100 (96.1–100.0) | 93/93 | 100 (40–100) | 4/4 | |
| Tyr (Y) | 75 (35–97) | 6/8 | 100 (95.9–100.0) | 89/89 | ||
CI, confidence interval.
Data are the number of samples with positive results concordant with results of the gold standard/total number of samples positive by the gold standard.
Data are number of samples with negative results concordant with results of the gold standard/total number of samples negative by the gold standard.
To explore the impact of peripheral blood parasite density on the performance of the PCR-PEXT-NALFIA, clinical and diagnostic information was analyzed from each patient for which the PEXT results did not agree with those of the gold standard tests. Seven patient DNA samples gave discrepant results for the pfcrt-76T test; in each case, the established qPCR assay identified the presence of both the K and T alleles, but the PEXT assay identified the K allele only. Only one of these seven individuals had a negative blood film. This suggests that the performance of the 76T test requires improvement to increase sensitivity. In 43 DNA samples, the PCR-PEXT-NALFIA did not detect any pfcrt alleles, despite successful genotyping with the qPCR method. All 43 of these samples came from patients noted as having “low parasitemia” in the MRL database. Microscopy results were available for 42 of the 43 patients; 29 of these were negative by blood film and thus were submicroscopic infections. In contrast, of 65 samples with a blood film available and showing perfect agreement between the PEXT and qPCR results for pfcrt, only 4.6% had a negative blood film. Therefore, samples in which PEXT failed to detect pfcrt alleles that were detected by the gold standard method were significantly more likely to come from a patient with a negative blood film, and thus subpatent P. falciparum infection (odds ratio [OR], 33.6; 95% confidence interval [CI], 8.37 to 186; P < 0.0001).
Much better agreement was seen for the pfmdr1 alleles, as the sensitivity of the PCR-PEXT-NALFIA was closer to that of the gold standard method (direct sequencing of nested-PCR products). For example, the PEXT result for the 86N allele agreed with that of sequencing for 109 samples; 24% of these were reported to have a negative blood film compared to 88.9% of the 18 samples in which the PEXT method failed to detect an allele successfully identified by sequencing (OR, 25.3; 95% CI, 5.22 to 234; P < 0.0001).
DISCUSSION
A procedure was developed for the detection of four SNPs in P. falciparum strains, which consisted of a PCR step, a primer extension (PEXT) step, and a lateral flow assay to detect specific amplicons (PCR-PEXT-NALFIA method). These polymorphisms, located in the codons 86, 184, and 1246 of the pfmdr1 gene and codon 76 of the pfcrt gene, have all been implicated in parasite drug responses to antimalarial drugs in vivo (8, 9, 23, 24).
Before comparing the results with those of the reference methods, the PCR-PEXT-NALFIA methods were first optimized. The optimal amounts of the components in the PEXT reaction mixture and the parameters in the PEXT program were dependent on the primer sequences. The choice of primer sequences was very limited due to the fixed positions of the SNPs. This partly explains the differences in the optimal conditions of the PEXT reactions between the 4 genotyping methods, such as the concentrations of PEXT primers, the lengths of A nucleotide spacers in the PEXT primers, and the addition of an extra PCR forward primer.
The acquired NALFIA signals might result from two kinds of products formed in the PEXT reaction: (i) a seminested-PCR product formed by the PEXT primer, the PCR forward primer, and biotinylated UTP nucleotides, and (ii) a primer extension product formed by the PEXT primer and biotinylated UTP nucleotides only. During the optimization experiments of the genotyping of pfcrt gene codon 76, increased NALFIA signals were found upon the addition of an extra PCR forward primer to the PEXT reaction mixture. However, the addition of PCR forward primers to the PEXT reaction mixtures of the other SNP genotyping assays did not result in better outcomes.
In a small set of 17 field samples, the newly developed PCR-PEXT-NALFIA methods showed very similar scores to those of qPCR (pfcrt-76) and direct sequencing (pfmdr1-86, pfmdr1-184, and pfmdr1-1246). Subsequently, the SNP detection methods were evaluated in a large sample set of 150 clinical samples. For all 4 genotyping assays, high specificities of 98% to 100% were found (Table 4). Sensitivities of 75% to 100% were found for almost all genotyping assays for which a PEXT result was obtained. However, a number of samples with low parasite density, as indicated by negative blood films, gave no result with the PEXT assay. It is concluded that the methodology provides a high level of diagnostic accuracy for DNA samples from patients with microscopy-positive P. falciparum infections and thus can inform treatment decisions in real time. Further optimization of the assays might improve the sensitivity to include subpatent parasite densities. In particular, the pfcrt-76T PCR-PEXT-NALFIA protocol should be further optimized to decrease the number of false-negative scores and to improve the sensitivity of detection of the T allele in mixed infections, where competition between the alleles for amplification primers is expected to occur. The genotyping of pfmdr1-1246 also may require further optimization, although the number of 1246Y alleles in the sample set was very low, and so the analysis lacked power to estimate the diagnostic accuracy of the PEXT method for this allele.
Despite the differences of the 4 PEXT methods, a next step would be to multiplex both the PCR and PEXT reactions. This would decrease the costs of the assay and reduce assay time. The PCR primers for amplifying the pfmdr1-86 and pfmdr1-184 codons were the same, because of the close proximity of the two codons in the pfmdr1 gene. Hence, the same PCR product could be used for both PEXT reactions. However, the PCRs of pfmdr1-1246 and pfcrt-76 had to be done with another primer set. Multiplexing of all PCRs into one reaction would include the three primer sets involved and would require the optimization of various PCR parameters (25). For this, the response surface methodology (RSM), especially the Box-Behnken design, would be a preferred method (26).
This article describes the use of NALFIA strips in which both a wild-type and a mutant genotype can be detected on one strip. The visualization of all PEXT products on 1 strip would need at least eight lines of different antibodies (as well as a control line). Even if it is possible, it would result in a complicated readout. An alternative to performing all visualization reactions of the PEXT products in one assay is to develop a nucleic acid microarray immunoassay (NAMIA), as was done for the detection of genes encoding virulence factors of Escherichia coli O157 and mastitis pathogens (27, 28). In the microarray assay, the number of analyzed PEXT products can be increased due to the higher level of complexity, e.g., a microarray of 100 spots on a 6- by 6-mm nitrocellulose pad. Another more rapid alternative for the detection of a microarray of different spots is the lateral flow microarray immunoassay (LMIA), as presented by Posthuma-Trumpie et al. (18). In the LMIA, a microarray of 5 by 5 spots can be accommodated on a lateral flow membrane, and the result can be read in 10 to 15 min.
Another possibility to reduce the total assay time is to apply a PCR machine that makes use of thin-walled PCR tubes, like the PIKO thermal cycler (Thermo Fisher Scientific, Vantaa, Finland) (17, 27, 28). In particular, performing the PCR step in the PIKO, using an accompanying fast and robust DNA polymerase, would save a lot of time. The PCR program in the PIKO is approximately 1.5 h faster than the PCR program in the thermal cycler used in this study.
In developing countries, the use of molecular techniques as diagnostic tools is not common; in most cases, these assays require expensive readout systems (e.g., qPCR) that can be performed only in well-organized laboratories by persons with specific knowledge and skills to understand and interpret the results. This study showed that once a conventional thermal cycler is available, the use of combined PCR-PEXT-NALFIA methods is potentially a cheaper and more rapid alternative for genotyping of SNPs in P. falciparum. Since PCR and PEXT reaction mixtures can be supplied in a lyophilized form, enabling a long shelf life, such methods can potentially be performed in developing countries without need for a cold chain and thus enable prompt and adequate medication for people suffering from malaria. The NALFIA device is a conventional lateral flow test that is very similar in format to established antigen-based rapid diagnostic tests for malaria. The routine production and marketing of the test by established diagnostic companies for use in tropical settings are thus very well feasible.
Improvements in pathogen detection have been achieved by replacing PCR with an isothermal amplification procedure, such as nucleic acid sequence-based amplification, loop-mediated isothermal amplification, strand displacement amplification, and rolling-circle amplification (29–32), but these approaches have not to date been successfully applied to SNP detection and discrimination of very similar genotypes in malarial parasites.
ACKNOWLEDGMENTS
This project was financially supported by the EU-funded FP7 project MALACTRES (Multidrug resistance in malaria under combination therapy: assessment of specific markers and development of innovative, rapid and simple diagnostics), grant 201889.
We thank all collaborators of the MALACTRES project for their fruitful discussions.
REFERENCES
- 1.WHO. 2014. Malaria fact sheet no. 94. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs094/en/. [Google Scholar]
- 2.Johnston SP, Pieniazek NJ, Xayavong MV, Slemenda SB, Wilkins PP, da Silva AJ. 2006. PCR as a confirmatory technique for laboratory diagnosis of malaria. J Clin Microbiol 44:1087–1089. doi: 10.1128/JCM.44.3.1087-1089.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safeukui I, Millet P, Boucher S, Melinard L, Fregeville F, Receveur MC, Pistone T, Fialon P, Vincendeau P, Fleury H, Malvy D. 2008. Evaluation of FRET real-time PCR assay for rapid detection and differentiation of Plasmodium species in returning travellers and migrants. Malar J 7:70. doi: 10.1186/1475-2875-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatti S, Gramegna M, Bisoffi Z, Raglio A, Gulletta M, Klersy C, Bruno A, Maserati R, Madama S, Scaglia M, Gispi Study Group . 2007. A comparison of three diagnostic techniques for malaria: a rapid diagnostic test (NOW Malaria), PCR and microscopy. Ann Trop Med Parasitol 101:195–204. doi: 10.1179/136485907X156997. [DOI] [PubMed] [Google Scholar]
- 5.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. 2000. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 6.Henriques G, Hallett RL, Beshir KB, Gadalla NB, Johnson RE, Burrow R, van Schalkwyk DA, Sawa P, Omar SA, Clark TG, Bousema T, Sutherland CJ. 3 July 2014. Directional selection at the pfmdr1, pfcrt, pfubp1 and pfap2mu loci of Plasmodium falciparum in Kenyan children treated with ACT. J Infect Dis. doi: 10.1093/infdis/jiu358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purfield A, Nelson A, Laoboonchai A, Congpuong K, McDaniel P, Miller R, Welch K, Wongsrichanalai C, Meshnick S. 2004. A new method for detection of pfmdr1 mutations in Plasmodium falciparum DNA using real-time PCR. Malar J 3:9. doi: 10.1186/1475-2875-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutherland CJ, Alloueche A, Curtis J, Drakeley CJ, Ord R, Duraisingh M, Greenwood BM, Pinder M, Warhurst D, Targett GA. 2002. Gambian children successfully treated with chloroquine can harbor and transmit Plasmodium falciparum gametocytes carrying resistance genes. Am J Trop Med Hyg 67:578–585. [DOI] [PubMed] [Google Scholar]
- 9.Humphreys GS, Merinopoulos I, Ahmed J, Whitty CJ, Mutabingwa TK, Sutherland CJ, Hallett RL. 2007. Amodiaquine and artemether-lumefantrine select distinct alleles of the Plasmodium falciparum mdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrob Agents Chemother 51:991–997. doi: 10.1128/AAC.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwok PY. 2001. Methods for genotyping single nucleotide polymorphisms. Annu Rev Genomics Hum Genet 2:235–258. doi: 10.1146/annurev.genom.2.1.235. [DOI] [PubMed] [Google Scholar]
- 11.Syvänen AC. 2001. Accessing genetic variation: genotyping single nucleotide polymorphisms. Nat Rev Genet 2:930–942. doi: 10.1038/35103535. [DOI] [PubMed] [Google Scholar]
- 12.Litos I, Emmanouilidou E, Glynou K, Laios E, Ioannou P, Christopoulos T, Kampa M, Castanas E, Gravanis A. 2007. Rapid genotyping of CYP2D6, CYP2C19 and TPMT polymorphisms by primer extension reaction in a dipstick format. Anal Bioanal Chem 389:1849–1857. doi: 10.1007/s00216-007-1593-4. [DOI] [PubMed] [Google Scholar]
- 13.Konstantou J, Ioannou P, Christopoulos T. 2007. Genotyping of single nucleotide polymorphisms by primer extension reaction and a dual-analyte bio/chemiluminometric assay. Anal Bioanal Chem 388:1747–1754. doi: 10.1007/s00216-007-1383-z. [DOI] [PubMed] [Google Scholar]
- 14.Litos IK, Ioannou PC, Christopoulos TK, Traeger-Synodinos J, Kanavakis E. 2009. Multianalyte, dipstick-type, nanoparticle-based DNA biosensor for visual genotyping of single-nucleotide polymorphisms. Biosens Bioelectron 24:3135–3139. doi: 10.1016/j.bios.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Kalogianni D, Boutsika L, Kouremenou P, Christopoulos T, Ioannou P. 2011. Carbon nano-strings as reporters in lateral flow devices for DNA sensing by hybridization. Anal Bioanal Chem 400:1145–1152. doi: 10.1007/s00216-011-4845-2. [DOI] [PubMed] [Google Scholar]
- 16.Shi MM. 2001. Enabling large-scale pharmacogenetic studies by high-throughput mutation detection and genotyping technologies. Clin Chem 47:164–172. [PubMed] [Google Scholar]
- 17.Noguera P, Posthuma-Trumpie G, van Tuil M, van der Wal F, de Boer A, Moers A, van Amerongen A. 2011. Carbon nanoparticles in lateral flow methods to detect genes encoding virulence factors of Shiga toxin-producing Escherichia coli. Anal Bioanal Chem 399:831–838. doi: 10.1007/s00216-010-4334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Posthuma-Trumpie GA, Wichers JH, Koets M, Berendsen LBJM, van Amerongen A. 2012. Amorphous carbon nanoparticles: a versatile label for rapid diagnostic (immuno)assays. Anal Bioanal Chem 402:593–600. doi: 10.1007/s00216-011-5340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Amerongen A, Koets M. 2005. Simple and rapid bacterial protein and DNA diagnostic methods based on signal generation with colloidal carbon particles, p 105–126. In van Amerongen A, Barug D, Lauwaars (ed), Rapid methods for biological and chemical contaminants in food and feed. Wageningen Academic Publishers, Wageningen, The Netherlands. [Google Scholar]
- 20.Gordon J, Michel G. 2008. Analytical sensitivity limits for lateral flow immunoassays. Clin Chem 54:1250–1251. doi: 10.1373/clinchem.2007.102491. [DOI] [PubMed] [Google Scholar]
- 21.Linares EM, Kubota LT, Michaelis J, Thalhammer S. 2012. Enhancement of the detection limit for lateral flow immunoassays: evaluation and comparison of bioconjugates. J Immunol Methods 375:264–270. doi: 10.1016/j.jim.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Mens PF, Moers AP, de Bes LM, Flint J, Sak JR, Keereecharoen L, van Overmeir C, Verweij J, Hallett RL, Wihokhoen B, Proux S, Schallig HD, van Amerongen A. 2012. Development, validation and evaluation of a rapid PCR-nucleic acid lateral flow immuno-assay for the detection of Plasmodium and the differentiation between Plasmodium falciparum and Plasmodium vivax. Malar J 11:279. doi: 10.1186/1475-2875-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutherland CJ, Haustein T, Gadalla N, Armstrong M, Doherty JF, Chiodini PL. 2007. Chloroquine-resistant Plasmodium falciparum infections among UK travellers returning with malaria after chloroquine prophylaxis. J Antimicrob Chemother 59:1197–1199. doi: 10.1093/jac/dkm104. [DOI] [PubMed] [Google Scholar]
- 24.Gadalla NB, Elzaki SE, Mukhtar E, Warhurst DC, El-Sayed B, Sutherland CJ. 2010. Dynamics of pfcrt alleles CVMNK and CVIET in chloroquine-treated Sudanese patients infected with Plasmodium falciparum. Malar J 9:74. doi: 10.1186/1475-2875-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henegariu O, Heerema NA, Dlouhy SR, Vance GH, Vogt PH. 1997. Multiplex PCR: critical parameters and step-by-step protocol. Biotechniques 23:504–511. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira SL, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão GC, da Silva EG, Portugal LA, dos Reis PS, Souza AS, dos Santos WN. 2007. Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta 597:179–186. doi: 10.1016/j.aca.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Noguera PS, Posthuma-Trumpie GA, van Tuil M, van der Wal FJ, de Boer A, Moers AP, van Amerongen A. 2011. Carbon nanoparticles as detection labels in antibody microarrays. Detection of genes encoding virulence factors in Shiga toxin-producing Escherichia coli. Anal Chem 83:8531–8536. doi: 10.1021/ac201823v. [DOI] [PubMed] [Google Scholar]
- 28.Mujawar LH, Moers A, Norde W, van Amerongen A. 2013. Rapid mastitis detection assay on porous nitrocellulose membrane slides. Anal Bioanal Chem 405:7469–7476. doi: 10.1007/s00216-013-7192-7. [DOI] [PubMed] [Google Scholar]
- 29.Chan AB, Fox JD. 1999. NASBA and other transcription-based amplification methods for research and diagnostic microbiology. Rev Med Microbiol 10:185–196. [Google Scholar]
- 30.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. 2000. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:e63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker GT, Fraiser MS, Schram JL, Little MC, Nadeau JG, Malinowski DP. 1992. Strand displacement amplification–an isothermal, in vitro DNA amplification technique. Nucleic Acids Res 20:1691–1696. doi: 10.1093/nar/20.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demidov VV. 2002. Rolling-circle amplification in DNA diagnostics: the power of simplicity. Expert Rev Mol Diagn 2:542–548. doi: 10.1586/14737159.2.6.542. [DOI] [PubMed] [Google Scholar]