Abstract
The relationships between antituberculosis drug exposure and treatment effects on humans receiving multidrug therapy are complex and nonlinear. In patients on treatment, an analysis of the rate of decline in the sputum bacillary burden reveals two slopes. The first is the α-slope, which is thought to reflect bactericidal effect, followed by a β-slope, which is thought to reflect sterilizing activity. We sought to characterize the effects of standard first-line treatment on sterilizing activity. Fifty-four patients receiving combination therapy for pulmonary tuberculosis in a clinical trial had drug concentrations measured and Mycobacterium tuberculosis isolates available for MIC identification. Sputum sample cultures were performed at baseline and weekly for 8 weeks. A time-to-event model based on the days to positivity in the liquid cultures was used to estimate the β-slope. The pharmacokinetic parameters of rifampin, isoniazid, ethambutol, and pyrazinamide were determined for each patient. Multivariate adaptive regression splines analyses, which simultaneously perform linear and nonlinear analyses, were used to identify the relationships between the predictors and the β-slope. The potential predictors examined included HIV status, lung cavitation, 24-h area under the concentration-time curve (AUC), peak drug concentration (Cmax), AUC/MIC ratio, Cmax/MIC ratio, and the time that that concentration persisted above MIC. A rifampin Cmax of >8.2 mg/liter and a pyrazinamide AUC/MIC of >11.3 were key predictors of the β-slope and interacted positively to increase the β-slope. In patients with a rifampin AUC of <35.4 mg · h/liter, an increase in the pyrazinamide AUC/MIC and/or ethambutol Cmax/MIC increased the β-slope, while increasing isoniazid Cmax decreased it, suggesting isoniazid antagonism. Antibiotic concentrations and MICs interact in a nonlinear fashion as the main drivers of a sterilizing effect. The results suggest that faster speeds of sterilizing effect might be achieved by omitting isoniazid and by increasing rifampin, pyrazinamide, and ethambutol exposures. However, isoniazid and ethambutol exposures may only be of importance when rifampin exposure is low. These findings need confirmation in larger studies. (This study has been registered at controlled-trials.com under registration no. ISRCTN80852505.)
INTRODUCTION
In active tuberculosis (TB) infection, ≥2 different subpopulations have been described: those that are actively replicating and are killed rapidly by antituberculosis drugs (1), and those that are nonreplicating, which can lie dormant in the human host for very long periods of time and are less sensitive to the bactericidal activities of drugs (2). Persisters were first defined in human disease and in animal models as the population of Mycobacterium tuberculosis that was left after kill by isoniazid and were demonstrated to be difficult to culture. They are now believed by some to reflect either very slowly growing or nonreplicating bacteria under hypoxic conditions, although this remains to be proven (3–8). The sterilizing activity of a drug is defined as its ability to kill either these nonreplicating bacteria or dormant bacteria under hypoxic conditions, as well as the ability to shorten antituberculosis therapy duration and reduce relapse (3, 9). Early bactericidal activity refers to the initial rapid kill of actively replicating mycobacteria (1). Another definition of sterilizing activity is the ability of a drug to prevent relapse (after the completion of treatment) in a long-term (≥18 months) study. The rates of decline in the M. tuberculosis burden in sputum during the first 14 days and 2 months of therapy are also used as surrogate markers for sterilizing activity (1, 10). The central problem in shortening antituberculosis therapy is finding drugs and doses that can increase the speed of the sterilizing effect. The sterilizing activity of standard therapy is weak compared to the potent bactericidal activity of the regimen during the initial 2 days of treatment (1). In clinical trials, sterilizing activity is measured by relapse. Using time to positivity (TTP) in liquid culture as a surrogate of bacillary burden (11) during the first 8 weeks of standard therapy, nonlinear mathematical models identified two bacterial subpopulations in 154 patients with pulmonary tuberculosis (12). The first subpopulation, in which bacteria were killed rapidly, with a half-life of 1.8 days, was 41-fold larger than the second. The second population, with bacterial kill rates described by the β-slope, declined, with a half-life of 39 days (12). In this study, the β-slope was used as a surrogate marker of sterilizing activity. Here, we investigated the effect of drug concentrations on the β-slope, with the assumption that the β-slope reflects sterilizing activity.
Several studies have shown the importance of antituberculosis drug concentrations on the rate of kill of M. tuberculosis in hollow fiber systems (13, 14), animal models of tuberculosis (15, 16), and patients (10, 11). Some clinical studies have also shown low drug concentrations to be associated with poorer outcomes in patients receiving combination therapy (17, 18). However, the magnitude and range of the relationships between drug exposure and sterilizing activity and the speed of the sterilizing effect have yet to be elucidated in tuberculosis patients on a multidrug regimen. In general, the relationship between drug concentrations and their effects in biological systems is nonlinear (19, 20), characterized by discontinuities, and of a higher order of complexity. Standard linear regression methods offer a limited approach to the analysis of such relationships. Here, we utilized multivariate adaptive regression splines (MARS) to identify factors that are predictive of sterilizing activity in patients with pulmonary tuberculosis. By allowing for possible nonlinear patterns in the data and being able to detect interactions between variables, MARS can uncover complex data structures often hidden in high-dimensional data (21). MARS has been used to successfully identify the predictors of disease progression or the efficacy of therapeutic interventions in a variety of medical contexts (22–24), and it outperforms other methods in identifying complex nonlinear disease-risk relationships (25). We used the β-slope as a measure of the sterilizing effect occurring during the first 8 weeks of therapy. Mathematical modeling has enabled the separation of the lower rate of kill of the persisting subpopulation from that of more susceptible organisms (12), thereby providing a powerful measure against which to evaluate the sterilizing activities of the drugs (i.e., using the β-slope).
MATERIALS AND METHODS
Study participants.
Patients were randomized to receive micronutrient or placebo intervention in a previously conducted prospective double-blind controlled study of 154 patients from Cape Town, South Africa (26). The study received ethical approval from the University of Cape Town ethics committee and was done in accordance with the Helsinki Declaration. The micronutrient intervention had no effect on the outcomes (26). A description of the primary study has been published (26). The patients were recruited at baseline and followed up weekly for 8 weeks after starting treatment. There were no study visits beyond these 8 weeks of treatment; hence, long-term outcomes were not available.
Patients with sputum smear-positive pulmonary tuberculosis were treated with weight-based doses of rifampin, isoniazid, pyrazinamide, and ethambutol as a fixed-dose combination (FDC) of tablets containing 150 mg of rifampin, 75 mg of isoniazid, 400 mg of pyrazinamide, and 275 mg of ethambutol (27). Patients weighing 38 to 54 kg received 3 tablets, those weighing 55 to 70 kg received 4 tablets, and those >70 kg received 5 tablets once daily. Of these 154 patients, 54 had drug plasma concentrations and baseline MICs determined for each drug. Consecutive patients were invited to participate in the pharmacokinetic study, for which separate consent was obtained. The analysis described here includes these 54 patients who were a random subset of the parent study.
Pharmacokinetic analyses.
Blood was drawn from patients for pharmacokinetic sampling ≥1 month after the patients commenced treatment. Each patient had between 4 and 8 samples drawn over a period of 7 h after the drugs had been administered. Twenty-four patients had pharmacokinetic samples drawn on a second occasion (one month after the first sampling had been done), thereby enabling the testing of within-subject variability of the drug exposures. Individual patient steady-state 24-h area under the concentration-time curve (AUC) and peak drug concentrations (Cmax) were identified from pharmacokinetic models implemented in NONMEM 7.2 (Ellicott City, MD). The models built using data from patients in this cohort have been published elsewhere for rifampin (28) and pyrazinamide (29), while those used to describe the pharmacokinetics of isoniazid (30) and ethambutol (31) were modified from the literature. Further details on the pharmacokinetic models are delineated in the supplemental material.
Determination of bacillary kill rates.
The sputum specimens were processed for culture on liquid medium using the Bactec MGIT 960 system (Becton Dickinson, Sparks, MD). Further details on the sputum processing can be found in the supplemental material. The TTP was recorded in days. These data were used to build a time-to-event model to describe the treatment response in patients, as outlined in our earlier work (12). In this model, the likelihood of having a positive sputum culture was estimated for patients who had a positive culture result at a particular time, while the survival (probability of no event) was estimated for patients who had a negative culture result. The hazard of a positive culture result was directly related to the bacillary load predicted from the model. A biexponential model described the decline in bacillary load using the α- and β-slopes, with the β-slope reflecting terminal sterilizing activity. Individual estimates of the β-slope were generated by the model for evaluating the effects of drug exposure on sterilizing activity in a MARS analysis.
MIC determination.
The Bactec MGIT 960 system with the EpiCenter software and TBeXiST application were used to determine the MICs of the M. tuberculosis isolates prior to therapy (32). The Bactec MGIT 960 SIRE kit was used to make stock solutions for isoniazid, rifampin, and ethambutol, while pyrazinamide was prepared from the Bactec MGIT 960 PZA kit (Becton Dickinson Biosciences, Sparks, MD). Further details can be found in the supplemental material.
Data analysis.
The MARS analysis was implemented using the Salford Predictive Modeler 7.0 (Salford Systems, San Diego, CA) to identify the drug exposures linked to the β-slope. MARS is a nonparametric regression data analysis technique that combines recursive partitioning with fitting of splines to variables in the data set. The method identifies the significant predictor variables, which enables splitting of the data into subregions of interest, while the spline fitting enables the determination of relationships within the subregions (33). This can be viewed as piecewise linear regression models in small ranges of values, with transition points at hinges, which are described by the lines of best fit for subregions of the data. The location and number of hinges are automatically determined by the data (33). Here, “interaction” refers to the effect of a drug on the dependent variable as a consequence of its association with exposure to another clinical factor, such as another drug. Such interactions can be additive, synergistic, or antagonistic. The splines and interactions are described by basis functions (BFs). MARS works by first fitting an overly large model with BFs (forward selection), as long as they improve the fit (higher R2), with no penalty for inclusion. This leads to overfitting. The next step is backward deletion, whereby MARS identifies one BF whose removal will have the least impact on the residual sum of squares; this pruning process is repeated with each BF in the model. BFs are thus eliminated based on generalized cross-validation error (GCV), which is the average squared residual multiplied by a penalty that is proportional to the number of BFs in the model (33). The MARS analysis is further explained in the supplemental material.
The potential predictors of the primary outcome (i.e., the β-slope) included in the MARS analysis were AUC, Cmax, AUC/MIC, Cmax/MIC, individual drug MICs, the percentage of the 24-h dosing interval that concentration exceeded MIC (%TMIC), HIV status, and the presence/absence of lung cavitation. The effects of the predictors on the β-slope were related in a series of BFs that describe the data in linear combination.
The secondary clinical outcome measured was 2-month sputum conversion (defined as both week-7 and week-8 culture results being negative after 8 weeks of treatment). Based on the MARS findings, standard statistical tests were used to determine the odds ratios of 2-month sputum conversion using contingency tables in Prism 4.0 (GraphPad Software, La Jolla, CA).
RESULTS
Patient demographics.
Of the 54 study participants, 63% were male, 13% were HIV positive, 88% had lung cavities present at baseline, and 28% had converted to ≥2 consecutive negative sputum culture results by the end of the follow-up period (8 weeks of treatment). The median (range) for weight, body mass index, and age were 52 (38 to 73) kg, 19 (14 to 29) kg/m2, and 28 (18 to 55) years, respectively. The clinical characteristics of this subset of patients did not differ substantially from those in the larger study of 154 patients (26).
Patient pharmacokinetic measures and MICs.
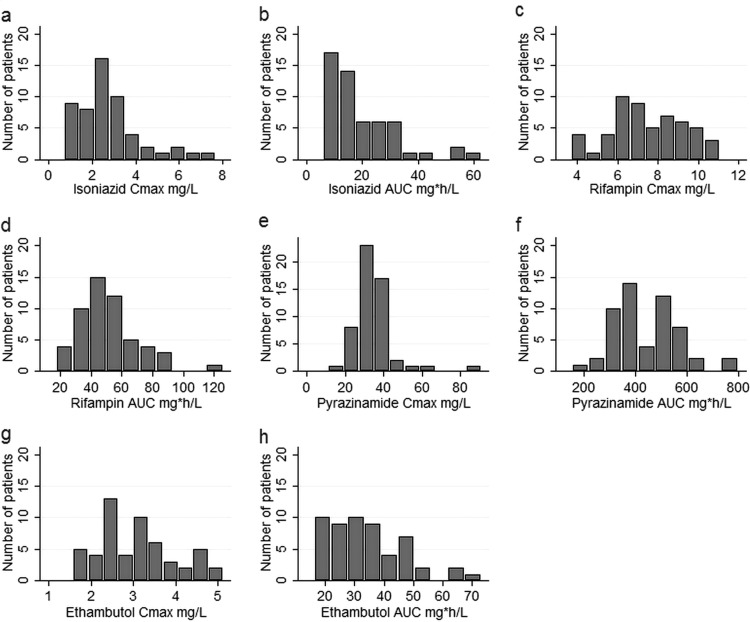
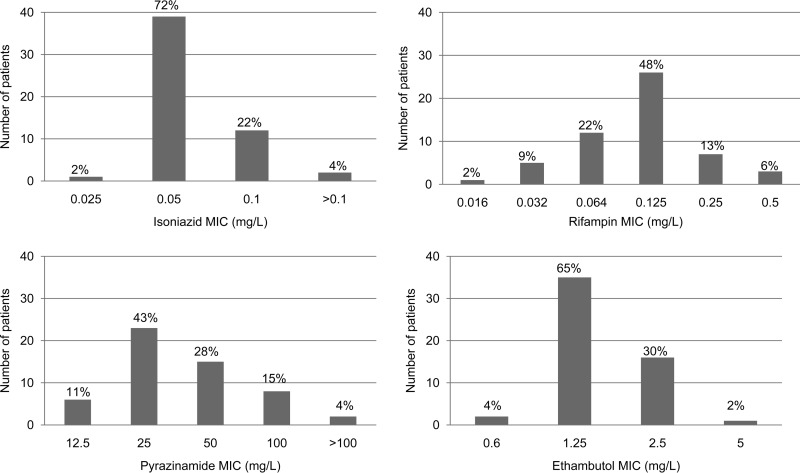
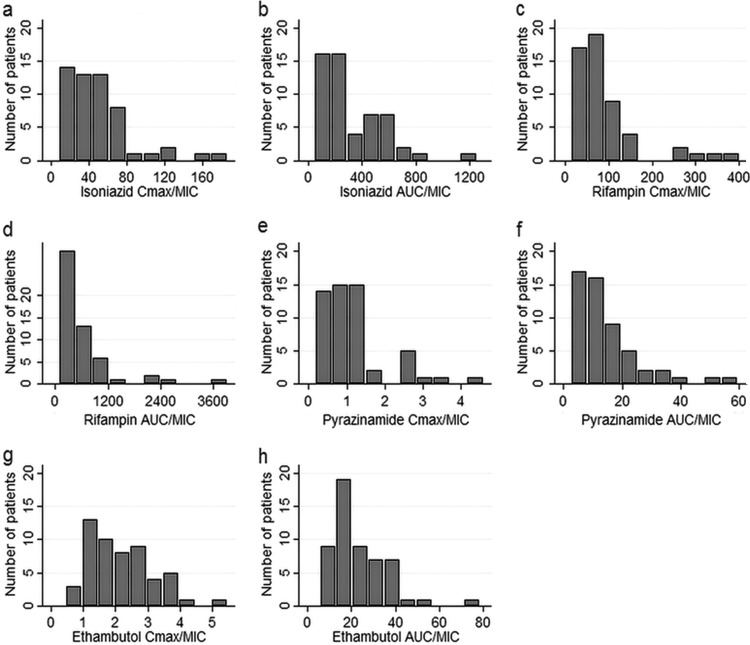
The final parameter estimates of the population pharmacokinetic models used to describe the pharmacokinetics of rifampin, isoniazid, pyrazinamide, and ethambutol are shown in Tables S1 to S4 in the supplemental material, respectively. The 4 drugs exhibited substantial pharmacokinetic variability, as shown in Fig. 1a to h. The ranges of the observed concentrations and the parameters are shown in Table 1; more details are shown in the supplemental material. Table S5 in the supplemental material shows that the primary pharmacokinetic measures of the different drugs did not significantly covary, indicating that multicolinearity would be unlikely to complicate the interpretation of the effects of the different drugs on the β-slope. Figure 2 shows the distribution of the MICs among the M. tuberculosis isolates. For purposes of calculations and data analysis, the 2 patients with an isoniazid MIC of >0.1 mg/liter were assumed to have an M. tuberculosis MIC of 0.2 mg/liter, and the 2 patients with a pyrazinamide MIC of >100 mg/liter were assumed to have an M. tuberculosis MIC of 200 mg/liter. When MIC variability was taken into account by indexing the AUC and Cmax in each patient to the MIC of the M. tuberculosis isolate in each patient, so as to take into account both microbial and pharmacokinetic variability, variability further increased, as shown in Fig. 3a to h. None of the MIC indexed parameters were normally distributed, apart from the ethambutol Cmax/MIC ratio (Shapiro-Wilk W test, P = 0.06).
FIG 1.
Histograms showing distribution of drug area under the concentration-time curve (24 h) and peak plasma concentration in study participants. The parameters are isoniazid Cmax (a) and AUC (b), rifampin Cmax (c) and AUC (d), pyrazinamide Cmax (e) and AUC (f), and ethambutol Cmax (g) and AUC (h).
TABLE 1.
Median (range) pharmacokinetic measures before and after adjustment for the MIC
| Drug | Cmax (mg/liter) | AUC (mg · h/liter) | Cmax/MIC | AUC/MIC | % time drug concn is above MIC (h) | MIC (mg/liter) |
|---|---|---|---|---|---|---|
| Isoniazid | 2.5 (0.7–7.7) | 14 (6.2–63) | 43 (8.2–187) | 272 (52–1,252) | 79 (54–100) | 0.05 (0.025–0.2) |
| Rifampin | 7.3 (3.7–11) | 48 (18–126) | 69 (13–401) | 454 (80–3,934) | 58 (26–100) | 0.125 (0.016–0.5) |
| Pyrazinamide | 34 (11–90) | 420 (153–798) | 1.0 (0.17–4.6) | 12 (2.6–60) | 0 (0–88) | 25 (12.5–200) |
| Ethambutol | 3.1 (1.6–5.1) | 32 (16–73) | 2.0 (0.46–5.5) | 20 (5.8–79) | 25 (0–73) | 1.25 (0.6–5) |
FIG 2.
Histograms showing distribution of MICs in M. tuberculosis isolates.
FIG 3.
Histograms showing distribution of drug area under the concentration-time curve (24 h) and peak plasma concentration adjusted by the corresponding MIC in study participants. The ratios shown are isoniazid Cmax/MIC (a) and AUC/MIC (b), rifampin Cmax/MIC (c) and AUC/MIC (d), pyrazinamide Cmax/MIC (e) and AUC/MIC (f), and ethambutol Cmax/MIC (g) and AUC/MIC (h).
MARS analysis for primary outcome.
The β-slope had a median (interquartile range) value of 0.46 week−1 (0.22 to 0.77 week−1). Five BFs were identified as significant predictors of the β-slope in the final MARS model and are shown in Table 2. This final model describing the effects of drug concentration and MIC on the β-slope was described by the equation β-slope = 0.49 − 0.80 × BF1 + 0.05 × BF2 + 0.71 × BF3 − 0.32 × BF4 + 0.02 × BF5. When a basis function has a positive coefficient, it means that higher drug exposure results in a steeper β-slope. If it has a negative coefficient, it means that higher drug exposure results in a shallower incline of the β-slope (i.e., poorer sterilizing activity). The R2 for the predictions of the β-slope based on the equation above against the actual β-slopes in patients was 0.85, which we consider a good fit. The cross-validated R2 (used to assess the reliability of the parameter estimates and robustness of the final model) was 0.72. The BFs are described in Table 2. The expressions are conditional at the hinges. BF1, for example, describes the effect of increasing rifampin AUC, which increases the β-slope until a hinge is seen at an AUC of 35.4 mg/liter, whereupon the effect of the basis function becomes zero. The multiplication of BFs describes interaction terms, taking into account the hinges. As an example, BF3 is conditional on BF1, whereby an increase in an ethambutol Cmax/MIC of >0.46 increases the β-slope if the rifampin AUC is <35.4 (BF1). Likewise, the pyrazinamide AUC/MIC ratio increases the β-slope when the rifampin AUC is <35.4 mg/liter (BF2), while the isoniazid Cmax reduces the β-slope under the same circumstances (BF4). Table 2 shows another interaction in which rifampin Cmax and pyrazinamide AUC/MIC interact positively to increase the β-slope if the rifampin Cmax is >8.2 mg/liter (BF5) and the pyrazinamide AUC/MIC is >11.3 (BF6). BF6 is an interaction term contained in BF5. In BF5, the expression “max(0, rifampin Cmax − 8.2 mg/liter) × BF6” means that the value of the whole expression is 0 if rifampin Cmax is ≤8.2 mg/liter; otherwise, the value is Cmax − 8.2 mg/liter, multiplied by BF6. If the pyrazinamide AUC/MIC is >11.3 and the rifampin Cmax is >8.2 mg/liter, exposures to the two drugs interact synergistically to enhance sterilizing activity.
TABLE 2.
Basis functions identified in final MARS model
| Basis function | Function | Coefficient in model | Interpretation |
|---|---|---|---|
| BF1 | max (0, 35.4 mg · h/liter − rifampin AUC) | −0.80 | Higher rifampin exposure results in increased β-slope, until the AUC reaches 35.4 mg · h/liter, whereupon the effect of the basis function becomes zero. The effect is exerted in 11 of the 54 patients with a rifampin AUC of <34.5 mg · h/liter. In the remaining patients, the basis function is 0. |
| BF2 | max (0, pyrazinamide AUC/MIC − 2.6) × BF1 | +0.05 | Higher pyrazinamide AUC/MIC results in increased β-slope, starting at 2.6. The effect is exerted in 11 of the 54 patients with a rifampin AUC of <35.4 mg · h/liter (because of interaction with BF1). In the remaining patients, the basis function is 0. |
| BF3 | max (0, ethambutol Cmax/MIC − 0.46) × BF1 | +0.71 | Higher ethambutol Cmax/MIC of >0.46 results in increased β-slope. The effect is exerted in 11 of the 54 patients with a rifampin AUC of <35.4 mg · h/liter (because of the interaction with BF1). In the remaining patients, the basis function is 0. |
| BF4 | max(0, isoniazid Cmax − 0.72 mg/liter) × BF1 | −0.32 | Higher isoniazid Cmax results in decreased β-slope. The effect is exerted in 11 of the 54 patients with a rifampin AUC of <35.4 mg · h/liter (because of the interaction with BF1). In the remaining patients, the basis function is 0. |
| BF5 | max (0, rifampin Cmax − 8.2 mg/liter) × BF6 | +0.02 | Higher rifampin Cmax (>8.2 mg/liter) results in increased β-slope. The effect is exerted in 12 of the 54 patients with rifampin Cmax >8.2 mg/liter and pyrazinamide AUC/MIC >11.3 (BF6). In the remaining patients, the basis function is 0. |
| BF6 | max (0, pyrazinamide AUC/MIC − 11.3) | Nil | Higher pyrazinamide AUC/MIC (>11.3) results in increased β-slope. The effect is exerted in 30 of the 54 patients with a pyrazinamide AUC/MIC of >11.3. In the remaining patients, the basis function is 0. BF6 serves solely to interact with rifampin Cmax (BF5). |
Odds of secondary outcome based on thresholds predicted by MARS.
Patients with 2-month sputum culture conversion (defined as both week-7 and week-8 culture results being negative after 8 weeks of treatment) had higher estimated β-slopes than patients who failed to convert (β-slope median, 1.1 versus 0.26 week−1; Mann-Whitney U test, P < 0.0001). Based on the threshold values predicted by MARS, contingency tables were generated to determine whether there were differences in the 2-month sputum conversion rates between patients with drug concentration exposures above and below those identified by MARS, with the results shown in Table 3. The rifampin Cmax threshold of 8.2 mg/liter was significantly associated with the 2-month sputum culture conversion (odds ratio [95% confidence interval {CI}], 3.8 [1.1 to 13.3]). The odds of a negative culture were further increased when a patient also achieved a pyrazinamide AUC/MIC ratio of >11.3 (odds ratio [95% CI], 6.0 [1.5 to 23.7]), confirming the positive interaction between pyrazinamide and rifampin exposures in their effect on treatment response.
TABLE 3.
Association between drug exposure above the MARS-predicted threshold and 2-month sputum culture conversion
| Drug threshold | Odds ratio of negative 2-month culture (95% CI)a | Sensitivity (%)b | Specificity (%)c |
|---|---|---|---|
| Rifampin Cmax >8.2 mg/liter and pyrazinamide AUC/MIC ratio >11.3 | 6.0 (1.5–23.7) | 47 | 87 |
| Rifampin Cmax >8.2 mg/liter regardless of pyrazinamide AUC/MIC ratio | 3.8 (1.1–13.3) | 60 | 72 |
| Pyrazinamide AUC/MIC ratio >11.3 regardless of rifampin Cmax | 2.9 (0.78–10.7) | 73 | 51 |
| Rifampin AUC alone | 1.0 (0.7–1.4) | ||
| Ethambutol Cmax/MIC regardless of rifampin Cmax | 0.80 (0.02–27) | ||
| Isoniazid Cmax regardless of rifampin Cmax | 0.45 (0.04–4.8) |
95% CI, 95% confidence interval.
Ability to correctly identify 2-month sputum culture conversion, i.e., the proportion of patients with culture conversion who were predicted to convert based on the MARS thresholds.
Ability to correctly identify failure to convert to negative sputum, i.e., the proportion of patients who failed to convert who were predicted to fail based on the MARS thresholds.
DISCUSSION
To our knowledge, this is the first report that investigates in totality the effects of antituberculosis drug exposure together with MIC, as well as the interaction between these drug exposures, on the sterilizing activity and 2-month sputum conversion in patients with pulmonary tuberculosis receiving a multidrug regimen. We identified the concentration thresholds at which the concentration-effect relationships occur in patients. Important predictors of sterilizing activity included rifampin AUC and Cmax, pyrazinamide AUC/MIC, ethambutol Cmax/MIC, and isoniazid Cmax. Ethambutol and isoniazid were significant predictors of sterilizing activity (as measured by the β-slope) only when the rifampin exposure was low. In patients with a rifampin Cmax of >8.2 mg/liter and a pyrazinamide AUC/MIC of >11.3, the rifampin and pyrazinamide exposures interacted synergistically to drive sterilizing activity. This points to the central role of these drugs and their concentration-dependent interactions in determining the activity of the regimen. On the other hand, in a distinct group of patients with a rifampin AUC of <35.4 mg · h/liter, increasing rifampin AUC, pyrazinamide AUC/MIC, or ethambutol Cmax/MIC significantly improved sterilizing activity. Conversely, among these patients with low rifampin exposures (AUC < 35.4 mg · h/liter), isoniazid Cmax antagonized sterilizing activity. Importantly, rifampin Cmax and AUC were correlated (Spearman's rho = 0.74; P < 0.001), and no patients with an AUC of <35.4 mg · h/liter had a rifampin Cmax of >8.2 mg/liter. Thus, the effects described were observed in two discrete groups of patients with low and high rifampin exposures. In a recent analysis of data from a different Cape Town cohort of 142 patients (19), the rifampin exposures were lower on average than those described in this study, and the sterilizing effect (defined as the absence of either relapse, treatment failure, or death for up to 2 years of follow-up) was best predicted by a pyrazinamide AUC of >363 mg · h/liter. MICs were not available in that study; however, if the median pyrazinamide MIC in our cohort, 25 mg/liter, or that from other cohorts, 37.5 mg/liter, is used to calculate the pyrazinamide AUC/MIC expected from that study, values of 9.7 to 14.5 are obtained, similar to our current findings (34, 35). Interestingly, if the 17.8-fold penetration into the lung is taken into consideration (36), our threshold pyrazinamide AUC/MIC of 11.3 in the plasma would result in an AUC/MIC of 201 at the site of effect in the lung. This value is comparable to the optimal AUC/MIC of 209 derived in the hollow fiber system (14). Overall, these results suggest that a faster sterilizing effect could be achieved by dosing rifampin and pyrazinamide to achieve concentrations associated with optimal effect, which could be used to design treatment regimens of shorter duration.
Another interesting finding is that isoniazid appeared to antagonize the sterilizing activities of rifampin and pyrazinamide, as reflected in the β-slope. We detected this effect only in patients with low rifampin exposures, which suggests that the effect is most evident in a relatively weak regimen. Thus, beyond the first few days of treatment, isoniazid is likely to be detrimental to sterilizing activity in a concentration-dependent manner. This finding is consistent with studies of murine tuberculosis, in which isoniazid demonstrated dose-dependent antagonism to the rifampin-pyrazinamide combination (37, 38). In the hollow fiber system, the shorter the interval between rifampin and isoniazid administration, the lower the bactericidal and sterilizing effects were, with the lowest kill rates occurring when the drugs were administered simultaneously (39). This too supports concentration-dependent antagonism. The molecular mechanism of antagonism is unclear. Isoniazid is a prodrug whose active moiety primarily targets the enoyl-acyl carrier protein reductase of the fatty acid synthase II (FASII) (40), and this might antagonize the effect of pyrazinamide on the pathway (41). These findings suggest that the effect of removing isoniazid from the regimen on long-term outcomes, such as relapse and emergence of resistance, should be evaluated in future studies. Some clinical studies have found a higher isoniazid AUC to be associated with positive treatment outcomes, while the effects of all the other drugs were not significant (42, 43). Therefore, the long-term role of isoniazid needs further evaluation. It must be noted that the only other companion drug in one of these studies was rifampin or rifapentine (43), suggesting that the possible antagonistic effect of isoniazid may be dependent upon the regimen.
In the landmark study by Jindani, Doré, and Mitchison (1) that examined the effects of various combinations of drugs on the number of M. tuberculosis CFU in sputum cultures over 14 days, ethambutol had a sterilizing effect and was judged to be antagonistic to the rifampin sterilizing effect (1). In our study, the ethambutol Cmax/MIC ratio was predicted to be positively correlated with the β-slope but only when rifampin exposure was low (AUC < 35.4 mg · h/liter). It may be that at a higher rifampin exposure, the effect of ethambutol is masked by the higher sterilizing effect of rifampin, so that the overall effect is less than that of adding the effects of two drugs, manifesting as apparent antagonism. Our results suggest that ethambutol may add a sterilizing effect in patients with low rifampin concentrations.
For the secondary outcome of 2-month sputum conversion, we confirmed the importance of rifampin Cmax and pyrazinamide AUC/MIC and demonstrate their significant positive interaction for 2-month sputum conversion. Until now, the pharmacokinetic predictors of 2-month culture conversion have been elusive. A rifampin Cmax of 8.2 mg/liter was found to be the threshold value in this study, which is within the same range as the 6.6 mg/liter value reported in another recent study (19), as well as the minimum Cmax of 8.0 mg/liter proposed by Peloquin (44), which was based on epidemiological data.
Many studies investigate pharmacokinetic variability without accounting for variability in the drug susceptibilities of the infecting strains. The inclusion of MIC information significantly strengthened the analysis, allowing definition of the roles of pyrazinamide and ethambutol. Our current study demonstrates that for pyrazinamide, for which AUC has been shown to be associated with a sterilizing effect (19), the inclusion of MIC data added to the ability to predict the effect on the β-slope and 2-month sputum conversion rate. The inclusion of MIC data also adds useful information that can aid in determining M. tuberculosis susceptibility breakpoints (epidemiological cutoffs) for clinical decision making (45).
Lung cavitation was not a significant predictor of β-slope. This finding is not unexpected, given that lung cavitation influences initial bacillary burden rather than kill rates, as we have shown before (12). The small proportion of patients with HIV infection (13%) may explain the failure to identify HIV infection as a significant covariate. However, other clinical studies have also shown that HIV infection does not appear to have an effect on sputum conversion rates (46, 47).
Our study has several limitations. First, the limited sample size in this study means that the results of the MARS model should be interpreted with caution, and our findings need confirmation in larger data sets. The findings related to isoniazid and ethambutol were limited to 11 patients who had low rifampin exposures. However, the use of a continuous measure of sterilizing activity, such as the β-slope, means that even in those confirmatory studies, relatively small sample sizes will be adequate, compared to those in studies employing dichotomous endpoints, such as relapse. A second possible concern is that our analysis had many possible predictors that might be colinear. However, in Table S5 in the supplemental material, we show that pharmacokinetic parameters were not colinear between the different drugs, thus obviating that concern. Moreover, where different basis functions were included for the same drug as those for rifampin, there was no overlap in the respective data subregions in which the effects of the different measures of a drug were apparent (e.g., there were no patients with a Cmax of >8.2 mg/liter and AUC of <35.4 mg · h/liter).
In summary, we show the effects of rifampin, pyrazinamide, isoniazid, and ethambutol concentrations and MICs on sterilizing activity. These effects were elucidated for each drug as part of a multidrug regimen in patients, and the findings are thus directly applicable to the clinical setting. In patients with low rifampin exposure, isoniazid had a detrimental effect on sterilizing activity, and clinical studies to confirm the effect of the removal of the drug from regimens are warranted. A rifampin Cmax of >8.2 mg/liter coupled with a pyrazinamide AUC/MIC ratio of >11.3 is associated with both higher sterilizing activity and higher 2-month sputum conversion rates. Last, the sterilizing effect of ethambutol is evident but only at low rifampin exposures. This work suggests that higher drug exposures (except for isoniazid) result in higher sputum conversion rates.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Clinical Infectious Diseases Research Initiative (CIDRI) Wellcome Trust Fund 412164 (to E.C.), the National Research Foundation (NRF) South Africa (2067444 and Research Council of Norway [RCN] 180353/S50), the Norwegian Programme for Development, Research, and Higher Education (NUFUPRO-2007/10183), Research Council of Norway (RCN) (183694/S50), the South African Medical Research Council, and the National Institutes of Health (R01AI079497) to J.G.P. and T.G.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03931-14.
REFERENCES
- 1.Jindani A, Doré CJ, Mitchison DA. 2003. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med 167:1348–1354. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- 2.Wayne LG, Hayes LG. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun 64:2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, Jasmer RM, Koppaka V, Menzies RI, O'Brien RJ, Reves RR, Reichman LB, Simone PM, Starke JR, Vernon AA, American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society . 2003. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 4.Canetti G, Grumbach F, Grosset J. 1960. Studies of bacillary populations in experimental tuberculosis of mice treated by isoniazid. Am Rev Respir Dis 82:295–313. [DOI] [PubMed] [Google Scholar]
- 5.Heifets L, Lindholm-Levy P. 1992. Pyrazinamide sterilizing activity in vitro against semidormant Mycobacterium tuberculosis bacterial populations. Am Rev Respir Dis 145:1223–1225. doi: 10.1164/ajrccm/145.5.1223. [DOI] [PubMed] [Google Scholar]
- 6.McCune RM, Feldmann FM, Lambert HP, McDermott W. 1966. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med 123:445–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCune RM, Feldmann FM, McDermott W. 1966. Microbial persistence. II. Characteristics of the sterile state of tubercle bacilli. J Exp Med 123:469–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchison DA. 1985. The action of antituberculosis drugs in short-course chemotherapy. Tubercle 66:219–225. doi: 10.1016/0041-3879(85)90040-6. [DOI] [PubMed] [Google Scholar]
- 9.Davies GR. 2010. Early clinical development of anti-tuberculosis drugs: science, statistics and sterilizing activity. Tuberculosis (Edinb) 90:171–176. doi: 10.1016/j.tube.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Rustomjee R, Lienhardt C, Kanyok T, Davies GR, Levin J, Mthiyane T, Reddy C, Sturm AW, Sirgel FA, Allen J, Coleman DJ, Fourie B, Mitchison DA, Gatifloxacin for TB (OFLOTUB) Study Team . 2008. A phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 12:128–138. [PubMed] [Google Scholar]
- 11.Pheiffer C, Carroll NM, Beyers N, Donald P, Duncan K, Uys P, van Helden P. 2008. Time to detection of Mycobacterium tuberculosis in BACTEC systems as a viable alternative to colony counting. Int J Tuberc Lung Dis 12:792–798. [PubMed] [Google Scholar]
- 12.Chigutsa E, Patel K, Denti P, Visser M, Maartens G, Kirkpatrick CMJ, McIlleron H, Karlsson MO. 2013. A time-to-event pharmacodynamic model describing treatment response in patients with pulmonary tuberculosis using days to positivity in automated liquid mycobacterial culture. Antimicrob Agents Chemother 57:789–795. doi: 10.1128/AAC.01876-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gumbo T, Louie A, Deziel MR, Liu W, Parsons LM, Salfinger M, Drusano GL. 2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother 51:3781–3788. doi: 10.1128/AAC.01533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gumbo T, Dona CS, Meek C, Leff R. 2009. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother 53:3197–3204. doi: 10.1128/AAC.01681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shandil RK, Jayaram R, Kaur P, Gaonkar S, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharath S, Balasubramanian V. 2007. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother 51:576–582. doi: 10.1128/AAC.00414-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayaram R, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharat S, Shandil RK, Kantharaj E, Balasubramanian V. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 47:2118–2124. doi: 10.1128/AAC.47.7.2118-2124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chideya S, Winston CA, Peloquin CA, Bradford WZ, Hopewell PC, Wells CD, Reingold AL, Kenyon TA, Moeti TL, Tappero JW. 2009. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis–Botswana. Clin Infect Dis 48:1685–1694. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiner M, Benator D, Burman W, Peloquin CA, Khan A, Vernon A, Jones B, Silva-Trigo C, Zhao Z, Hodge T, Tuberculosis Trials Consortium . 2005. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin Infect Dis 40:1481–1491. doi: 10.1086/429321. [DOI] [PubMed] [Google Scholar]
- 19.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. 2013. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 208:1464–1473. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP. 2002. Nonlinear systems in medicine. Yale J Biol Med 75:247–260. [PMC free article] [PubMed] [Google Scholar]
- 21.Salford Systems. 2001. MARS user guide. Salford Systems, San Diego, CA. [Google Scholar]
- 22.Lee YC, Lee TS, Lee WJ, Lin YC, Lee CK, Liew PL. 2012. Predictors of anemia after bariatric surgery using multivariate adaptive regression splines. Hepatogastroenterology 59:1378–1380. doi: 10.5754/hge10155. [DOI] [PubMed] [Google Scholar]
- 23.Koba M, Baczek T. 2013. The evaluation of multivariate adaptive regression splines for the prediction of antitumor activity of acridinone derivatives. Med Chem 9:1041–1050. doi: 10.2174/1573406411309080005. [DOI] [PubMed] [Google Scholar]
- 24.Lin HY, Wang W, Liu YH, Soong SJ, York TP, Myers L, Hu JJ. 2008. Comparison of multivariate adaptive regression splines and logistic regression in detecting SNP-SNP interactions and their application in prostate cancer. J Hum Genet 53:802–811. doi: 10.1007/s10038-008-0313-z. [DOI] [PubMed] [Google Scholar]
- 25.York TP, Eaves LJ, van den Oord EJ. 2006. Multivariate adaptive regression splines: a powerful method for detecting disease-risk relationship differences among subgroups. Stat Med 25:1355–1367. doi: 10.1002/sim.2292. [DOI] [PubMed] [Google Scholar]
- 26.Visser ME, Grewal HM, Swart EC, Dhansay MA, Walzl G, Swanevelder S, Lombard C, Maartens G. 2010. The effect of vitamin A and zinc supplementation on treatment outcomes in pulmonary tuberculosis: a randomized controlled trial. Am J Clin Nutr 93:93–100. doi: 10.3945/ajcn.110.001784. [DOI] [PubMed] [Google Scholar]
- 27.WHO. 2003. Treatment of tuberculosis: guidelines for national programmes, 3rd ed. WHO/CDS/TB/2003.313. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/HQ/2003/WHO_CDS_TB_2003.313_eng.pdf. [Google Scholar]
- 28.Chigutsa E, Visser ME, Swart EC, Denti P, Pushpakom S, Egan D, Holford NH, Smith PJ, Maartens G, Owen A, McIlleron H. 2011. The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: dosing implications. Antimicrob Agents Chemother 55:4122–4127. doi: 10.1128/AAC.01833-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chigutsa E, McIlleron H, Holford N. 2010. Parallel first order and mixed order elimination of pyrazinamide in South African patients with tuberculosis, abstr. 1946 Population Approach Group Europe (PAGE), 8 to 11 June 2010, Berlin, Germany. [Google Scholar]
- 30.Wilkins JJ, Langdon G, McIlleron H, Pillai G, Smith PJ, Simonsson US. 2011. Variability in the population pharmacokinetics of isoniazid in South African tuberculosis patients. Br J Clin Pharmacol 72:51–62. doi: 10.1111/j.1365-2125.2011.03940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jönsson S, Davidse A, Wilkins J, Van der Walt JS, Simonsson US, Karlsson MO, Smith P, McIlleron H. 2011. Population pharmacokinetics of ethambutol in South African tuberculosis patients. Antimicrob Agents Chemother 55:4230–4237. doi: 10.1128/AAC.00274-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Springer B, Lucke K, Calligaris-Maibach R, Ritter C, Böttger EC. 2009. Quantitative drug susceptibility testing of Mycobacterium tuberculosis by use of MGIT 960 and EpiCenter instrumentation. J Clin Microbiol 47:1773–1780. doi: 10.1128/JCM.02501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman JH. 1991. Multivariate adaptive regression splines. Ann Statist 19:1–67. doi: 10.1214/aos/1176347963. [DOI] [PubMed] [Google Scholar]
- 34.Gumbo T. 2010. New susceptibility breakpoints for first-line antituberculosis drugs based on antimicrobial pharmacokinetic/pharmacodynamic science and population pharmacokinetic variability. Antimicrob Agents Chemother 54:1484–1491. doi: 10.1128/AAC.01474-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salfinger M, Heifets LB. 1988. Determination of pyrazinamide MICs for Mycobacterium tuberculosis at different pHs by the radiometric method. Antimicrob Agents Chemother 32:1002–1004. doi: 10.1128/AAC.32.7.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conte JE Jr, Golden JA, McIver M, Zurlinden E. 2006. Intrapulmonary pharmacokinetics and pharmacodynamics of high-dose levofloxacin in healthy volunteer subjects. Int J Antimicrob Agents 28:114–121. doi: 10.1016/j.ijantimicag.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Almeida D, Nuermberger E, Tasneen R, Rosenthal I, Tyagi S, Williams K, Peloquin C, Grosset J. 2009. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob Agents Chemother 53:4178–4184. doi: 10.1128/AAC.00830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grosset J, Truffot-Pernot C, Lacroix C, Ji B. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother 36:548–551. doi: 10.1128/AAC.36.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srivastava S, Sherman C, Meek C, Leff R, Gumbo T. 2011. Pharmacokinetic mismatch does not lead to emergence of isoniazid- or rifampin-resistant Mycobacterium tuberculosis but to better antimicrobial effect: a new paradigm for antituberculosis drug scheduling. Antimicrob Agents Chemother 55:5085–5089. doi: 10.1128/AAC.00269-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Um KS, Wilson T, Collins D, de Lisle G, Jacobs WR Jr. 1994. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227–230. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- 41.Zimhony O, Cox JS, Welch JT, Vilcheze C, Jacobs WR Jr. 2000. Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis. Nat Med 6:1043–1047. doi: 10.1038/79558. [DOI] [PubMed] [Google Scholar]
- 42.Sloan DJ, Schipani A, Waterhouse D, Stone L, Mwandumba HC, Butterworth AE, Heyderman RS, Corbett EL, Khoo S, Davies G. 2014. Pharmacokinetic variability in TB therapy: associations with HIV and effect on outcome, abstr 106. Conference on Retroviruses and Opportunistic infections (CROI), 3 to 6 March 2014, Boston, MA. [Google Scholar]
- 43.Weiner M, Burman W, Vernon A, Benator D, Peloquin CA, Khan A, Weis S, King B, Shah N, Hodge T, Tuberculosis Trials Consortium . 2003. Low isoniazid concentrations and outcome of tuberculosis treatment with once-weekly isoniazid and rifapentine. Am J Respir Crit Care Med 167:1341–1347. doi: 10.1164/rccm.200208-951OC. [DOI] [PubMed] [Google Scholar]
- 44.Peloquin CA. 2002. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 62:2169–2183. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 45.Gumbo T, Chigutsa E, Pasipanodya J, Visser M, van Helden PD, Sirgel FA, McIlleron H. 2014. The pyrazinamide susceptibility breakpoint above which combination therapy fails. J Antimicrob Chemother 69:2420–2425. doi: 10.1093/jac/dku136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brindle RJ, Nunn PP, Githui W, Allen BW, Gathua S, Waiyaki P. 1993. Quantitative bacillary response to treatment in HIV-associated pulmonary tuberculosis. Am Rev Respir Dis 147:958–961. doi: 10.1164/ajrccm/147.4.958. [DOI] [PubMed] [Google Scholar]
- 47.Senkoro M, Mfinanga SG, Mørkve O. 2010. Smear microscopy and culture conversion rates among smear positive pulmonary tuberculosis patients by HIV status in Dar es Salaam, Tanzania. BMC Infect Dis 10:210. doi: 10.1186/1471-2334-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.