Abstract
The development of resistance to antimalarials is a major challenge for global malaria control. Artemisinin-based combination therapies, the newest class of antimalarials, are used worldwide but there have been reports of artemisinin resistance in Southeast Asia. In February through May 2013, we conducted open-label, nonrandomized therapeutic efficacy studies of artemether-lumefantrine (AL) and dihydroartemisinin-piperaquine (DP) in Zaire and Uíge Provinces in northern Angola. The parasitological and clinical responses to treatment in children with uncomplicated Plasmodium falciparum monoinfection were measured over 28 days, and the main outcome was a PCR-corrected adequate clinical and parasitological response (ACPR) proportion on day 28. Parasites from treatment failures were analyzed for the presence of putative molecular markers of resistance to lumefantrine and artemisinins, including the recently identified mutations in the K13 propeller gene. In the 320 children finishing the study, 25 treatment failures were observed: 24 in the AL arms and 1 in the DP arm. The PCR-corrected ACPR proportions on day 28 for AL were 88% (95% confidence interval [CI], 78 to 95%) in Zaire and 97% (91 to 100%) in Uíge. For DP, the proportions were 100% (95 to 100%) in Zaire, and 100% (96 to 100%) in Uíge. None of the treatment failures had molecular evidence of artemisinin resistance. In contrast, 91% of AL late-treatment failures had markers associated with lumefantrine resistance on the day of failure. The absence of molecular markers for artemisinin resistance and the observed efficacies of both drug combinations suggest no evidence of artemisinin resistance in northern Angola. There is evidence of increased lumefantrine resistance in Zaire, which should continue to be monitored.
INTRODUCTION
Successful malaria control and elimination programs rely on the availability of effective antimalarials. However, resistance to antimalarials poses a threat to the global effort in malaria control. Widespread resistance to conventional antimalarials, such as chloroquine, sulfadoxine-pyrimethamine, and mefloquine, motivated the World Health Organization (WHO) to recommend the use of artemisinin-based combination therapy (ACT) for the treatment of uncomplicated Plasmodium falciparum malaria (1). Although ACTs were introduced only in the 1990s, studies have documented the emergence of resistance to artemisinins in limited areas of the Greater Mekong Subregion (2–10), and a recent dispatch reported a case of frank artemisinin treatment failure in a traveler from Angola (11).
Due to the risk of the emergence and spread of antimalarial drug resistance, the WHO recommends that countries where malaria is endemic routinely retest the efficacy of first-line antimalarials using a standardized protocol (12). Reduced efficacy of ACTs can be due to resistance to the fast-acting artemisinin derivative, the longer-lasting partner drug, or both. Resistance to the artemisinin derivative is typically characterized by delayed parasite clearance. In contrast, resistance to the partner drug more often manifests as recrudescent infections, occurring more than 7 days after the start of treatment, following initial parasite clearance. As a complement to clinical evidence, parasites from treatment failures can be tested for the presence of molecular markers of resistance to the component drugs. Resistance to the commonly used partner drug lumefantrine has been tentatively associated with certain specific mutations in the pfmdr1 gene (13). For artemisinin resistance, a promising molecular marker in the propeller domain of the kelch protein, K13, was recently identified (9). Mutations in this gene have been found in resistant parasites in samples from Southeast Asia, the same region where resistance to chloroquine and sulfadoxine-pyrimethamine first arose and then spread to Africa. Mutations in this gene have also been found in a small proportion of samples from Africa, but none of these matched mutations associated with delayed clearance in Southeast Asia (10).
Angola, a country in Southern Africa where malaria is endemic, began recommending ACTs as first-line treatment for uncomplicated malaria in 2005. Currently, three ACTs are equally recommended for use in Angola: artemether-lumefantrine (AL), artesunate-amodiaquine (ASAQ), and dihydroartemisinin-piperaquine (DP). The most recent data on the efficacy of ACTs in Angola comes from a 2004 in vivo therapeutic efficacy study that found 100% PCR-corrected efficacy of ASAQ and AL in Huambo Province in central Angola (14).
Here, we report the results of back-to-back in vivo studies of AL and DP carried out in both Zaire and Uíge Provinces in northern Angola in 2013 designed to measure the therapeutic efficacy of each drug for the treatment of uncomplicated P. falciparum malaria. In addition, we report the results of screening the treatment failures for the presence of molecular markers associated with lumefantrine and artemisinin resistance.
MATERIALS AND METHODS
We followed the standard 28-day WHO protocol for in vivo therapeutic efficacy studies (12). The study was designed and powered to independently measure the efficacy of AL and DP in each province, for a total of four arms. The study was not designed to compare the two drugs; there was no randomization, and in each province patients were first enrolled in the AL arm until the target number was reached, and then patients were enrolled in the DP arm.
Study location and population.
Malaria transmission in Zaire Province, on the Atlantic coast in the northwest corner of the country, is classified as mesoendemic stable, while in Uíge Province, directly to the west of Zaire, transmission is classified as hyperendemic. Both provinces border the Democratic Republic of the Congo to the north. Enrollment and follow-up of patients took place between February and May of 2013 and coincided with the peak malaria transmission season.
In Zaire Province, the study took place in the outpatient clinics of the M'Banza Congo Municipal Hospital and the 11 de Novembro Maternal and Child Health Center, both located in the provincial capital. In Uíge Province, participants were enrolled and followed in the outpatient clinic of the General Hospital of Uíge, the main referral hospital for the province. Participants were children between 6 and 108 months (9 years) of age and were restricted to those living in close proximity to the clinics (generally within 5 km). To achieve a precision of 5% assuming an expected efficacy of 95%, the minimal sample size was calculated to be 73 for each arm, and 100 participants were planned to be enrolled to allow for loss to follow-up.
Study procedures.
Children in the specified age category coming into the outpatient clinics with fever or history of fever within 24 h were identified and screened for eligibility to participate in the study. Children were eligible to participate if they had uncomplicated P. falciparum monoinfection with a parasite density of between 2,000 and 100,000 asexual parasites/μl. Children were excluded from participating if they weighed less than 5 kg, were malnourished (defined as a weight for age Z-score of <3), were severely anemic (hemoglobin less than 5 g/dl), reported having taken drugs with antimalarial activity in the previous 2 weeks, had general danger signs or signs of severe malaria, or if their guardians were unwilling or unable to bring them back for all follow-up visits.
The first 100 children enrolled in each province were treated with AL (Coartem-D dispersible tablets; Novartis, Basel, Switzerland). The next 100 children were treated with DP (Duo-cotecxin; Beijing Holley-Cotec, Beijing, China). The manufacturers' weight-based dosing schedules were followed. If the child vomited within a half hour of taking the medication, the entire dose was repeated. If the child vomited between a half hour and an hour after taking the medication, a half-dose was given. Children who vomited more than once in 24 h were excluded. While all three doses of DP were directly observed in the clinic, three of the AL doses were observed in the clinic, and the three evening doses were given at home. The children's parents or guardians were given a dose to administer at night, together with a reserve dose in case of vomiting. Patients with cell phones were called twice in the evening during treatment days—once to remind them about giving the dose and 1 h later to inquire about vomiting.
Clinical and parasitological responses to treatment were assessed over a 28-day follow-up, with clinic visits including a clinical exam on days 0, 1, 2, 3, 7, 14, 21, and 28. The drugs were administered on days 0, 1, and 2. Parasitemia was measured through microscopy on days 0, 2, 3, 7, 14, 21, and 28 of follow-up. Slides were read by two technicians posted in each of the three participating health facilities, following standard WHO procedures (12). A subset of slides was quality controlled during periodic supervisory visits by an external reader. Hemoglobin was measured on days 0, 14, and 28 using a HemoCue (AB Leo Diagnostics, Helsinborg, Sweden) machine. Dried blood spots were collected on days 0, 7, 14, 21, and 28 for potential molecular analysis and were stored in individual plastic bags with desiccant at room temperature for later transport to CDC laboratories in Atlanta, GA.
Participants were classified as early treatment failures if they developed signs of severe malaria in the presence of parasitemia up to day 3, if day 2 parasitemia was higher than day 0 parasitemia, if day 3 parasitemia was higher than 25% of day 0 parasitemia in the absence of fever, or if there was any parasitemia on day 3 in the presence of fever. Participants were classified as late treatment failures if they did not demonstrate early treatment failure and had at least 1 blood slide positive for asexual P. falciparum parasites after day 3.
Patients meeting criteria of treatment failure were treated with intravenous quinine and removed from further participation in the study. Patients negative for malaria on day 28 and not having previously met any treatment failure criteria were classified as having an adequate treatment response.
Laboratory procedures.
Genomic DNA was isolated from dried blood spots using the QIAamp DNA blood minikit (Qiagen, Valencia, CA) per the manufacturer's instruction. Confirmation of P. falciparum infection and DNA quality assessment were conducted using photoinduced electron transfer (PET)-PCR (15).
A panel of seven neutral microsatellite markers on six chromosomes was chosen to determine the genotype on the parasite genetic background and compare the genotype profiles of parasites collected at the time of enrollment and at the time of failure (16–18). The fragments were amplified by PCR, separated on an ABI3130xl genetic analyzer, and scored using GeneMapper v3.1 (Applied Biosystems, Foster City, CA) according to previously described methods (19). An allele was considered different when the length of the allele was more than 2 bp different. Patient samples from day 0 and day of failure sharing allele sizes for at least six of seven neutral microsatellite loci were considered recrudescent infections (20, 21). The multiplicity of infection was defined as the maximum number of alleles at a single locus from a single sample.
Putative markers of lumefantrine and artemisinin resistance were also examined for all treatment failures on day 0 and day of failure (no known molecular markers for piperaquine exist). The pfmdr1 copy number was estimated by real-time PCR following a previously described method (22). Two regions of pfmdr1, covering codons 86 to 184 and 1034 to 1246, were amplified and analyzed for polymorphisms by direct sequencing using a previously described method (23). The pfmdr1 haplotypes were constructed for treatment failures from the pfmdr1 sequences. Previously reported polymorphisms associated with artemisinin resistance on chromosome 10 (MAL10-688956) and chromosome 13 (MAL13-1718319) (8) were also assayed by PET-PCR and pyrosequencing. In addition, the recently identified artemisinin resistance markers on the kelch K13-propeller gene were assayed using Sanger sequencing (see the methods in the supplemental material).
Statistical and sequence analyses.
Double data entry was done daily using Epi Info 7 (CDC, Atlanta, Georgia). All statistical analysis was done using R version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria). All sequence analysis was done using Geneious Pro R7 (Biomatters, Inc., Auckland, New Zealand).
The main outcome analyzed was the 28-day PCR-corrected proportion of adequate clinical and parasitological response (ACPR), defined as the fraction of participants that finished the study that were classified as having adequate treatment responses, excluding any patients with PCR-determined reinfection or any patients excluded over the course of the study. Secondary outcomes included (i) PCR-uncorrected 28-day ACPR, where cases of reinfection were included as treatment failures, and (ii) parasite clearance rates for day 3, defined as the proportion of evaluable patients without any asexual parasites on that day.
Kaplan-Meier estimates of the survival function for each arm were calculated, and P values corresponding to the significance of differences in time to failure between provinces were calculated from the log rank test. For this analysis, data from patients lost to follow-up or patients excluded from the per-protocol analysis due to protocol violation were included up until the final visit before exclusion and censure. In the PCR-corrected analysis, cases of reinfections were censured at the day of reinfection. The PCR-corrected and PCR-uncorrected cumulative success rates on day 28 were estimated using the Kaplan-Meier estimator, with confidence intervals calculated using the Greenwood method.
Ethical considerations.
Official human subject oversight approval to conduct the evaluation was obtained from the Centers for Disease Control and Prevention in Atlanta, GA, and the National Malaria Control Program in Angola. Written informed consent was obtained from participants' parents or guardians before enrollment.
RESULTS
Screening.
The target enrollment numbers of 100 children in each arm were reached within 2 months in both provinces. Malaria positivity in screened children ranged from 47% to 69% in the four arms (Table 1). At least 5% of tested children had a high parasitemia (>100,000 parasites/μl) in each arm. In order to enroll 100 children for each arm, we needed to screen between 188 and 329 children for each arm. The most common cause of exclusion during screening was the presence of no parasitemia.
TABLE 1.
Characteristics of patients at enrollment in therapeutic efficacy studies in Zaire and Uíge Provinces, Angola, in 2013
| Parameter |
n (%) in study arm |
|||
|---|---|---|---|---|
| AL |
DP |
|||
| Zaire | Uíge | Zaire | Uíge | |
| Initial screen | 305 | 188 | 329 | 230 |
| Slide read | 287 (94) | 173 (92) | 326 (99) | 222 (97) |
| Parasitemia (parasites/ml) | ||||
| Negative for plasmodium | 112 (39) | 53 (31) | 174 (53) | 78 (35) |
| Low (<2,000) | 23 (8.0) | 10 (5.8) | 20 (6.1) | 15 (6.7) |
| Target parasitemia (2,000–100,000) | 102 (36) | 101 (58) | 104 (32) | 102 (46) |
| High parasitemia (>100,000) | 50 (17) | 9 (5.2) | 28 (8.6) | 28 (13) |
Outcomes.
The rates of loss to follow-up were 11% and 10% in the AL arms in Zaire and Uíge, respectively, and 20% and 9% in the DP arms in Zaire and Uíge, respectively (Table 2). There were a total of 32 patients excluded from the study after enrollment. Reasons for this included the incorrect dose taken or dose not taken at home (12), persistent vomiting (8), taking another antimalarial after enrollment (7), incorrect enrollment of a child not meeting the inclusion criteria (3), and development of signs of severe illness in the absence of parasitemia (2).
TABLE 2.
Sample size by study arm in therapeutic efficacy studies in Zaire and Uíge Provinces, Angola, in 2013
| Parameter |
n (%) in study arm |
|||
|---|---|---|---|---|
| AL |
DP |
|||
| Zaire | Uíge | Zaire | Uíge | |
| Total enrolled | 101 | 99 | 102 | 100 |
| Lost to follow-up | 11 (11) | 10 (10) | 20 (20) | 9 (9) |
| Excluded/censured | 11 (11) | 11 (11) | 2 (2) | 8 (8) |
| Finished study | 79 (78) | 78 (79) | 80 (78) | 83 (83) |
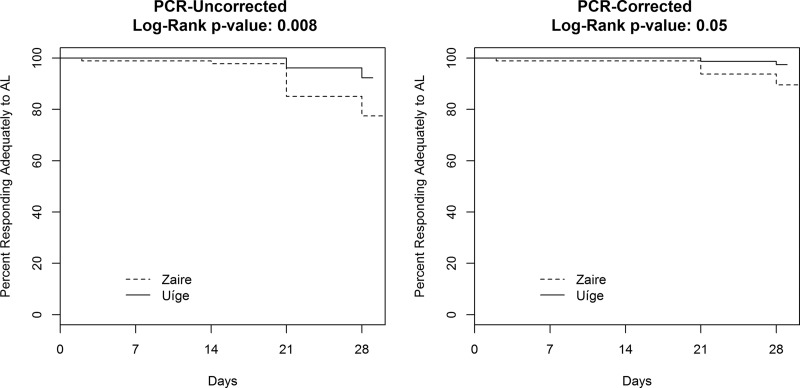
This resulted in 79 and 78 children finishing the study in the AL arms in Zaire and Uíge, respectively, and 80 children in the DP Zaire arm and 83 children in the DP Uíge arm (Table 3). There were 25 treatment failures documented during the study: 24 in the AL arms and 1 in the DP arms. One of the treatment failures was an early treatment failure from the AL Zaire arm; in this case the child's hemoglobin (initially 5.5 g/dl) dropped below 5 g/dl on day 2, with a parasitemia of 615 parasites/μl. The PCR-uncorrected ACPR rates were 77.2% (95% confidence interval [CI], 66 to 86%) in the AL Zaire arm, 92.3% (84 to 97%) in the AL Uíge arm, 98.8% (93 to 100%) in the DP Zaire arm, and 100% (96 to 100%) in the DP Uíge arm. Time to failure was significantly shorter in the AL Zaire arm than in the AL Uíge arm in the PCR-uncorrected analysis, with a P value of 0.008 (Fig. 1). The PCR-uncorrected cumulative success rates on day 28 for each study arm closely matched the ACPR proportion on day 28 (Table 3).
TABLE 3.
Response to treatment in therapeutic efficacy studies in Zaire and Uíge Provinces, Angola, in 2013
| Parameter | Result in study arm |
|||
|---|---|---|---|---|
| AL |
DP |
|||
| Zaire (n = 79) | Uíge (n = 78) | Zaire (n = 80) | Uíge (n = 83) | |
| ACPR, n (%) | 61 (77) | 72 (92) | 79 (99) | 83 (100) |
| Treatment failure, n (%) | 18 (23) | 6 (7.7) | 1 (1.2) | 0 (0) |
| Early | 1 (1.3) | 0 (0) | 0 (0) | 0 (0) |
| Late | 17 (22) | 6 (7.7) | 1 (1.2) | 0 (0) |
| Day 14 | 1 (1.3) | 0 (0) | 0 (0) | 0 (0) |
| Day 21 | 10 (13) | 3 (3.8) | 0 (0) | 0 (0) |
| Day 28 | 6 (7.6) | 3 (3.8) | 1 (1.2) | 0 (0) |
| Reinfections | 10 (13) | 4 (5.1) | 1 (1.2) | 0 (0) |
| Recrudescence | 7 (8.9) | 2 (2.6) | 0 (0) | 0 (0) |
| Day 3 clearance, % (95% CI) | 100 (96–100) | 97.6 (92–100) | 100 (96–100) | 100 (96–100) |
| Cumulative success rate on day 28, % (95% CI) | ||||
| PCR uncorrected | 77.4 (69–87) | 92.3 (87–98) | 98.8 (96–100) | 100a |
| PCR corrected | 89.6 (83–97) | 97.4 (94–100) | 100a | 100a |
| Proportion of ACPR on day 28, % (95% CI) | ||||
| PCR uncorrected | 77.2 (66–86) | 92.3 (84–97) | 98.8 (93–100) | 100 (96–100) |
| PCR corrected | 88.4 (78–95) | 97.3 (91–100) | 100 (95–100) | 100 (96–100) |
Confidence intervals were not calculated for the Kaplan-Meier estimator when the rate was 100%.
FIG 1.
PCR-uncorrected and PCR-corrected survival functions for time until failure from a 2013 therapeutic efficacy study of AL in Zaire and Uíge Provinces, Angola. DP data are not shown as efficacy was uniformly high.
Using microsatellite genotyping analysis (see Table S1 in the supplemental material), we were able to classify 15 of the 24 late treatment failures as reinfections and 9 as recrudescent infections, with 7 of the recrudescent infections occurring in the AL Zaire arm and the remaining 2 in the AL Uíge arm. This translated to 28-day PCR-corrected ACPR proportions of 88.4% (95% CI, 78 to 95%) in the AL Zaire arm, 97.3% (91 to 100%) in the AL Uíge arm, 100% (95 to 100%) in the DP Zaire arm, and 100% (96 to 100%) in the DP Uíge arm. The survival curves for the AL arms in the two provinces were also significantly different in the PCR-corrected analysis, with a P value of 0.05 (Fig. 1). The PCR-corrected cumulative success rates on day 28 for each study arm closely matched the ACPR proportion on day 28 (Table 3).
For AL, day 3 clearance rates were 100% (95% CI, 96 to 100%) in Zaire and 97.6% (92 to 100%) in Uíge. For DP, all patients in both provinces cleared their parasitemia by day 3 (Table 3).
Molecular analyses.
The 25 treatment failures were further characterized using previously identified molecular markers associated with drug resistance to either lumefantrine or artemisinin (Table 4). All failures had a single copy of pfmdr1. Sequencing of the pfmdr1 gene only showed mutations at codons 86, 184, and 1246. Haplotypes constructed from these codons reflected haplotypes previously associated with lumefantrine resistance (13). While six different pfmdr1 haplotypes were observed in day 0 samples, only three haplotypes were present in day of failure samples. The NFD haplotype was found on day of failure in four recrudescences (44% of AL recrudescences) and four reinfections (31% of AL reinfections). The NYD haplotype was found on day of failure in five recrudescences (56%) and eight reinfections (54%). The YYD haplotype was only found on the day of failure in two reinfections (15%).
TABLE 4.
Molecular characteristics of 25 observed treatment failures during therapeutic efficacy studies in Zaire and Uíge Provinces, Angola, in 2013
| Patient ID no. | Treatment arma | Classificationb | MOIc | Resistance marker(s) on day 0 and day of failured |
||||
|---|---|---|---|---|---|---|---|---|
|
pfmdr1e |
SNPf |
K13 | ||||||
| CN | Haplotype | Chr 10 | Chr 13 | |||||
| B313g | AL-Z | ETF | 2 | 1 | NYD | wt | wt | wt |
| A111 | AL-U | RECR | 1 | 1 | NFD | wt | wt | wt |
| A145 | AL-U | RECR | 1 | 1 | NYD | wt | wt | wt |
| B314 | AL-Z | RECR | 1 | 1 | NYD | wt | wt | wt |
| B384 | AL-Z | RECR | 1 | 1 | NYD | wt | wt | wt |
| B385 | AL-Z | RECR | 2 | 1 | NFD | wt | wt | wt |
| B399 | AL-Z | RECR | 2 | 1 | NFD | wt | wt | wt |
| B404 | AL-Z | RECR | 2 | 1 | NFD | wt | wt | wt |
| B416 | AL-Z | RECR | 2 | 1 | NYD | wt | wt | wt |
| B422 | AL-Z | RECR | 2/1 | 1 | NYD+NFD/NYD | wt | wt | wt |
| A114 | AL-U | REIN | 1 | 1 | NYD | wt | wt | wt |
| A115 | AL-U | REIN | 2/1 | 1 | YFD/YYD | wt | wt | wt |
| A144 | AL-U | REIN | 4/1 | 1 | NYD | wt | wt | wt |
| A171 | AL-U | REIN | 1/2 | 1 | YYD/NFD | wt | wt | wt |
| B304 | AL-Z | REIN | 1 | 1 | NFD | wt | wt | wt |
| B312 | AL-Z | REIN | 1 | 1 | NYD | wt | wt | wt |
| B371 | AL-Z | REIN | 2 | 1 | NFD | wt | wt | wt |
| B375 | AL-Z | REIN | 1/2 | 1 | NFD/YYD | wt | wt | wt |
| B386 | AL-Z | REIN | 1 | 1 | YYY/NYD | wt | wt | wt |
| B387 | AL-Z | REIN | 1 | 1 | NFY/NYD | wt | wt | wt |
| B398 | AL-Z | REIN | 1 | 1 | NYD | wt | wt | wt |
| B402 | AL-Z | REIN | 2/1 | 1 | YYY/NYD | wt | wt | wt |
| B421 | AL-Z | REIN | 1/2 | 1 | NYD/NFD | wt | wt | wt |
| B423 | AL-Z | REIN | 1/2 | 1 | NFD/NYD | wt | wt | wt |
| C560 | DP-Z | REIN | 1 | 1 | YYD | wt | wt | wt |
AL, artemether-lumefantrine; DP, dihydroartemisinin-piperaquine; Z, Zaire; U, Uíge.
ETF, early treatment failure; RECR, recrudescence; REIN, reinfection.
Multiplicities of infection (MOI) either are the same for day 0 and the day of failure or are reported as day 0 MOI/day of failure MOI.
Resistance markers either are the same for day 0 and the day of failure or are reported as day 0/day of failure. wt, wild type.
“CN” represents the P. falciparum multidrug resistance gene 1 copy number, and “haplotype” represents the haplotype constructed from mutations N86Y, Y184F, and D1246Y.
Shown are the chromosome 10 (Chr 10) SNP at position 688956 and Chr 13 SNP at position 1718319 associated with artemisinin resistance.
Molecular data for early treatment failure are available for day 0 only.
All isolates, both on day 0 and the day of treatment failure, were wild type at loci on chromosomes 10 and 13, where single-nucleotide polymorphisms (SNPs) had originally been shown to be associated with artemisinin resistance. The kelch K13 propeller gene, the site of the recently identified molecular markers of artemisinin resistance, was wild type for all day 0 and day of treatment failure isolates.
Conclusions.
The 28-day PCR-corrected efficacies for the AL Uíge arm and both DP arms are consistent with previously reported data for AL (24, 25) and DP (26, 27) and with high clinical efficacy estimates measured in African children prior to the widespread use of ACT. The point estimate for the 28-day PCR-corrected ACPR proportion in the AL Zaire arm of 88.4% is below the 90% WHO target efficacy for first-line treatment policy (12), but the 95% confidence interval of 78 to 95% is wide and includes the 95% secondary WHO target efficacy for ACTs. While the efficacies of DP are uniformly high in both provinces, AL is significantly less efficacious in Zaire Province than in Uíge Province.
The true efficacy of AL could be underestimated here, as we were not able to observe the three evening doses. However, previous studies comparing the efficacy of fully supervised and unsupervised (five out of six doses taken at home) dosing did not find significant differences in measured efficacies (28, 29).
Meta-analyses show that DP has higher PCR-corrected efficacies than AL, at least in the African setting (30). Moreover, DP has consistently been shown to have a lower reinfection rate in 28-day follow-up studies (30), likely due to the difference in elimination half-lives between piperaquine (23 days in adults) and lumefantrine (4.5 days in adults) (31, 32).
Because of nonrandom assignment to the AL and DP arms, we cannot statistically compare the efficacies of the two drugs in this setting. Participants were enrolled in the DP arms only after the AL enrollment was finished, and consequently the follow-up for the DP arms systematically occurred later in the transmission period. Therefore, the smaller number of reinfections in the DP arm might be due to a lower transmission intensity and a lower risk of reinfection. The 28-day follow-up period might also mean some DP treatment failures went unobserved because of the longer half-life of piperaquine.
Our finding of a single pfmdr1 copy number is similar to those from previous studies from Africa, where a single pfmdr1 copy number has been found in isolates from patients who experienced AL treatment failure (13). We found high rates of two pfmdr1 haplotypes containing mutations previously associated with lumefantrine resistance, NFD and NYD, in isolates from reinfections and recrudescences from the AL arms in both provinces. Our results are consistent with data from Uganda showing selection of the 86N, 184F, and 1246D mutations following treatment with AL (33, 34). Malmberg et al. recently reported that parasites with the pfmdr1 NFD and NYD haplotypes could tolerate 15- and 7-fold-higher lumefantrine blood concentrations, respectively, than parasites with the YYY haplotype (35). Overall, 100% of day of failure isolates from recrudescences and 87% of day of failure reinfections in the AL arms had either an NFD or NYD haplotype.
The pfmdr1 results, together with the uniformly wild-type chromosome 10 and 13 and kelch K13 propeller data and parasite clearance rates, suggest that the observed failure rates of AL are likely due to lumefantrine resistance in circulating parasites. The significant difference in efficacy of AL between provinces suggests that rates of lumefantrine resistance might be higher in Zaire than Uíge.
The measured efficacy and parasite clearance rates of both drugs, combined with the confirmed absence of putative molecular markers of artemisinin resistance, are consistent with a parasite population still exquisitely sensitive to artemisinins. There is sufficient evidence for the continued use of AL and DP in northern Angola. Although the measured efficacy of AL in Zaire Province is below the 90% threshold at which WHO suggests changing treatment policy, a single estimate with wide confidence intervals and limited in time and space does not necessarily provide sufficient evidence for policy change. However, the efficacy of AL and molecular markers of resistance should be monitored in Zaire Province and at other sites across the country due to the apparent existence of lumefantrine resistance in the parasite population and the potential for selective pressure to intensify it. A follow-up therapeutic efficacy study of AL is planned to take place in Zaire Province and other sites in early 2015. These data should help provide a more comprehensive picture of antimalarial resistance and inform decisions regarding national treatment policy in Angola.
Supplementary Material
ACKNOWLEDGMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This work was supported by the Centers for Disease Control and Prevention and the President's Malaria Initiative.
The authors declare they do not have any commercial or other associations that might pose a conflict of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04181-14.
REFERENCES
- 1.World Health Organization. 2012. Guidelines for the treatment of malaria. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wongsrichanalai C, Meshnick SR. 2008. Declining artesunate-mefloquine efficacy against falciparum malaria on the Cambodia-Thailand border. Emerg Infect Dis 14:716–719. doi: 10.3201/eid1405.071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM, Artemisinin Resistance in Cambodia 1 Study Consortium . 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 5.Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. 2012. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satimai W, Sudathip P, Vijaykadga S, Khamsiriwatchara A, Sawang S, Potithavoranan T, Sangvichean A, Delacollette C, Singhasivanon P, Kaewkungwal J, Lawpoolsri S. 2012. Artemisinin resistance containment project in Thailand. II. Responses to mefloquine-artesunate combination therapy among falciparum malaria patients in provinces bordering Cambodia. Malar J 11:300. doi: 10.1186/1475-2875-11-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheeseman IH, Miller BA, Nair S, Nkhoma S, Tan A, Tan JC, Al Saai S, Phyo AP, Moo CL, Lwin KM, McGready R, Ashley E, Imwong M, Stepniewska K, Yi P, Dondorp AM, Mayxay M, Newton PN, White NJ, Nosten F, Ferdig MT, Anderson TJ. 2012. A major genome region underlying artemisinin resistance in malaria. Science 336:79–82. doi: 10.1126/science.1215966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takala-Harrison S, Clark TG, Jacob CG, Cummings MP, Miotto O, Dondorp AM, Fukuda MM, Nosten F, Noedl H, Imwong M, Bethell D, Se Y, Lon C, Tyner SD, Saunders DL, Socheat D, Ariey F, Phyo AP, Starzengruber P, Fuehrer HP, Swoboda P, Stepniewska K, Flegg J, Arze C, Cerqueira GC, Silva JC, Ricklefs SM, Porcella SF, Stephens RM, Adams M, Kenefic LJ, Campino S, Auburn S, MacInnis B, Kwiatkowski DP, Su XZ, White NJ, Ringwald P, Plowe CV. 2013. Genetic loci associated with delayed clearance of Plasmodium falciparum following artemisinin treatment in Southeast Asia. Proc Natl Acad Sci U S A 110:240–245. doi: 10.1073/pnas.1211205110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B. 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Hong N. 2014. Severe malaria not responsive to artemisinin derivatives in man returning from Angola to Vietnam. Emerg Infect Dis 20:1199–1202. doi: 10.3201/eid2007.140155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. 2009. Methods for surveillance of antimalarial drug efficacy. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 13.Nzila A, Okombo J, Ohuma E, Al-Thukair A. 2012. Update on the in vivo tolerance and in vitro reduced susceptibility to the antimalarial lumefantrine. J Antimicrob Chemother 67:2309–2315. doi: 10.1093/jac/dks252. [DOI] [PubMed] [Google Scholar]
- 14.Guthmann J, Cohuet S, Rigutto C, Fortes F, Saraiva N, Kiguli J, Kyomuhendo J, Francis M, Noël F, Mulemba M. 2006. High efficacy of two artemisinin-based combinations (artesunate+ amodiaquine and artemether+ lumefantrine) in Caala, Central Angola. Am J Trop Med Hyg 75:143. [PubMed] [Google Scholar]
- 15.Lucchi NW, Narayanan J, Karell MA, Xayavong M, Kariuki S, DaSilva AJ, Hill V, Udhayakumar V. 2013. Molecular diagnosis of malaria by photo-induced electron transfer fluorogenic primers: PET-PCR. PLoS One 8:e56677. doi: 10.1371/journal.pone.0056677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson TJ, Haubold B, Williams JT, Estrada-Franco JG, Richardson L, Mollinedo R, Bockarie M, Mokili J, Mharakurwa S, French N, Whitworth J, Velez ID, Brockman AH, Nosten F, Ferreira MU, Day KP. 2000. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol Biol Evol 17:1467–1482. doi: 10.1093/oxfordjournals.molbev.a026247. [DOI] [PubMed] [Google Scholar]
- 17.Anderson TJ, Su XZ, Bockarie M, Lagog M, Day KP. 1999. Twelve microsatellite markers for characterization of Plasmodium falciparum from finger-prick blood samples. Parasitology 119:113–125. doi: 10.1017/S0031182099004552. [DOI] [PubMed] [Google Scholar]
- 18.McCollum AM, Mueller K, Villegas L, Udhayakumar V, Escalante AA. 2007. Common origin and fixation of Plasmodium falciparum dhfr and dhps mutations associated with sulfadoxine-pyrimethamine resistance in a low-transmission area in South America. Antimicrob Agents Chemother 51:2085–2091. doi: 10.1128/AAC.01228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffing SM, Mixson-Hayden T, Sridaran S, Alam MT, McCollum AM, Cabezas C, Marquino Quezada W, Barnwell JW, De Oliveira AM, Lucas C, Arrospide N, Escalante AA, Bacon DJ, Udhayakumar V. 2011. South American Plasmodium falciparum after the malaria eradication era: clonal population expansion and survival of the fittest hybrids. PLoS One 6:e23486. doi: 10.1371/journal.pone.0023486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenhouse B, Myrick A, Dokomajilar C, Woo JM, Carlson EJ, Rosenthal PJ, Dorsey G. 2006. Validation of microsatellite markers for use in genotyping polyclonal Plasmodium falciparum infections. Am J Trop Med Hyg 75:836–842. [PMC free article] [PubMed] [Google Scholar]
- 21.Shaukat AM, Gilliams EA, Kenefic LJ, Laurens MB, Dzinjalamala FK, Nyirenda OM, Thesing PC, Jacob CG, Molyneux ME, Taylor TE. 2012. Clinical manifestations of new versus recrudescent malaria infections following anti-malarial drug treatment. Malar J 11:1–6. doi: 10.1186/1475-2875-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price RN, Uhlemann AC, Brockman A, McGready R, Ashley E, Phaipun L, Patel R, Laing K, Looareesuwan S, White NJ, Nosten F, Krishna S. 2004. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malmberg M, Ngasala B, Ferreira PE, Larsson E, Jovel I, Hjalmarsson A, Petzold M, Premji Z, Gil JP, Bjorkman A, Martensson A. 2013. Temporal trends of molecular markers associated with artemether-lumefantrine tolerance/resistance in Bagamoyo district, Tanzania. Malar J 12:103. doi: 10.1186/1475-2875-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falade C, Makanga M, Premji Z, Ortmann C-E, Stockmeyer M, de Palacios PI. 2005. Efficacy and safety of artemether–lumefantrine (Coartem) tablets (six-dose regimen) in African infants and children with acute, uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg 99:459–467. doi: 10.1016/j.trstmh.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Makanga M, Bassat Q, Falade CO, Premji ZG, Krudsood S, Hunt P, Walter V, Beck H-P, Marrast A-C, Cousin M. 2011. Efficacy and safety of artemether-lumefantrine in the treatment of acute, uncomplicated Plasmodium falciparum malaria: a pooled analysis. Am J Trop Med Hyg 85:793. doi: 10.4269/ajtmh.2011.11-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nambozi M, Van Geertruyden J-P, Hachizovu S, Chaponda M, Mukwamataba D, Mulenga M, Ubben D, D'Alessandro U. 2011. Safety and efficacy of dihydroartemisinin-piperaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Zambian children. Malar J 10:50. doi: 10.1186/1475-2875-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zwang J, Ashley EA, Karema C, D'Alessandro U, Smithuis F, Dorsey G, Janssens B, Mayxay M, Newton P, Singhasivanon P. 2009. Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One 4:e6358. doi: 10.1371/journal.pone.0006358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piola P, Fogg C, Bajunirwe F, Biraro S, Grandesso F, Ruzagira E, Babigumira J, Kigozi I, Kiguli J, Kyomuhendo J. 2005. Supervised versus unsupervised intake of six-dose artemether-lumefantrine for treatment of acute, uncomplicated Plasmodium falciparum malaria in Mbarara, Uganda: a randomised trial. Lancet 365:1467–1473. doi: 10.1016/S0140-6736(05)66416-1. [DOI] [PubMed] [Google Scholar]
- 29.Ngasala BE, Malmberg M, Carlsson AM, Ferreira PE, Petzold MG, Blessborn D, Bergqvist Y, Gil JP, Premji Z, Björkman A. 2011. Efficacy and effectiveness of artemether-lumefantrine after initial and repeated treatment in children <5 years of age with acute uncomplicated Plasmodium falciparum malaria in rural Tanzania: a randomized trial. Clin Infect Dis 52:873–882. doi: 10.1093/cid/cir066. [DOI] [PubMed] [Google Scholar]
- 30.Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. 2009. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst Rev 2009:CD007483. doi: 10.1002/14651858.CD007483.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hung TY, Davis TM, Ilett KF, Karunajeewa H, Hewitt S, Denis MB, Lim C, Socheat D. 2004. Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria. Br J Clin Pharmacol 57:253–262. doi: 10.1046/j.1365-2125.2003.02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ezzet F, Mull R, Karbwang J. 1998. Population pharmacokinetics and therapeutic response of CGP 56697 (artemether+ benflumetol) in malaria patients. Br J Clin Pharmacol 46:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conrad MD, LeClair N, Arinaitwe E, Wanzira H, Kakuru A, Bigira V, Muhindo M, Kamya MR, Tappero JW, Greenhouse B. 2014. Comparative impacts over 5 years of artemisinin-based combination therapies on P. falciparum polymorphisms that modulate drug sensitivity in Ugandan children. J Infect Dis 210:344–353. doi: 10.1093/infdis/jiu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mbogo GW, Nankoberanyi S, Tukwasibwe S, Baliraine FN, Nsobya SL, Conrad MD, Arinaitwe E, Kamya M, Tappero J, Staedke SG. 2014. Temporal changes in prevalence of molecular markers mediating antimalarial drug resistance in a high malaria transmission setting in Uganda. Am J Trop Med Hyg 91:54–61. doi: 10.4269/ajtmh.13-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malmberg M, Ferreira PE, Tarning J, Ursing J, Ngasala B, Bjorkman A, Martensson A, Gil JP. 2013. Plasmodium falciparum drug resistance phenotype as assessed by patient antimalarial drug levels and its association with pfmdr1 polymorphisms. J Infect Dis 207:842–847. doi: 10.1093/infdis/jis747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.