LETTER
As of 23 July 2014, 837 laboratory-confirmed cases of Middle East respiratory syndrome (MERS-CoV) infection, including 291 deaths, had been reported to the WHO (http://www.who.int/csr/disease/coronavirus_infections/en/), raising concerns about its pandemic potential and calling for the development of vaccines and therapeutics against MERS-CoV infection.
We previously identified peptidic HIV-1 and severe acute respiratory syndrome coronavirus (SARS-CoV) fusion inhibitors (1, 2), which led to the development of MERS-CoV spike (S) protein-mediated cell-cell fusion and six-helix bundle (6-HB) formation assays. Using these assays, we identified a peptide from the MERS-CoV S protein HR2 region, termed HR2P, that inhibited 6-HB formation, cell-cell fusion, and MERS-CoV replication (3). To identify small-molecule MERS-CoV fusion inhibitors, we used a cell-cell fusion assay to screen 1,280 compounds from an FDA-approved drug library obtained from MicroSource Discovery Systems, Inc. (Gaylordsville, CT), but none of the compounds at 10 μM could significantly inhibit MERS-CoV S-mediated membrane fusion.
Most recently, de Wilde et al. (4) and Dyall et al. (5) used a cytopathogenic effect assay to screen several hundred compounds from FDA-approved drug libraries and identified a series of compounds that inhibit the replication of both MERS-CoV and SARS-CoV in the low micromolar range. Although the mechanisms of action have not been defined, both groups suggested that some of the drugs, such as chlorpromazine (a clathrin-mediated endocytosis inhibitor), might block virus entry (4, 5).
Coronavirus enters the target cell via endocytosis or plasma membrane fusion, while the latter is the main pathway for MERS-CoV entry (3). To determine whether these reported MERS-CoV replication inhibitors also block virus entry via plasma membrane fusion, we tested 16 compounds with MERS-CoV replication-inhibiting activity available in the FDA-approved drug library from MicroSource and ribavirin and mycophenolic acid (Sigma-Aldrich), which were reported to inhibit MERS-CoV replication (6), for inhibitory activity on MERS-CoV S-mediated cell-cell fusion using HR2P as a control. A cell-cell fusion inhibition assay was performed as we described before (3). Briefly, Huh-7 cells were used as target cells and 293T cells, which simultaneously express MERS-CoV S protein and enhanced green fluorescent protein (293T/MERS/EGFP), were used as effector cells. 293T/MERS/EGFP cells were cocultured with HR2P or compounds at graded concentrations (initial concentration of 40 μM) for 30 min, added to Huh-7 cells, and then incubated at 37°C for 2 to 4 h (3). As expected, HR2P inhibited cell-cell fusion with an IC50 (half-maximal inhibitory concentration) of ∼1 μM and effectively blocked 6-HB formation. In contrast, most of these compounds exhibited no significant inhibitory activity at 40 μM, except for the three neurotransmitter inhibitors (chlorpromazine, promethazine, and fluphenazine), which were moderate inhibitors of cell-cell fusion with IC50s of about 20, 20, and 29 μM, respectively (Table 1).
TABLE 1.
Inhibitory activities of the reported MERS-CoV replication inhibitors available in the FDA-approved drug library from MicroSource and the control HR2P peptide on MERS-CoV S-mediated cell-cell fusion and 6-HB formation
| Drug | Original function | IC50(s) (μM)a for inhibition of: |
CC50 (μM)a,b | Inhibition of 6-HB formation at 40 μMa,c | ||
|---|---|---|---|---|---|---|
| MERS-CoV replication | Cell-cell fusion | Clathrin-mediated endocytosis | ||||
| Lopinavir | HIV protease inhibitor | 17.10 (4) | >40 | >40 | >40 | − |
| Loperamide | Opioid receptor agonist | 5.90 (4) | >40 | >40 | >40 | − |
| Chloroquine diphosphate | Antiparasitic agent | 4.10 (4), 6.28 (5) | >40 | >40 | >40 | − |
| Hydroxychloroquine sulfate | Antiparasitic agent | 8.28 (5) | >40 | >40 | >40 | − |
| Amodiaquine dihydrochloride | Antiparasitic agent | 6.21 (5) | >40 | >40 | >40 | − |
| Chlorpromazine hydrochloride | Neurotransmitter inhibitor | 8.80 (4); 9.51 (5) | 23.33 ± 2.89 | 7.24 ± 2.55 | >40 | − |
| Promethazine hydrochloride | Neurotransmitter inhibitor | 11.80 (5) | 16.67 ± 7.22 | 7.48 ± 4.53 | >40 | − |
| Fluphenazine hydrochloride | Neurotransmitter inhibitor | 5.86 (5) | 15.00 ± 4.33 | 3.23 ± 2.79 | ∼40 | − |
| Thiothixene | Neurotransmitter inhibitor | 9.30 (5) | >40 | 5.74 ± 2.51 | >40 | − |
| Astemizole | Neurotransmitter inhibitor | 4.88 (5) | >40 | 3.48 ± 1.34 | 28.63 ± 1.94 | − |
| Triflupromazine hydrochloride | Neurotransmitter inhibitor | 5.76 (5) | >40 | 3.32 ± 1.51 | 33.58 ± 2.37 | − |
| Clomipramine hydrochloride | Neurotransmitter inhibitor | 9.33 (5) | >40 | 8.79 ± 2.35 | >40 | − |
| Emetine dihydrochloride | Antibacterial agent | 0.01 (5) | >40 | >5d | <5 | − |
| Tamoxifen citrate | Estrogen receptor inhibitor | 10.12 (5) | >40 | 7.46 ± 2.74 | >40 | − |
| Cycloheximide | Protein processing inhibitor | 0.19 (5) | >40 | >40 | >40 | − |
| Dasatinib | Kinase signaling inhibitor | 5.47 (5) | >40 | >40 | >40 | − |
| Ribavirin | Nucleoside analogue | 9.99 (6) | >40 | >40 | >40 | − |
| Mycophenolic acid | Immunosuppressant agent | 0.17 (6) | >40 | >40 | >40 | − |
| HR2P | Fusion inhibitor | 0.60 (3) | 1.64 ± 0.75 | 14.28 ± 5.57 | >40 | + |
Samples were tested in triplicate, and the experiment was repeated twice. The data are presented as means ± standard deviations. The data of IC50s for inhibiting MERS-CoV replication were adapted from the references, and the numbers of the corresponding references are shown in parentheses. If a compound had no more than 50% inhibition at 40 μM, its IC50 was recorded as >40 μM.
If a compound had no more than 50% cytotoxicity at 40 μM, its CC50 was recorded as >40 μM.
If a compound could or could not inhibit 6-HB formation at 40 μM, its activity was recorded as + or −, respectively.
No inhibitory activity at its CC50 (5 μM).
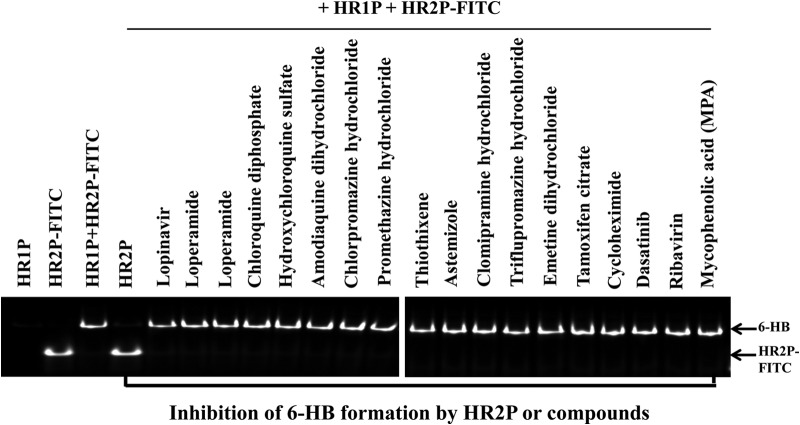
Subsequently, we determined the inhibitory activities of these compounds (40 μM) on 6-HB formation between HR1P and HR2P-fluorescein isothiocyanate (FITC) by using a fluorescence native polyacrylamide gel electrophoresis (FN-PAGE) assay adapted from the FN-PAGE assay for testing of HIV fusion inhibitors (7). As expected, HR1P showed no band because it carries net positive charges, thus migrating up and off the gel under native electrophoresis conditions, which is consistent with the results of HR1 peptides from HIV-1 (7) and SARS-CoV (2), while HR2P-FITC showed a band at a lower position. The mixture of HR1P and HR2P-FITC showed a band at a higher position, suggesting the formation of an HR1P/HR2P-FITC complex, possibly the 6-HB band (Fig. 1). In the presence of HR2P, the upper band disappeared while the lower HR2P-FITC band was displayed, suggesting that HR2P binds to HR1P and blocks 6-HB formation between HR2P-FITC and HR1P. However, none of the MERS-CoV replication inhibitors at 40 μM could block 6-HB formation by HR2P-FITC and HR1P (Fig. 1 and Table 1).
FIG 1.
Inhibition of MERS-CoV S protein 6-HB formation by the HR2P peptide and various compounds. The inhibitory activities of the HR2P peptide and the compounds shown on 6-HB formation between HR1P and HR2P-FITC were detected by using an FN-PAGE assay. Briefly, HR1P (20 μM) was incubated with HR2P (40 μM) or each of the compounds tested (40 μM) at 37°C for 30 min before the addition of HR2P-FITC (20 μM). Tris-glycine native sample buffer (Invitrogen, Carlsbad, CA) was add to the mixture at a ratio of 1:1. The samples were then loaded onto a precast gel (10 cm by 10 cm; 25 μl/well), and electrophoresis was carried out with a constant voltage of 125 V at room temperature for 2 h. The fluorescence bands in the gel were then imaged by the FluorChem 8800 Imaging System using a transillumination UV light source with an excitation wavelength of 302 nm and a fluorescence filter with an emission wavelength of 520 nm (7).
The cytotoxicity of these MERS-CoV replication inhibitors to the Huh-7 cells that were used as target cells in the cell-cell fusion assay was determined by using cell counting kit 8 (Dojindo, Kumamoto, Japan) as previously described (3). Except for emetine dihydrochloride, triflupromazine hydrochloride, and clomipramine hydrochloride with CC50s (the concentration of a compound causing 50% cytotoxicity) of 28.63, 33.58, and <5 μM, respectively, none of the compounds exhibited cytotoxicity at 40 μM (Table 1).
We then tested the inhibitory activity of the 16 MERS-CoV replication inhibitors on MERS-CoV pseudovirus-based, clathrin-mediated endocytosis by using an assay adapted from the method for testing of SARS-CoV inhibitors as previously described (8). The pseudotyped MERS-CoV was constructed as describe before (3). Huh7 cells were incubated with chlorpromazine hydrochloride (as a positive control) and other MERS-CoV replication inhibitors at graded concentrations for 1 h and then infected with the MERS-CoV pseudovirus for an additional 12 h. After extensive washes with phosphate-buffered saline to remove the virus and compounds, the cells were further incubated for 48 h before the luciferase activities were determined as described previously (8). HR2P was included as a control. In addition to chlorpromazine, promethazine, and fluphenazine, all of the other neurotransmitter inhibitors also exhibited inhibitory activity against clathrin-mediated endocytosis of MERS-CoV with IC50s in the range of 3.23 to 8.79 μM. Unexpectedly, HR2P and tamoxifen citrate, an estrogen receptor inhibitor, also displayed some inhibitory activity on clathrin-mediated endocytosis of MERS-CoV with IC50s of 14.28 and 7.46 μM, respectively, while other MERS-CoV replication inhibitors had no significant inhibitory activity at a concentration of 40 μM (Table 1).
Poste et al. (9) reported that the neurotransmitter inhibitors chlorpromazine, promethazine, and fluphenazine also inhibited herpes simplex virus-induced cell fusion without impairing virus replication, suggesting that their weak cell-cell fusion inhibitory activity may not contribute to their inhibition of MERS-CoV replication. Indeed, de Wilde et al. (4) demonstrated that chlorpromazine inhibited MERS-CoV replication at both an early and a postentry stage, indicating that endocytosis is unlikely to be the sole antiviral mechanism. HR2P also exhibited inhibitory activity in the pseudovirus-based, clathrin-mediated endocytosis assay possibly because the HR2P peptide on the cell surface may be engulfed by the plasma membrane into endosomes, where the peptide inhibits endosomal membrane fusion in a way similar to that in which the HIV fusion inhibitor enfuvirtide inhibits HIV endocytosis (10).
In conclusion, some of the reported MERS-CoV replication inhibitors from the FDA-approved drug libraries could inhibit clathrin-mediated endocytosis, but most of them do not block MERS-CoV fusion with the target cell membrane (only three of these showed moderate inhibitory activity) and none of them inhibits 6-HB, suggesting that their mechanisms of action are different from that of the MERS-CoV fusion inhibitor HR2P. Therefore, the use of HR2P in combination with these reported MERS-CoV replication inhibitors may have a synergistic effect against MERS-CoV infection.
REFERENCES
- 1.Jiang SB, Lin K, Strick N, Neurath AR. 1993. HIV-1 inhibition by a peptide. Nature 365:113. doi: 10.1038/365113a0. [DOI] [PubMed] [Google Scholar]
- 2.Liu SW, Xiao GF, Chen YB, He YX, Niu JK, Escalante CR, Xiong HB, Farmar J, Debnath AK, Tien P, Jiang SB. 2004. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet 363:938–947. doi: 10.1016/S0140-6736(04)15788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu L, Liu Q, Zhu Y, Chan KH, Qin LL, Li Y, Wang Q, Chan JFW, Du LY, Yu F, Ma CQ, Ye S, Yuen KY, Zhang RG, Jiang SB. 2014. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat Commun 5:3067. doi: 10.1038/ncomms4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, S van Nieuwkoop Bestebroer TM, BG van den Hoogen Neyts J, Snijder EJ. 2014. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother 58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyall J, Coleman CM, Hart BJ, Venkataraman T, Holbrook MR, Kindrachuk J, Johnson RF, Olinger GG, Jahrling PB, Laidlaw M, Johansen LM, Lear-Rooney CM, Glass PJ, Hensley LE, Frieman MB. 2014. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother 58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan JFW, Chan KH, Kao RYT, To KKW, Zheng BJ, Li CPY, Li PTW, Dai J, Mok FKY, Chen HL, Hayden FG, Yuen KY. 2013. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect 67:606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S, Zhao Q, Jiang S. 2003. Determination of the HIV-1 gp41 fusogenic core conformation modeled by synthetic peptides: applicable for identification of HIV-1 fusion inhibitors. Peptides 24:1303–1313. doi: 10.1016/j.peptides.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Inoue Y, Tanaka N, Tanaka Y, Inoue S, Morita K, Zhuang M, Hattori T, Sugamura K. 2007. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J Virol 81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poste G, Reeve P. 1972. Inhibition of virus-induced cell fusion by local anesthetics and phenothiazine tranquilizers. J Gen Virol 16:21–28. doi: 10.1099/0022-1317-16-1-21. [DOI] [PubMed] [Google Scholar]
- 10.Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. 2009. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell 137:433–444. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]