Abstract
The treatment of progressive vaccinia in individuals has involved antiviral drugs, such as cidofovir (CDV), brincidofovir, and/or tecovirimat, combined with vaccinia immune globulin (VIG). VIG is costly, and its supply is limited, so sparing the use of VIG during treatment is an important objective. VIG sparing was modeled in immunosuppressed mice by maximizing the treatment benefits of CDV combined with VIG to determine the effective treatments that delayed the time to death, reduced cutaneous lesion severity, and/or decreased tissue viral titers. SKH-1 hairless mice immunosuppressed with cyclophosphamide and hairless SCID mice (SHO strain) were infected cutaneously with vaccinia virus. Monotherapy, dual combinations (CDV plus VIG), or triple therapy (topical CDV, parenteral CDV, and VIG) were initiated 2 days postinfection and were given every 3 to 4 days through day 11. The efficacy assessment included survival rate, cutaneous lesion severity, and viral titers. Delays in the time to death and the reduction in lesion severity occurred in the following order of efficacy: triple therapy had greater efficacy than double combinations (CDV plus VIG or topical plus parenteral CDV), which had greater efficacy than VIG alone. Parenteral administration of CDV or VIG was necessary to suppress virus titers in internal organs (liver, lung, and spleen). The skin viral titers were significantly reduced by triple therapy only. The greatest efficacy was achieved by triple therapy. In humans, this regimen should translate to a faster cure rate, thus sparing the amount of VIG used for treatment.
INTRODUCTION
Serious, life-threatening, progressive vaccinia infections have arisen in individuals after receipt of a smallpox vaccination, usually in military personnel or their household contacts, such as young children (1–3). A primary component of the treatment of such infections is vaccinia immune globulin (VIG) (4, 5). VIG is expensive and difficult to produce in large quantities. The U.S. government maintains a small stockpile of the material that can be severely depleted, even by just one treated individual. As an example, in 2012, the case of an immunosuppressed military vaccinee with a severe progressive vaccinia infection was reported. The individual was successfully treated, but treatment required 341 vials of VIG over the course of 75 days in addition to oral and topical treatments with tecovirimat (ST-246) and brincidofovir (CMX001, an orally active prodrug of cidofovir [CDV]) (4).
Many animal models exist for studying the treatment of infections with vaccinia virus, including those using mice, prairie dogs, rabbits, and nonhuman primates (6–8). Mice are most often used because of their availability in large numbers and relatively low cost. Several studies in mice have reported VIG as a single treatment for severe vaccinia virus infections (9–12). Two reports addressed the use of CDV in combination with VIG to treat progressive vaccinia infections in severe combined immunodeficient (SCID) mice (13, 14). These investigations were performed using vaccine strains of vaccinia virus to infect the animals at the base of the dorsal side of the tail. The combination treatments (antiviral drug plus VIG) were more effective than VIG alone in reducing cutaneous lesion severity and in delaying the time to death. Vaccinia infections in SCID mice are difficult to treat due to the profound immunosuppressive state of the mice, and a cure is not generally possible unless treatment is initiated very early postexposure (13). The delayed-treatment SCID mouse model may be a good representation of the treatment of vaccinia infections in immunocompromised humans, who require aggressive antiviral/VIG therapy (3–5). In an alternative mouse model, we developed a progressive vaccinia virus cutaneous infection model in normal hairless mice (SHK-1 strain) that were immunosuppressed with cyclophosphamide (15). Because the mice were hairless, the expansion of the primary lesion areas and the broad dissemination of satellite lesions were easily quantifiable. This model was used to study antiviral treatments that used several compounds administered by the intraperitoneal (i.p.) and topical routes (15–17), and it was used in the present investigation. Recently, Charles River Laboratories developed SCID (SHO strain) hairless mice that were also used in this research. Athymic nude mice are also hairless, but vaccinia virus infections in them are nonprogressive, and the animals recover (18).
In 2012, the Centers for Disease Control and Prevention (CDC) sponsored a workshop to discuss animal models and research performed to study the treatment of progressive vaccinia with antivirals combined with VIG. There, it was noted that only two published studies have been performed with combinations of VIG and an antiviral drug (i.e., CDV) (13, 14), clearly indicating that more research is warranted. Subsequently, the present research was conducted, focusing on ways to maximize the treatment benefit in vaccinia-infected immunosuppressed mice by evaluating several drug combinations to better understand the contribution of each compound with regard to treatment efficacy. Since severely immunosuppressed mice cannot be cured of vaccinia virus infection except by very early postexposure intervention (13), studying ways to shorten the disease course (i.e., time to cure) is not possible, although it an important goal in human therapy. Instead, we investigated ways to improve the treatment efficacy, as measured by delays in mortality, reductions in cutaneous lesion severity, and decreased viral titers. This research may lead to better methods for treating progressive vaccinia in humans while allowing for diminished VIG usage.
(This work was previously presented at the 27th International Conference on Antiviral Research, Raleigh, NC, 12 to 16 May 2014.)
MATERIALS AND METHODS
Ethical treatment of animals.
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Utah State University. Death as an endpoint was approved by the IACUC, with the provision that the animals whose body weight decreased below 30% of their initial weight or were found moribund were humanely euthanized. The work was performed in the AAALAC-accredited Laboratory Animal Research Center of the university in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals, the Animal Welfare Act, and other applicable public laws and regulations.
Animals.
Female 6- to 7-week-old specific-pathogen-free SKH-1 hairless mice were obtained from Charles River Laboratories (Wilmington, MA). They were quarantined at least 3 days prior to initiation of the infection. The animals were maintained on standard rodent chow and tap water in the Laboratory Animal Research Center of Utah State University. For one study, 6- to 7-week-old male SCID (SHO strain) hairless mice (Charles River Laboratories) were used. Male SHO mice were used instead of female mice because they are considerably less expensive. They were maintained on irradiated rodent chow and with autoclaved bedding, cages, and water.
Virus.
Vaccinia virus (WR strain, ATCC VR-119) was originally purchased from the American Type Culture Collection (ATCC, Manassas, VA). The virus was propagated in African green monkey kidney (MA-104) cells (from the ATCC) for use in these studies.
Test compounds.
CDV was obtained from a local pharmacy as a liquid preparation at 75 mg/ml (presumably in physiological saline). It was diluted into Dermovan (obtained from a local pharmacy) to equal a 0.5% weight/weight topical formulation. The topical placebo control consisted of Dermovan diluted with water (similar to the water content of the 0.5% CDV cream). CDV was also diluted in physiological saline for parenteral administration. VIG (VIG-IV; Cangene Corp., Winnipeg, Manitoba, Canada) was kindly provided by the CDC (Atlanta, GA). According to the packaging material, the protein content was at a concentration of 50 mg/ml and contained >50,000 U/vial of the vaccinia virus antibody. Cyclophosphamide, an immunosuppressive agent, was obtained from Sigma-Aldrich (St. Louis, MO).
Experiment design.
SKH-1 mice were anesthetized with ketamine/xylazine (50/5 mg/kg of body weight) by i.p. injection. They were scratched using a 27-gauge needle in order to penetrate the dermal layer in the hip and shoulder areas (i.e., two sites) on one side of the body. The area of each scratched site was about 25 mm2 (5 mm by 5 mm) and consisted of 4 to 5 scratches in one direction. A 20-μl volume of vaccinia virus (containing approximately 2.5 × 105 PFU of virus) was placed on each wounded area and remained there while the mice rested (5 to 10 min) under the influence of the anesthesia. Immunosuppression was accomplished by i.p. treatment of the mice with cyclophosphamide (100 mg/kg/day) every 4 days starting 1 day before virus challenge. The methods employed were as originally published (15). The procedures for using the SHO (SCID) hairless mice were similar to those used for the SKH-1 mice, except that greater care was exercised in terms of animal handling, feeding, watering, and bedding to ensure a well-sanitized state.
CDV-containing and placebo creams (50 to 100 μl) were applied with a spatula to each primary lesion site twice daily on days 2, 5, 8, and 11. Satellite lesions were not treated. CDV was also administered to some animals by i.p. injection once per day on days 2, 5, 8, and 11. The placebo controls (topical cream and i.p. saline) were administered at the same times. VIG was administered by i.p. injection once per day on days 2, 6, and 10. The placebo control (i.p. saline) was administered at the same times. On days 5 to 11, when only certain groups received i.p. drug treatments (either CDV or VIG), the other groups were treated i.p. with saline. In this manner, all the mice were treated by i.p. injection on each day (days 2, 5, 8, and 11) of treatment. The treatment regimens were modeled after the work of Fisher et al. (13) (who treated until day 15 postinfection), except that we treated only until day 11.
The mice were evaluated for time to death (moribund animals were sacrificed and counted as dead on the following day), primary lesion sizes, the number of satellite lesions, and tissue virus titers. The primary lesion areas were determined by measuring each lesion (hip and shoulder, recorded separately) with a millimeter ruler. The numbers of satellite lesions were counted per animal. For virus titer determinations, the primary lesions were excised, and the livers, lungs, and spleens were removed. All tissues were weighed, and the weights were recorded. The tissues were stored frozen at −80°C until homogenization in a cell culture medium. The organs were homogenized by placing them in Stomacher bags containing 1 ml of medium and rolling a pipette over them to disrupt the tissues. The skin samples were homogenized in 1 ml of medium using sterilized mortars and pestles. The homogenized samples were refrozen at −80°C until titration for the virus was performed. Titrations were performed by plaque assays in Vero cells in 12-well microplates by diluting samples in 10-fold increments. After 3 days of incubation at 37°C, the cells were fixed and stained with 0.2% crystal violet in 5% buffered formalin for 15 min. The dye was removed by aspiration, and the wells were rinsed with water. The plaque numbers were counted with the aid of a plaque viewer (Bellco, Vineland, NJ). Virus titers are expressed as log10 PFU per gram of tissue. Because there were two primary lesion sites per animal, there were twice as many data points for cutaneous lesion areas and skin virus titer determinations compared to those of other measures.
Statistical analysis.
Rates of survival were compared among all groups using the Mantel-Cox log-rank test. Pairwise comparisons were made using the Gehan-Breslow-Wilcoxon test with a Bonferroni-corrected threshold for significance based upon the total number of treatment groups. The mean primary lesion areas, the mean numbers of satellite lesions, and the mean body weights were statistically analyzed over the course of the infection by a two-way analysis of variance (ANOVA) followed by Tukey's multiple-comparison test. This test was performed only for days on which all groups had some survivors. The mean viral titer comparisons were evaluated by a one-way ANOVA with Tukey's multiple-comparison test. The analyses were performed using Prism 6.0 software (GraphPad Software, San Diego, CA).
RESULTS
Determining doses of CDV and VIG for treatment of immunosuppressed SKH-1 mice.
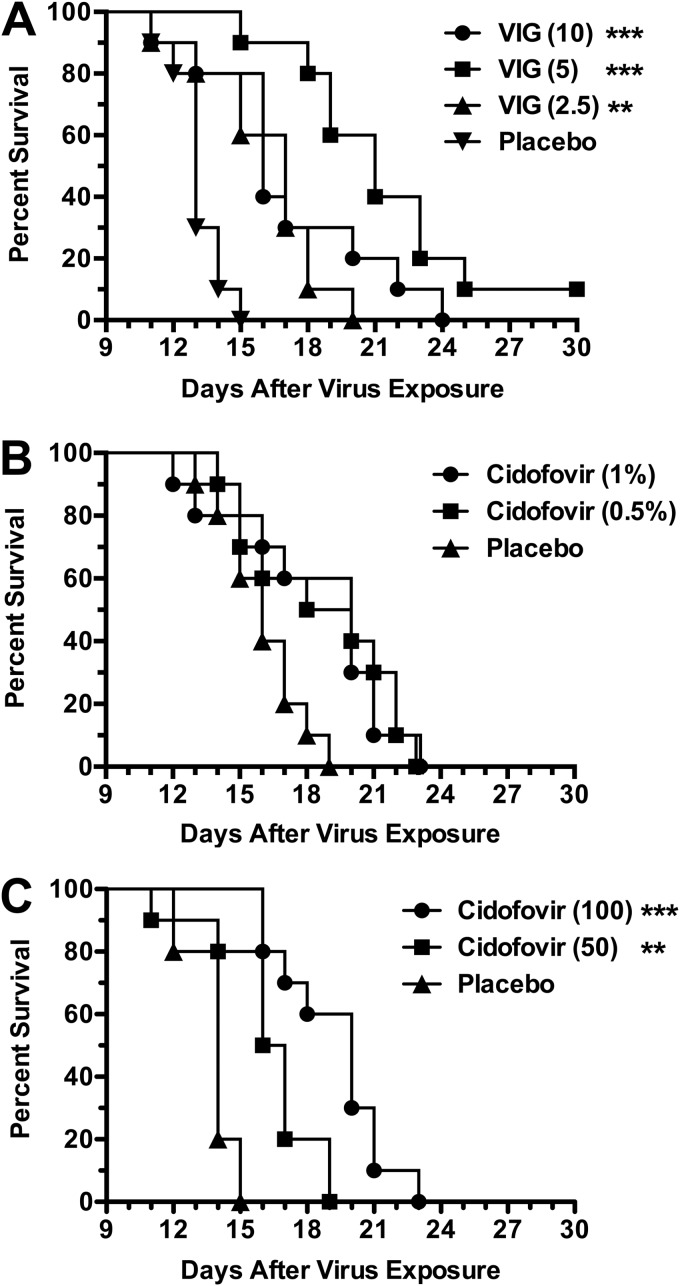
Prior to performing drug combination experiments, it was necessary to determine the doses of CDV (both topical and parenteral) and VIG to use as monotherapy. As seen in Fig. 1A, the best protection with VIG was seen with the 5-mg/mouse dose. The 2.5- and 10-mg/mouse doses were not quite as protective but were still significantly better than placebo. These results were from a single experiment, and repeat experiments may not support a conclusion that the 10-mg dose is less active than the 5-mg dose used in this model. The topical CDV treatments of 0.5% and 1% creams provided nearly the same survival rate increases (Fig. 1B), neither of which were significantly different than that of placebo due to some early deaths. CDV administered parenterally was more beneficial at 100 mg/kg/day than at the lower dose (Fig. 1C). From these experiments, it was decided that the doses of 5 mg/mouse VIG, 0.5% topical CDV, and 50 mg/kg/day parenteral CDV would be appropriate for the subsequent drug combination studies.
FIG 1.
Dose-response effects of VIG (A), topical CDV (B), and parenteral CDV (C) treatments on survival during cutaneous vaccinia virus infection in cyclophosphamide-immunosuppressed SHK-1 hairless mice. The mice were infected cutaneously with virus on scarified skin of the hip and shoulder regions (2.5 × 105 PFU/site in 20-μl volumes). VIG (mg/mouse) was administered parenterally once daily on days 2, 6, and 10 after infection. Topical CDV (% drug in cream formulation) was applied twice per day (at 12-h intervals) on days 2, 5, 8, and 11 after virus exposure. Parenteral CDV (mg/kg/day) was given once per day on days 2, 5, 8, and 11. The placebos consisted of parenteral saline and topical cream, administered at the same times as the other treatments. Cyclophosphamide (100 mg/kg/day) was given i.p. once per day every 4 days starting 1 day prior to virus challenge. Ten mice were in each treatment group. **, P < 0.01, and ***, P < 0.001 (both compared to placebo).
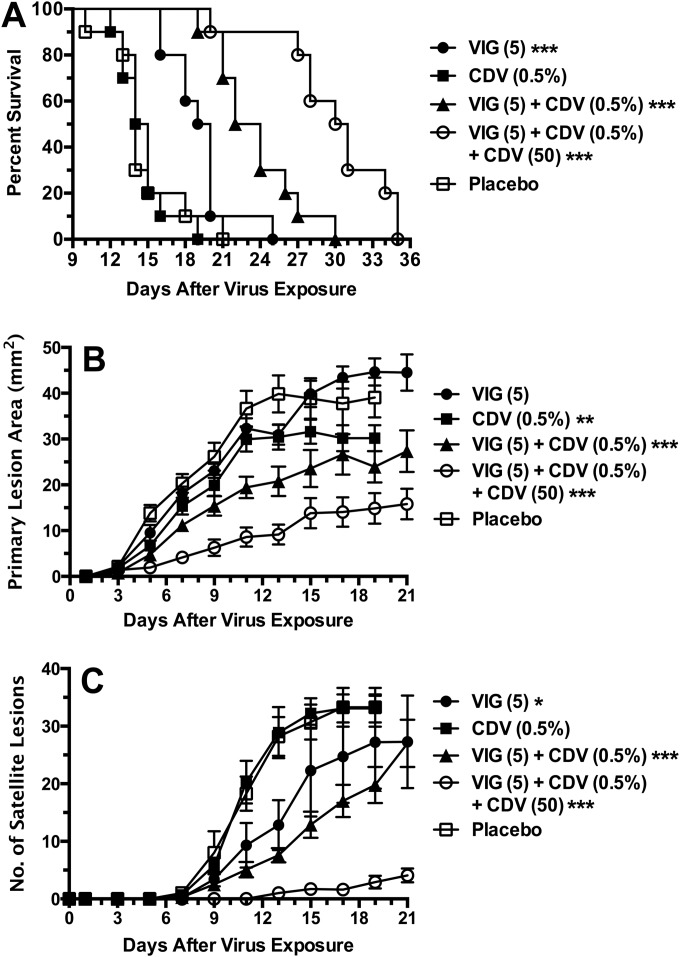
Combinations of CDV and VIG to treat cutaneous vaccinia infections in immunosuppressed SKH-1 mice.
Monotherapy with VIG or topical CDV was compared with double combinations and with triple combinations that contained topical CDV, parenteral CDV, and VIG (Fig. 2). Topical CDV alone had no effect in delaying the time to death (Fig. 2A). Treatment with VIG alone resulted in a significant prolongation of survival relative to that with placebo. Additional benefits were afforded by the combination of 0.5% CDV cream and VIG. Maximal benefits were achieved by combining CDV cream, parenteral CDV, and VIG. A statistically significant (P < 0.01) difference was noted between mice in the triple-therapy group and mice in the groups treated with the double combination of VIG and topical CDV. The primary lesion areas and numbers of satellite lesions were significantly reduced by treatments in the following order of efficacy: triple therapy was more effective than CDV cream plus VIG, which was more effective than CDV cream, which was more effective than VIG (Fig. 2B and C). VIG treatment alone did not reduce the primary lesion areas, and topical CDV alone did not reduce the numbers of satellite lesions. Triple therapy was superior to the other treatment regimens (P < 0.01).
FIG 2.
Effects of topical CDV and parenteral VIG treatments (each used alone and in combination) and triple therapy (by adding parenteral CDV) on survival rates (A), primary lesion areas (B), and numbers of satellite lesions (C) during a cutaneous vaccinia virus infection in cyclophosphamide-immunosuppressed SHK-1 hairless mice. Treatment regimens were the same as those described for Fig. 1. Standard error bars are shown in panels B and C. Ten mice were in each treatment group. *, P < 0.05, **, P < 0.01, and ***, P < 0.001 (all compared to placebo).
Parenteral CDV alone was compared with VIG alone, with a combination of parenteral CDV with VIG, and with a triple combination that included topical CDV (Fig. 3). All treatments were superior to the placebo (Fig. 3A); triple therapy efficacy was greater than or equal to that of parenteral CDV plus VIG, which was more effective than VIG alone, which was more effective than parenteral CDV alone. In this experiment, a significant difference between the triple therapy and the VIG-parenteral CDV combination was not observed (P = 0.29). The efficacies of these two combination treatment regimens exceeded those of the CDV and VIG monotherapies (P < 0.001). The primary lesion areas and the numbers of satellite lesions were significantly reduced by treatments with the dual and triple therapies (Fig. 3B and C). However, the triple therapy was superior to all others in reducing the primary lesion areas and the numbers of satellite lesions (P < 0.001), and VIG plus parenteral CDV was significantly more effective than monotherapy (P < 0.001).
FIG 3.
Effects of parenteral CDV and VIG treatments (each used alone and in combination) and triple therapy (by adding topical CDV) on survival rates (A), primary lesion areas (B), and numbers of satellite lesions (C) during a cutaneous vaccinia virus infection in cyclophosphamide-immunosuppressed SHK-1 hairless mice. The treatment regimens were the same as those described for Fig. 1. Standard error bars are shown in panels B and C. Ten mice were in each treatment group. ***, P < 0.001, compared to placebo.
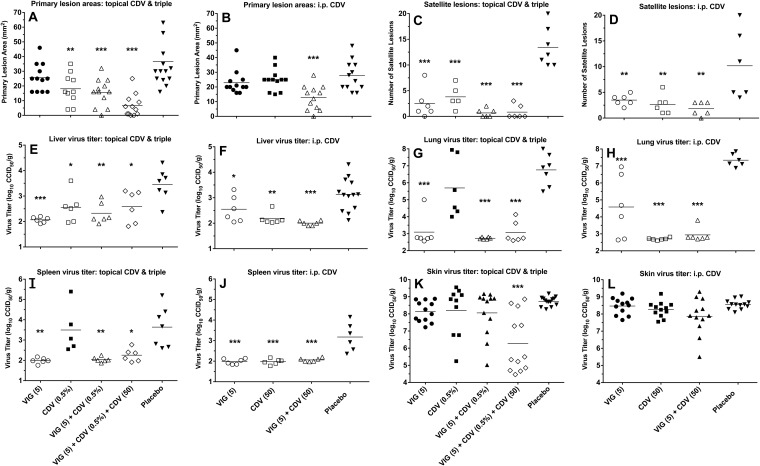
Effects of treatment on cutaneous lesions and viral titers.
Infected immunosuppressed SKH-1 mice were treated intermittently for 11 days with combinations of CDV cream, parenteral CDV, and VIG. On day 12 of the infection, the animals were evaluated for lesion development, and then they were sacrificed to determine tissue viral titers. On this study date, the primary lesion areas were not significantly reduced by parenteral CDV or VIG alone (Fig. 4A and B). The following treatments were effective: topical CDV, topical CDV plus VIG, parenteral CDV plus VIG, and triple therapy containing CDV cream, parenteral CDV, and VIG. The triple therapy gave the greatest reduction in primary lesion areas, although the results were not significantly different from those of the other treatments. All drug treatments significantly reduced the numbers of satellite lesions (Fig. 4C and D), but none was more effective than another. The virus titers in the liver were significantly reduced by all drug treatments compared to those of the placebo, and none was more effective than another (Fig. 4E and F). The virus titers in the lung were not reduced by CDV cream treatment, but the other treatments were effective (Fig. 4G and H). As shown in Fig. 4H, VIG alone was significantly less effective than CDV or VIG plus CDV (P < 0.05). The virus titers in the spleen were not affected by topical CDV, whereas all other treatments were equally effective in reducing that viral titer (Fig. 4I and J). Finally, the virus titers in the skin lesions were reduced significantly only by triple therapy consisting of CDV cream, parenteral CDV, and VIG (Fig. 4K and L).
FIG 4.
Effects of topical CDV, parenteral CDV, and VIG (each used alone and in double or triple combination) treatments on primary lesion areas (A and B), numbers of satellite lesions (C and D), and tissue virus titers (E through L) on day 12 of a cutaneous vaccinia virus infection in cyclophosphamide-immunosuppressed SHK-1 hairless mice. The treatment regimens were the same as those described for Fig. 1. Mean values are represented by the horizontal bars. Six mice were in each treatment group. With two primary lesions per mouse, there were 12 measurements for the primary lesion areas and the skin virus titers. Open symbols represent data sets that were significantly different than those in the placebo group. *, P < 0.05, **, P < 0.01, and ***, P < 0.001. CCID50, 50% cell culture infectious dose.
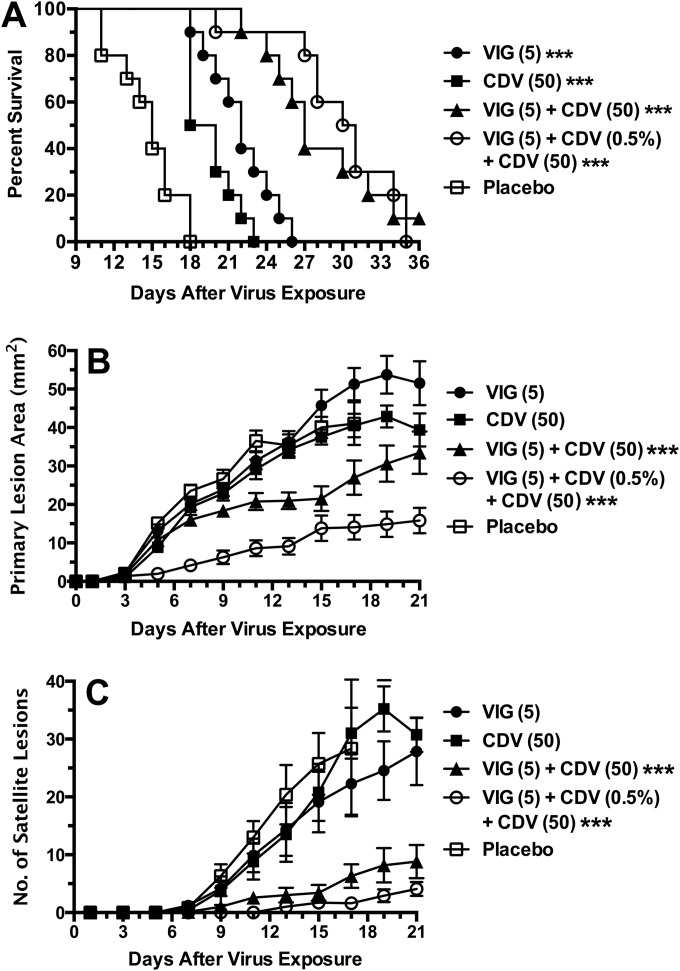
Treatment of cutaneous vaccinia virus infection in SHO (SCID) hairless mice.
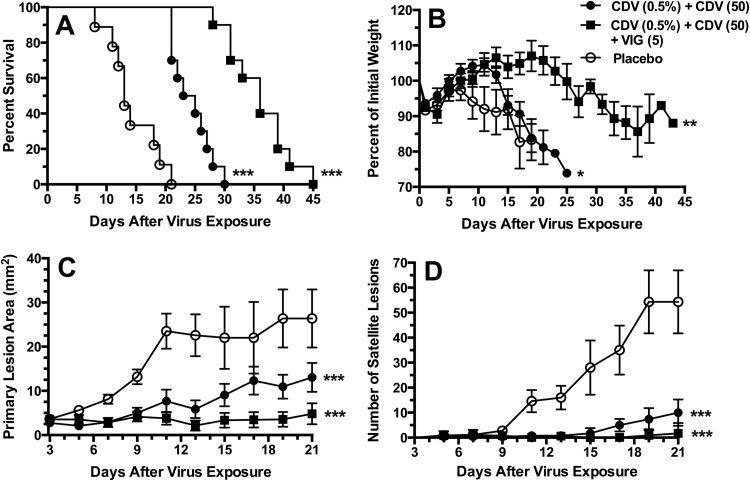
We next asked whether antiviral therapy alone can replace VIG usage altogether. For this experiment, SHO mice were selected as a new immunosuppressed hairless mouse model in which cyclophosphamide treatment can be eliminated. As seen in Fig. 5A, SHO mice treated with placebo succumbed from infection between 9 and 21 days, with a mean (± standard deviation) day of death of 14.6 ± 4.2 days. A significant delay in death was observed with topical CDV combined with parenteral CDV treatment (mean day of death, 25.8 ± 2.6 days). An even greater improvement was observed by treatments with topical CDV, parenteral CDV, and VIG (mean day of death, 33.3 ± 10.7 days). The triple therapy was superior to the double combination containing CDV (P < 0.001). In addition, weight loss did not occur as rapidly in mice treated with the triple therapy (Fig. 5B). The greatest reduction in the primary lesion areas was achieved with the triple therapy regimen (Fig. 5C), which differed significantly (P < 0.001) from that in the other groups. The numbers of satellite lesions were considerably lower with the double and triple therapy regimens (Fig. 5D) than with the monotherapies, and both the double and triple therapy regimens were similarly effective. Thus, these results demonstrate the importance of VIG in contributing to the overall treatment benefit.
FIG 5.
Effects of double combinations of CDV (topical plus parenteral treatment) and triple therapy (by adding VIG) on survival rates (A), body weights (B), primary lesion areas (C), and numbers of satellite lesions (D) during a cutaneous vaccinia virus infection in SHO (SCID hairless) mice. The treatment regimens were the same as those described for Fig. 1 except that no cyclophosphamide was given. Standard error bars are shown in panels B, C, and D. Ten mice were in each treatment group. *, P < 0.05, **, P < 0.01, and ***, P < 0.001 (all compared to placebo).
DISCUSSION
The treatment of progressive vaccinia infections in patients with compromised immune systems has been a daunting challenge (2–5). As the number of individuals who have been successfully treated has increased, greater understanding has been gained regarding the use of combination chemotherapy involving antiviral compounds plus VIG. Because the amount of stockpiled VIG is small, an effort to limit VIG usage per patient treated (but not at the cost of treatment efficacy) is of prime importance. This work and other published studies show that greater treatment benefits can be accomplished by the combination of VIG with an effective antiviral agent (13, 14). Sparing VIG usage may entail a reduction of either the daily VIG dose given to patients or of the number of total doses given because the time to cure is shorter. When considering human treatment, it is unlikely that reducing the administered VIG dose during treatment will be an option. Rather, VIG will likely be administered (in combination with antiviral chemotherapy) to achieve a more profound effect that would shorten the disease course. This was the focus of the present investigation in which we used different combinations of CDV with VIG. To accomplish this, we investigated parenteral and topical CDV treatments alone and combined with VIG. The results demonstrated the important contribution made by topical CDV, parenteral CDV, and VIG in the triple combination. We also demonstrated that parenteral CDV or VIG alone delayed the time to death, whereas topically administered CDV was minimally effective by itself. Double combinations of CDV and VIG prolonged survival, and triple therapy with topical CDV, parenteral CDV, and VIG was the most effective.
Two other published studies compared the effects of CDV combined with VIG in immunocompromised (SCID) mice (13, 14). In the study by Hanlon et al., CDV (administered subcutaneously) combined with VIG (given intramuscularly) was more effective than VIG alone in reducing vaccinia tail lesion severity in mice (14). They did not report time to death determinations, so their results cannot be fully compared with those of the present work. Fisher et al. reported the times to death in SCID mice following infections at the base of the tail with a vaccine strain of vaccinia virus (13). Parenteral CDV plus VIG alone was superior to VIG alone. Topical CDV plus VIG was more effective than the other regimen. In that study, CDV was applied topically at a 1% concentration and VIG was administered at 10 mg/mouse; these were twice the doses used in our experiments. Nonetheless, the observations of the two prior reports agreed with the findings in this study with regard to dual combinations of CDV plus VIG. Neither of the two prior publications addressed the efficacy of a triple regimen using VIG, parenteral cidofovir, and topical cidofovir, which was shown here to be the most efficacious treatment.
In these combination studies, a dose of 5 mg/mouse (or 200 to 250 mg/kg) was given per treatment. This equates to an equivalent dose in humans of 16 to 20 mg/kg, which is higher than the normal 5-mg/kg dose usually given to humans. It is understood that the treatment of mice cannot be directly compared to that in humans. Higher doses or more frequent administration of each drug could have been used to provide greater efficacy in the mouse model. For example, Fisher et al. treated SCID mice at a dose of 10 mg/animal (13). However, higher doses of VIG and/or CDV may not have allowed us to understand the contribution of each substance to the overall treatment effect. Using the cyclophosphamide immunosuppression model entailed using an aggressive strain of vaccinia virus (with the WR strain preferred) so that the time course of infection was not unduly prolonged. Other published studies investigating the effects of VIG (either alone or combined with CDV) have used SCID mice (9, 11–14), CD-1 mice in a tail lesion model (12), and BALB/c mice infected intranasally with vaccinia (WR strain) (10). Rabbits have been used to study experimental antibody preparations in a rabbitpox virus infection model (9).
We report here for the first time the use of a newly developed mouse (SHO strain) that is both SCID and hairless for studying progressive vaccinia infections. This mouse model has advantages over SKH-1 hairless mice that are immunosuppressed with cyclophosphamide by alleviating cyclophosphamide toxicity that occurs after prolonged treatment. However, SHO mice are expensive, which may limit the size of a study. In hairless mice (SKH-1 or SHO), we were able to quantify lesion sizes and dissemination, which is not easily done in furry animals.
Fisher et al. studied the treatment of progressive vaccinia in SCID mice and attempted to understand how long-term survival was possible in animals with combined cellular and humoral immunodeficiencies (13). They concluded that innate effectors may clear the virus in the absence of T- and B-cell functions. High levels of antibodies (such as VIG) combined with antiviral treatment may delay or limit infection to the extent that these innate effectors will be operative. Neyts et al. (19) reasoned that virus replication in the skin is important for viral spread to internal organs. Thus, reduction in skin virus titers is important for effective treatment. Here, we showed that treatment with parenteral CDV or VIG alone was effective in reducing liver, lung, and spleen viral titers but not virus titers in the skin. Topical CDV treatment at the dosage used caused a reduction in the liver virus titer but no significant reduction in the lung, spleen, or skin virus titers. Importantly, the only treatment that resulted in a significant skin virus titer reduction was topical and parenteral CDV combined with VIG. This treatment was most effective in delaying the time to death and in reducing viral lesion severity. It is possible that some of the increased benefit of the triple combination (i.e., adding topical CDV to the VIG plus parenteral CDV treatment regimen) came from the topical CDV that entered the circulatory system and increased the overall CDV level. If the mice ingested some of the topically applied CDV, drug uptake into the bloodstream would be negligible because of the very low oral bioavailability of CDV (20). Fisher et al. found that topical CDV plus VIG was more effective than parenteral CDV plus VIG but did not speculate as to why (13).
Three potent antiviral compounds have been used to treat humans, namely, CDV, CMX001, and ST-246. The present experiments evaluated only CDV combined with VIG, but the other compounds definitely merit investigation. It may be particularly important to determine the topical benefits of CMX001 and ST-246 as part of the overall treatment regimen. ST-246 has already been used topically (and orally) in combination with VIG and CMX001 to treat a human case of progressive vaccinia (4). However, no experimental data demonstrating that ST-246 is effective topically have been published. In addition, drug efficacy by a topical route is dependent on the vehicle. We used Dermovan for preparing topical CDV based upon the experience of other investigators (13, 21). There may be other vehicles that are superior. CDV causes renal toxicity in humans when administered parenterally, and probenecid is coadministered to diminish this effect (22, 23). What is not understood is how much topical CDV can be applied without causing renal toxicity. CMX001 is more hydrophobic than CDV, so its topical application may require a different vehicle.
By evaluating mortality, lesion progression, and viral titers in two models of progressive vaccinia, it was evident that a topical antiviral treatment is an important component of the overall treatment regimen. The triple therapy consisting of topical CDV, parenteral CDV, and VIG was the only one that provided a significant reduction in skin lesion virus titers, and it provided the greatest survival benefit and reduction in lesion severity and dissemination. Reduction of the amount of VIG used during treatment of humans should be possible by maximizing the efficacies of the treatment modalities for each drug combination used.
ACKNOWLEDGMENTS
We thank Inger Damon at the Centers for Disease Control and Prevention (Atlanta, GA) for providing VIG for these studies.
We have no conflicts of interest to report.
This work was supported in part by contracts HHSN272201000039I/HHSN27200006/A30 and HHSN272201000039I/HHSN27200007/A43 from the Virology Branch, Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, USA.
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the NIAID.
REFERENCES
- 1.Bray M. 2003. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res 58:101–114. doi: 10.1016/S0166-3542(03)00008-1. [DOI] [PubMed] [Google Scholar]
- 2.Bray M, Wright ME. 2003. Progressive vaccinia. Clin Infect Dis 36:766–774. doi: 10.1086/374244. [DOI] [PubMed] [Google Scholar]
- 3.Wertheimer ER, Olive DS, Brundage JF, Clark LL. 2012. Contact transmission of vaccinia virus from smallpox vaccinees in the United States, 2003–2011. Vaccine 30:985–988. doi: 10.1016/j.vaccine.2011.12.049. [DOI] [PubMed] [Google Scholar]
- 4.Lederman ER, Davidson W, Groff HL, Smith SK, Warkentien T, Li Y, Wilkins KA, Karem KL, Akondy RS, Ahmed R, Frace M, Shieh WJ, Zaki S, Hruby DE, Painter WP, Bergman KL, Cohen JI, Damon IK. 2012. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis 206:1372–1385. doi: 10.1093/infdis/jis510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vora S, Damon I, Fulginiti V, Weber SG, Kahana M, Stein SL, Gerber SI, Garcia-Houchins S, Lederman E, Hruby D, Collins L, Scott D, Thompson K, Barson JV, Regnery R, Hughes C, Daum RS, Li Y, Zhao H, Smith S, Braden Z, Karem K, Olson V, Davidson W, Trindade G, Bolken T, Jordan R, Tien D, Marcinak J. 2008. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis 46:1555–1561. doi: 10.1086/587668. [DOI] [PubMed] [Google Scholar]
- 6.Smee DF. 2008. Progress in the discovery of compounds inhibiting orthopoxviruses in animal models. Antivir Chem Chemother 19:115–124. [DOI] [PubMed] [Google Scholar]
- 7.Smee DF. 2013. Orthopoxvirus inhibitors that are active in animal models: an update from 2008 to 2012. Future Virol 8:891–901. doi: 10.2217/fvl.13.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smee DF, Sidwell RW. 2003. A review of compounds exhibiting anti-orthopoxvirus activity in animal models. Antiviral Res 57:41–52. doi: 10.1016/S0166-3542(02)00199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crickard L, Babas T, Seth S, Silvera P, Koriazova L, Crotty S. 2012. Protection of rabbits and immunodeficient mice against lethal poxvirus infections by human monoclonal antibodies. PLoS One 7:e48706. doi: 10.1371/journal.pone.0048706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Law M, Putz MM, Smith GL. 2005. An investigation of the therapeutic value of vaccinia-immune IgG in a mouse pneumonia model. J Gen Virol 86:991–1000. doi: 10.1099/vir.0.80660-0. [DOI] [PubMed] [Google Scholar]
- 11.McCausland MM, Benhnia MR, Crickard L, Laudenslager J, Granger SW, Tahara T, Kubo R, Koriazova L, Kato S, Crotty S. 2010. Combination therapy of vaccinia virus infection with human anti-H3 and anti-B5 monoclonal antibodies in a small animal model. Antivir Ther 15:661–675. doi: 10.3851/IMP1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shearer JD, Siemann L, Gerkovich M, House RV. 2005. Biological activity of an intravenous preparation of human vaccinia immune globulin in mouse models of vaccinia virus infection. Antimicrob Agents Chemother 49:2634–2641. doi: 10.1128/AAC.49.7.2634-2641.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher RW, Reed JL, Snoy PJ, Mikolajczyk MG, Bray M, Scott DE, Kennedy MC. 2011. Postexposure prevention of progressive vaccinia in SCID mice treated with vaccinia immune globulin. Clin Vaccine Immunol 18:67–74. doi: 10.1128/CVI.00280-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanlon CA, Niezgoda M, Shankar V, Niu HS, Koprowski H, Rupprecht CE. 1997. A recombinant vaccinia-rabies virus in the immunocompromised host: oral innocuity, progressive parenteral infection, and therapeutics. Vaccine 15:140–148. doi: 10.1016/S0264-410X(96)00163-6. [DOI] [PubMed] [Google Scholar]
- 15.Smee DF, Bailey KW, Wong MH, Wandersee MK, Sidwell RW. 2004. Topical cidofovir is more effective than is parenteral therapy for treatment of progressive vaccinia in immunocompromised mice. J Infect Dis 190:1132–1139. doi: 10.1086/422696. [DOI] [PubMed] [Google Scholar]
- 16.Smee DF, Bailey KW, Wong MH, Tarbet EB. 2011. Topical treatment of cutaneous vaccinia virus infections in immunosuppressed hairless mice with selected antiviral substances. Antivir Chem Chemother 21:201–208. doi: 10.3851/IMP1734. [DOI] [PubMed] [Google Scholar]
- 17.Tarbet EB, Larson D, Anderson BJ, Bailey KW, Wong MH, Smee DF. 2011. Evaluation of imiquimod for topical treatment of vaccinia virus cutaneous infections in immunosuppressed hairless mice. Antiviral Res 90:126–133. doi: 10.1016/j.antiviral.2011.03.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quenelle DC, Collins DJ, Kern ER. 2004. Cutaneous infections of mice with vaccinia or cowpox viruses and efficacy of cidofovir. Antiviral Res 63:33–40. doi: 10.1016/j.antiviral.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neyts J, De Clercq E. 1993. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine for the treatment of lethal vaccinia virus infections in severe combined immune deficiency (SCID) mice. J Med Virol 41:242–246. doi: 10.1002/jmv.1890410312. [DOI] [PubMed] [Google Scholar]
- 20.Wachsman M, Petty BG, Cundy KC, Jaffe HS, Fisher PE, Pastelak A, Lietman PS. 1996. Pharmacokinetics, safety and bioavailability of HPMPC (cidofovir) in human immunodeficiency virus-infected subjects. Antiviral Res 29:153–161. doi: 10.1016/0166-3542(95)00829-2. [DOI] [PubMed] [Google Scholar]
- 21.Toro JR, Wood LV, Patel NK, Turner ML. 2000. Topical cidofovir: a novel treatment for recalcitrant molluscum contagiosum in children infected with human immunodeficiency virus 1. Arch Dermatol 136:983–985. doi: 10.1001/archderm.136.8.983. [DOI] [PubMed] [Google Scholar]
- 22.Lacy SA, Hitchcock MJ, Lee WA, Tellier P, Cundy KC. 1998. Effect of oral probenecid coadministration on the chronic toxicity and pharmacokinetics of intravenous cidofovir in cynomolgus monkeys. Toxicol Sci 44:97–106. doi: 10.1093/toxsci/44.2.97. [DOI] [PubMed] [Google Scholar]
- 23.Polis MA, Spooner KM, Baird BF, Manischewitz JF, Jaffe HS, Fisher PE, Falloon J, Davey RT Jr, Kovacs JA, Walker RE, et al. . 1995. Anticytomegaloviral activity and safety of cidofovir in patients with human immunodeficiency virus infection and cytomegalovirus viruria. Antimicrob Agents Chemother 39:882–886. doi: 10.1128/AAC.39.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]