Abstract
We present here the first evidence that granzyme B acts against Plasmodium falciparum (50% inhibitory concentration [IC50], 1,590 nM; 95% confidence interval [95% CI], 1,197 to 2,112 nM). We created a novel antimalarial fusion protein consisting of granzyme B fused to a merozoite surface protein 4 (MSP4)-specific single-chain Fv protein (scFv), which targets the enzyme to infected erythrocytes, with up to an 8-fold reduction in the IC50 (176 nM; 95% CI, 154 to 202 nM). This study confirms the therapeutic efficacies of recombinant antibody-mediated antimalarial immunotherapeutics based on granzyme B.
TEXT
Malaria is caused by parasites of the genus Plasmodium and remains one of the most widespread and dangerous infectious diseases, causing ∼627,000 deaths worldwide per year (1). Among the six species that infect humans, Plasmodium falciparum causes the severest form of the disease (2). There is no effective vaccine against these parasites (3, 4), and resistances are emerging against a wide variety of antimalarial drugs (5). It was recently shown in vitro that natural killer (NK) cells can eliminate erythrocytes infected with P. falciparum (6) and that this is associated with the production of the serine protease granzyme B (Gb) (7). In vivo, NK cells are essential for protection against plasmodial infections in mice (8), and increased levels of circulating Gb are found in naturally infected humans (9).
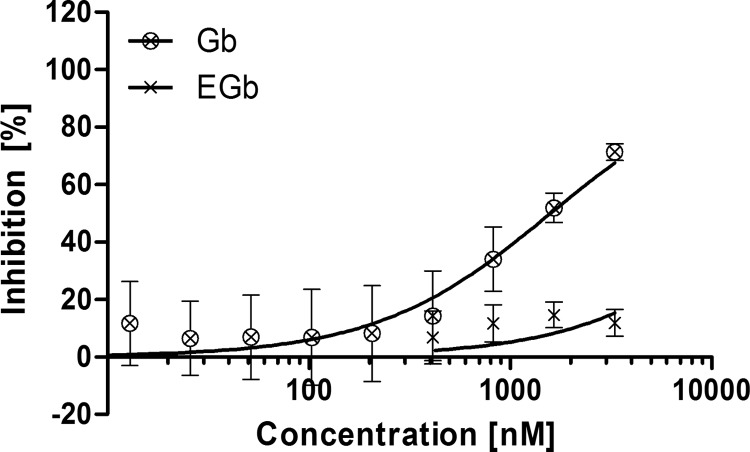
We therefore investigated the antimalarial effect of recombinant Gb on P. falciparum (strain 3D7A) in a standardized 72-h drug susceptibility assay starting with synchronized ring-stage parasites (10). Gb was produced in HEK293 cells with an N-terminal protective peptide fused to an enterokinase cleavage site (EGb) to suppress the enzymatic activity in the host cells, as previously described (11). Activity was restored by the enzymatic removal of this peptide using 0.02 U of recombinant enterokinase (Novagen; Merck) per μg of protein (12). The restored enzymatic activity was confirmed using a colorimetric activity assay (13). Parasite growth was specifically inhibited by activated Gb, with a half-maximal inhibitory concentration (IC50) of 1,590 nM (95% confidence interval [95% CI], 1,197 to 2,112 nM, calculated using the Hill equation in GraphPad Prism version 5). Undigested (inactive) EGb showed no inhibition (Fig. 1 and Table 1). To our knowledge, this is the first time that the antimalarial activity of Gb has been directly confirmed in vitro.
FIG 1.
The 72-h drug susceptibility assay using P. falciparum 3D7A. The susceptibility of P. falciparum 3D7A toward active Gb (⊗) and inactive EGb (×) was determined in a 72-h drug susceptibility assay starting at the ring stage. The data represent the mean ± standard deviation (SD) from two experiments, using technical duplicates.
TABLE 1.
IC50s of all tested samples in the drug susceptibility assays
| Proteina | Duration of drug susceptibility assay (h) | No. of replication cycles (stages) | P. falciparum strain | IC50 (nM)b | 95% CI (nM) | Relative enzymatic activity (AU)e | Activity-corrected IC50 (95% CI) (nM) |
|---|---|---|---|---|---|---|---|
| Gb | 72 | 1.5 (ring-schizont- schizont | 3D7A | 1,590 | 1,197–2,112 | 1.09 | 1,733 (1,305–2,302) |
| EGb | 3D7A | >3,000c | 0 | NAd | |||
| 2.44IgG1 | 48 | 1 (schizont-schizont) | 3D7A | >25,000c | NA | NA | |
| Gb-2.44 | 3D7A | 176 | 154–202 | 1 | 176 (154–202) | ||
| Gb-H22 | 3D7A | 1,103 | 949–1,283 | 0.95 | 1,048 (902–1,219) | ||
| Gb-Ki4 | 3D7A | 1,500 | 1,180–1,905 | 0.85 | 1,275 (1,003–1,619) | ||
| Gb | 3D7A | 970 | 852–1,104 | 1.09 | 1,057 (929–1,203) | ||
| EGb-2.44 | 3D7A | >4,000c | 0 | NA | |||
| EGb-H22 | 3D7A | >4,000c | 0 | NA | |||
| EGb-Ki4 | 3D7A | >4,000c | 0 | NA | |||
| Gb-2.44 | 30 | 0.5 (ring-schizont) | 3D7A | 1,773 | 1,496–2,102 | 1 | 1,773 (1,496–2,102) |
| Gb-H22 | 3D7A | 3,668 | 2,804–4,798 | 0.95 | 3,485 (2,664–4,558) | ||
| Gb | 3D7A | 2,143 | 1,426–3,220 | 1.09 | 2,336 (1,554–3,510) | ||
| Gb-2.44 | 48 | 1 (schizont-schizont) | K1 | 386 | 306–488 | 1 | 386 (306–488) |
| Gb-H22 | K1 | 3,497 | 2,368–5,166 | 0.95 | 3,322 (2,250–4,908) | ||
| Gb-Ki4 | K1 | >2,000c | 0.85 | NA |
Gb, active granzyme B; EGb, inactive granzyme B with retained N-terminal protective peptide and enterokinase cleavage site; 2.44IgG1, MSP4-specific full-size chimeric antibody; Gb-2.44, MSP4-specific Gb-scFv fusion protein; Gb-H22 and Gb-Ki4, P. falciparum-unrelated Gb-scFv fusion proteins.
If no inhibition of parasite growth was determined at the highest applied concentration, this highest applied concentration was given.
NA, not applicable.
AU, arbitrary units.
We developed a strategy to target Gb to the parasite and thus reduce the required dose. Targeted toxin delivery via the parasite transferrin receptor has already been reported (14, 15). Although some authors claim to have identified and characterized this receptor (16, 17), others argue that iron uptake by the parasite is nonspecific and that the P. falciparum transferrin receptor remains elusive (18). Promising alternative targets include the merozoite surface proteins (MSPs), especially MSP1, MSP2, MSP4, and MSP8, which bear glycosylphosphatidylinositol (GPI) anchors and therefore are not completely shed during merozoite invasion. Some also contain immunogenic epidermal growth factor (EGF)-like domains near the C-terminal GPI anchors, which serve as ideal targets for specific antibodies (19–22). Because EGF-like domains are less variable between strains and even species (23), they are ideal targets for antibody-based approaches. Recently, it was shown that MSP4 is imported into newly infected erythrocytes without significant processing, and it remains there for up to 5 h (24). MSP4-specific antibodies or their fragments are therefore attractive candidates for guiding Gb into the infected erythrocyte.
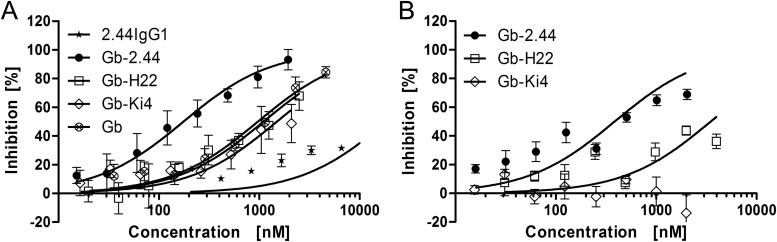
We generated a single-chain variable fragment (scFv) from an MSP4EGF-like domain-specific murine antibody, 2.44IgG1 (S. Kapelski, A. Boes, H. Spiegel, M. de Almeida, T. Klockenbring, A. Reimann, R. Fischer, S. Barth, and R. Fendel, unpublished data), by splicing by overlap extension (SOE)-PCR using a glycine-serine linker peptide and fusing it to the SerpinB9-resistant EGbR201K mutant (12). This was expressed in HEK293-6E cells using a vector based on pTT5 (25) modified with an expression cassette designed for EGb-scFv fusion proteins (26, 27). Two unrelated EGb-scFv fusion constructs named EGb-H22 (targeting human CD64) (11) and EGb-Ki4 (targeting human CD30) (13) were used as negative controls. Following enterokinase-mediated activation, the Gb-scFv fusion proteins were used in a 48-h drug susceptibility assay, similar to the standard invasion inhibition assay used for the evaluation of antibodies (28). We also used 2.44IgG1 as a full-size control antibody in the assay. The proteins were added to synchronous schizont-stage P. falciparum 3D7A parasites growing in 96-well half-area microtiter cell culture plates at a parasitemia level of 0.05% and a final hematocrit level of 1.5%, in a total volume of 50 μl per well. After incubation for 48 h, inhibition was determined as described previously (29). The IC50 of Gb-2.44 was 176 nM (95% CI, 154 to 202 nM), which was 5- to 8-fold lower than that of Gb, Gb-H22, and Gb-Ki4, each of which showed IC50s of ∼1,000 nM (Fig. 2A and Table 1). Undigested controls (EGb-2.44, EGb-H22, EGb-Ki4, and EGb) and antibody 2.44IgG1 showed no effect on parasite growth (Table 1). Similar experiments were carried out using the multidrug-resistant strain P. falciparum K1, resulting in a similar IC50 for Gb-2.44, which were again substantially lower than that of Gb-Ki4 and of Gb-H22 (Fig. 2B and Table 1).
FIG 2.
The 48-h drug susceptibility assay using strain P. falciparum 3D7A and the multidrug-resistant strain P. falciparum K1. The inhibition of parasite growth mediated by Gb fused to an MSP4-specific scFv was determined in a 48-h drug susceptibility assay from schizonts to schizont stage. The schizonts were incubated in the presence of the MSP4-specific fusion Gb-2.44 (●), two unrelated fusions named Gb-H22 (□) and Gb-Ki4 (◇), nonfused Gb (⊗), and the chimeric MSP4-specific antibody 2.44IgG1 (*) for 48 h. (A) The assay was carried out on a standard laboratory strain, P. falciparum 3D7A, and the data represent the mean ± SD from three (Gb-2.44 and Gb-H22), two (Gb-Ki4 and Gb), or one (2.44IgG1) experiment, each in technical triplicates. (B) Multidrug-resistant strain P. falciparum K1 was used, and the data represent the mean ± SD from two experiments, each in technical triplicates (Gb-2.44), or one experiment using technical triplicates (Gb-H22 and Gb-Ki4).
The various fusion partners of the Gb constructs might have an influence on enzymatic activity. Therefore, the enzymatic activity of each construct was determined by a colorimetric assay (13), and the relative activity to Gb-2.44 was calculated. All IC50s were corrected by this factor (Table 1).
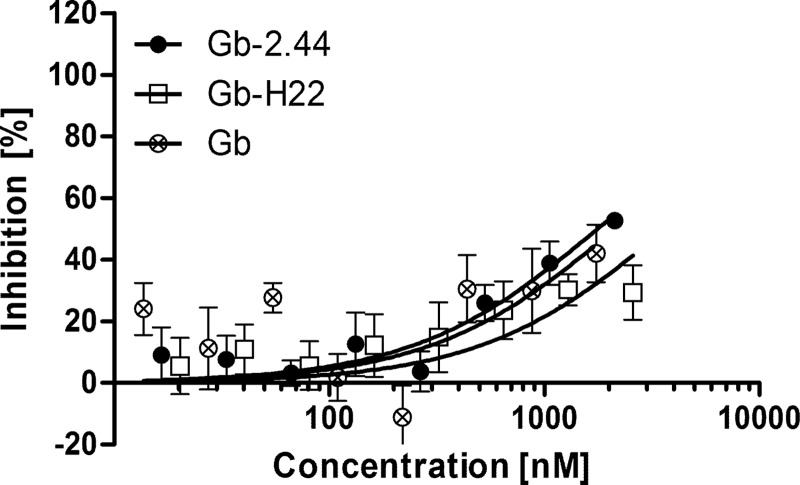
The dependency of the enhanced inhibition of parasite growth was assessed in a 30-h drug susceptibility assay. Here, the fusion proteins were added to synchronous ring-stage parasites at a 0.2% parasitemia level (to achieve an equal parasitemia level at the assay endpoint in the wells without any parasite inhibition) and 1.5% hematocrit and incubated until mature schizonts were present, omitting a schizont rupture and merozoite invasion step. Thus, without possible coimport of the P. falciparum-specific Gb-2.44 into the newly infected erythrocyte along with the merozoite, the Gb fusion proteins all showed similar levels of inhibition (Fig. 3 and Table 1).
FIG 3.
The 30-h drug susceptibility assay using strain P. falciparum 3D7A. The dependency of growth inhibition on the coimport of Gb-2.44 (●) was determined by adding the fusion protein and the unrelated fusion Gb-H22 (□) or nonfused Gb (⊗) to synchronous ring-stage parasites and incubating them for 30 h, when mature schizonts but no rings were present to exclude the potential coimport step. The data represent the mean ± SD from two experiments, using technical triplicates.
Of note, the IC50s determined in the various assays are not equivalent, as the drug exposure time of the parasites has a direct influence on the IC50. Therefore, we strictly perform intra-assay comparisons of IC50s.
We have therefore provided direct confirmation that Gb has antiparasitic activity, and we demonstrated its potential therapeutic use against malarial infections. The concept of recombinant immunotoxins has been extensively investigated in the field of targeted tumor therapy with various bacterial and human effector domains (30–32), including Gb (11, 13, 27, 33). After binding to a disease-specific cell surface antigen, these immunotoxins are internalized, e.g., by receptor-mediated endocytosis, released from endosomal compartments into the cytoplasm, and efficiently kill the malignant cell by their catalytic activity. In the case of Gb, the corresponding human cytolytic fusion protein proteolytically cleaves certain caspases to induce apoptosis (34). Comparably, P. falciparum bears one designated metacaspase, P. falciparum MCA-1 (35), which may be the target of Gb. Another hypothesis is that Gb induces eryptosis in infected erythrocytes and thus kills the parasite (7).
Taken together, we provide solid evidence that Gb-containing malaria-specific fusion proteins are valuable drug candidates acting against multidrug-resistant P. falciparum strains. The IC50 of these novel immunotherapeutic agents in the drug susceptibility assay lies within the range for a hit candidate as a new therapeutic agent, as suggested by the Medicines for Malaria Venture (MMV) (http://www.mmv.org/research-development/essential-information-scientists). The 50% effective concentration [EC50] of recombinant Gb in vitro (using Jurkat cells) is ∼8 μM (36); thus, the estimated selectivity index of our human antimalarial fusion protein is >40.
In further studies, we will investigate the underlying mechanism of its antimalarial activity in order to increase the specificity and further reduce the inhibitory concentration.
ACKNOWLEDGMENTS
This study was partly financed by the Fraunhofer Future Foundation (grant 125-300004). S.K. received the RFwN Ph.D. grant from RWTH Aachen University.
The following reagents were obtained through the MR4 as part of the BEI Resources Repository, NIAID, NIH: MRA-151 for P. falciparum 3D7A, deposited by D. Walliker, and MRA-159 for P. falciparum K1, deposited by D. E. Kyle.
We acknowledge the receipt of nonfused granzyme B from Sonja Schiffer (Institute for Applied Medical Engineering, Aachen, Germany) and Grit Hehmann-Titt (Pharmedartis GmbH, Aachen) and the receipt of the murine hybridoma strain 2.44 from Alexander Boes. We acknowledge the regional blood bank of the University Hospital Aachen for providing us with O+ blood preparations for the P. falciparum culture.
We thank Richard M. Twyman for critical revision of the manuscript.
REFERENCES
- 1.WHO. 2013. World malaria report 2013. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/world_malaria_report_2013/wmr2013_no_profiles.pdf?ua=1. [Google Scholar]
- 2.Keeling PJ, Rayner JC. 25 June 2014. The origins of malaria: there are more things in heaven and earth. Parasitology. doi: 10.1017/S0031182014000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz L, Brown GV, Genton B, Moorthy VS. 2012. A review of malaria vaccine clinical projects based on the WHO rainbow table. Malar J 11:11. doi: 10.1186/1475-2875-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crompton PD, Pierce SK, Miller LH. 2010. Advances and challenges in malaria vaccine development. J Clin Invest 120:4168–4178. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mavoungou E, Luty AJ, Kremsner PG. 2003. Natural killer (NK) cell-mediated cytolysis of Plasmodium falciparum-infected human red blood cells in vitro. Eur Cytokine Netw 14:134–142. [PubMed] [Google Scholar]
- 7.Böttger E, Multhoff G, Kun JF, Esen M. 2012. Plasmodium falciparum-infected erythrocytes induce granzyme B by NK cells through expression of host-Hsp70. PLoS One 7:e33774. doi: 10.1371/journal.pone.0033774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon JB, Forbes MG, Solomon GR. 1985. A possible role for natural killer cells in providing protection against Plasmodium berghei in early stages of infection. Immunol Lett 9:349–352. doi: 10.1016/0165-2478(85)90061-6. [DOI] [PubMed] [Google Scholar]
- 9.Hermsen CC, Konijnenberg Y, Mulder L, Loé C, van Deuren M, van der Meer JW, van Mierlo GJ, Eling WM, Hack CE, Sauerwein RW. 2003. Circulating concentrations of soluble granzyme A and B increase during natural and experimental Plasmodium falciparum infections. Clin Exp Immunol 132:467–472. doi: 10.1046/j.1365-2249.2003.02160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreidenweiss A, Kremsner PG, Mordmüller B. 2008. Comprehensive study of proteasome inhibitors against Plasmodium falciparum laboratory strains and field isolates from Gabon. Malar J 7:187. doi: 10.1186/1475-2875-7-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stahnke B, Thepen T, Stöcker M, Rosinke R, Jost E, Fischer R, Tur MK, Barth S. 2008. Granzyme B-H22(scFv), a human immunotoxin targeting CD64 in acute myeloid leukemia of monocytic subtypes. Mol Cancer Ther 7:2924–2932. doi: 10.1158/1535-7163.MCT-08-0554. [DOI] [PubMed] [Google Scholar]
- 12.Losasso V, Schiffer S, Barth S, Carloni P. 2012. Design of human granzyme B variants resistant to serpin B9. Proteins 80:2514–2522. doi: 10.1002/prot.24133. [DOI] [PubMed] [Google Scholar]
- 13.Schiffer S, Hansen HP, Hehmann-Titt G, Huhn M, Fischer R, Barth S, Thepen T. 2013. Efficacy of an adapted granzyme B-based anti-CD30 cytolytic fusion protein against PI-9-positive classical Hodgkin lymphoma cells in a murine model. Blood Cancer J 3:e106. doi: 10.1038/bcj.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surolia N. 2000. Receptor-mediated targeting of toxins to intraerythrocytic parasite Plasmodium falciparum. Adv Drug Deliv Rev 41:163–170. doi: 10.1016/S0169-409X(99)00063-0. [DOI] [PubMed] [Google Scholar]
- 15.Surolia N, Misquith S. 1996. Cell surface receptor directed targeting of toxin to human malaria parasite, Plasmodium falciparum. FEBS Lett 396:57–61. doi: 10.1016/0014-5793(96)01065-4. [DOI] [PubMed] [Google Scholar]
- 16.Haldar K, Henderson CL, Cross GA. 1986. Identification of the parasite transferrin receptor of Plasmodium falciparum-infected erythrocytes and its acylation via 1,2-diacyl-sn-glycerol. Proc Natl Acad Sci U S A 83:8565–8569. doi: 10.1073/pnas.83.22.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez MH, Jungery M. 1986. A protein on Plasmodium falciparum-infected erythrocytes functions as a transferrin receptor. Nature 324:388–391. doi: 10.1038/324388a0. [DOI] [PubMed] [Google Scholar]
- 18.Clark M, Fisher NC, Kasthuri R, Cerami Hand C. 2013. Parasite maturation and host serum iron influence the labile iron pool of erythrocyte stage Plasmodium falciparum. Br J Haematol 161:262–269. doi: 10.1111/bjh.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia Y, Puentes A, Curtidor H, Cifuentes G, Reyes C, Barreto J, Moreno A, Patarroyo ME. 2007. Identifying merozoite surface protein 4 and merozoite surface protein 7 Plasmodium falciparum protein family members specifically binding to human erythrocytes suggests a new malarial parasite-redundant survival mechanism. J Med Chem 50:5665–5675. doi: 10.1021/jm070773z. [DOI] [PubMed] [Google Scholar]
- 20.Marshall VM, Silva A, Foley M, Cranmer S, Wang L, McColl DJ, Kemp DJ, Coppel RL. 1997. A second merozoite surface protein (MSP-4) of Plasmodium falciparum that contains an epidermal growth factor-like domain. Infect Immun 65:4460–4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drew DR, Sanders PR, Crabb BS. 2005. Plasmodium falciparum merozoite surface protein 8 is a ring-stage membrane protein that localizes to the parasitophorous vacuole of infected erythrocytes. Infect Immun 73:3912–3922. doi: 10.1128/IAI.73.7.3912-3922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackman MJ, Heidrich HG, Donachie S, McBride JS, Holder AA. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med 172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burns JM Jr, Belk CC, Dunn PD. 2000. A protective glycosylphosphatidylinositol-anchored membrane protein of Plasmodium yoelii trophozoites and merozoites contains two epidermal growth factor-like domains. Infect Immun 68:6189–6195. doi: 10.1128/IAI.68.11.6189-6195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyle MJ, Langer C, Chan JA, Hodder AN, Coppel RL, Anders RF, Beeson JG. 2014. Sequential processing of merozoite surface proteins during and after erythrocyte invasion by Plasmodium falciparum. Infect Immun 82:924–936. doi: 10.1128/IAI.00866-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durocher Y, Perret S, Kamen A. 2002. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res 30:E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stöcker M, Tur MK, Sasse S, Krüssmann A, Barth S, Engert A. 2003. Secretion of functional anti-CD30-angiogenin immunotoxins into the supernatant of transfected 293T-cells. Protein Expr Purif 28:211–219. doi: 10.1016/S1046-5928(02)00709-X. [DOI] [PubMed] [Google Scholar]
- 27.Schiffer S, Letzian S, Jost E, Mladenov R, Hristodorov D, Huhn M, Fischer R, Barth S, Thepen T. 2013. Granzyme M as a novel effector molecule for human cytolytic fusion proteins: CD64-specific cytotoxicity of Gm-H22(scFv) against leukemic cells. Cancer Lett 341:178–185. doi: 10.1016/j.canlet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Remarque EJ, Faber BW, Kocken CH, Thomas AW. 2008. A diversity-covering approach to immunization with Plasmodium falciparum apical membrane antigen 1 induces broader allelic recognition and growth inhibition responses in rabbits. Infect Immun 76:2660–2670. doi: 10.1128/IAI.00170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noedl H, Bronnert J, Yingyuen K, Attlmayr B, Kollaritsch H, Fukuda M. 2005. Simple histidine-rich protein 2 double-site sandwich enzyme-linked immunosorbent assay for use in malaria drug sensitivity testing. Antimicrob Agents Chemother 49:3575–3577. doi: 10.1128/AAC.49.8.3575-3577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tur MK, Huhn M, Jost E, Thepen T, Brümmendorf TH, Barth S. 2011. In vivo efficacy of the recombinant anti-CD64 immunotoxin H22(scFv)-ETA′ in a human acute myeloid leukemia xenograft tumor model. Int J Cancer 129:1277–1282. doi: 10.1002/ijc.25766. [DOI] [PubMed] [Google Scholar]
- 31.Huhn M, Sasse S, Tur MK, Matthey B, Schinköthe T, Rybak SM, Barth S, Engert A. 2001. Human angiogenin fused to human CD30 ligand (Ang-CD30L) exhibits specific cytotoxicity against CD30-positive lymphoma. Cancer Res 61:8737–8742. doi: 10.1016/S0959-8049(01)80351-8. [DOI] [PubMed] [Google Scholar]
- 32.Hristodorov D, Mladenov R, Pardo A, Pham AT, Huhn M, Fischer R, Thepen T, Barth S. 2013. Microtubule-associated protein tau facilitates the targeted killing of proliferating cancer cells in vitro and in a xenograft mouse tumour model in vivo. Br J Cancer 109:1570–1578. doi: 10.1038/bjc.2013.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberoi P, Jabulowsky RA, Bähr-Mahmud H, Wels WS. 2013. EGFR-targeted granzyme B expressed in NK cells enhances natural cytotoxicity and mediates specific killing of tumor cells. PLoS One 8:e61267. doi: 10.1371/journal.pone.0061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Cheung LH, Hittelman WN, Rosenblum MG. 2003. Targeted delivery of human pro-apoptotic enzymes to tumor cells: in vitro studies describing a novel class of recombinant highly cytotoxic agents. Mol Cancer Ther 2:1341–1350. [PubMed] [Google Scholar]
- 35.Meslin B, Barnadas C, Boni V, Latour C, De Monbrison F, Kaiser K, Picot S. 2007. Features of apoptosis in Plasmodium falciparum erythrocytic stage through a putative role of PfMCA1 metacaspase-like protein. J Infect Dis 195:1852–1859. doi: 10.1086/518253. [DOI] [PubMed] [Google Scholar]
- 36.Bird CH, Sun J, Ung K, Karambalis D, Whisstock JC, Trapani JA, Bird PI. 2005. Cationic sites on granzyme B contribute to cytotoxicity by promoting its uptake into target cells. Mol Cell Biol 25:7854–7867. doi: 10.1128/MCB.25.17.7854-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]