Abstract
The relatively short half-lives of most β-lactams suggest that continuous infusion of these time-dependent antimicrobials may be favorable compared to short-term infusion. Nevertheless, only limited solid-tissue pharmacokinetic data are available to support this theory. In this study, we randomly assigned 12 pigs to receive cefuroxime as either a short-term or continuous infusion. Measurements of cefuroxime were obtained every 30 min in plasma, subcutaneous tissue, and bone. For the measurements in solid tissues, microdialysis was applied. A two-compartment population model was fitted separately to the drug concentration data for the different tissues using a nonlinear mixed-effects regression model. Estimates of the pharmacokinetic parameters and time with concentrations above the MIC were derived using Monte Carlo simulations. Except for subcutaneous tissue in the short-term infusion group, the tissue penetration was incomplete for all tissues. For short-term infusion, the tissue penetration ratios were 0.97 (95% confidence interval [CI], 0.67 to 1.39), 0.61 (95% CI, 0.51 to 0.73), and 0.45 (95% CI, 0.36 to 0.56) for subcutaneous tissue, cancellous bone, and cortical bone, respectively. For continuous infusion, they were 0.53 (95% CI, 0.33 to 0.84), 0.38 (95% CI, 0.23 to 0.57), and 0.27 (95% CI, 0.13 to 0.48) for the same tissues, respectively. The absolute areas under the concentration-time curve were also lower in the continuous infusion group. Nevertheless, a significantly longer time with concentrations above the MIC was found for continuous infusion up until MICs of 4, 2, 2, and 0.5 μg/ml for plasma and the same three tissues mentioned above, respectively. For drugs with a short half-life, like cefuroxime, continuous infusion seems to be favorable compared to short-term infusion; however, incomplete tissue penetration and high MIC strains may jeopardize the continuous infusion approach.
INTRODUCTION
The relatively short half-lives of most β-lactams suggest that extended infusion (EI) or continuous infusion (CI) of these time-dependent antimicrobials may be favorable compared to short-term infusion (STI). Nevertheless, different meta-analyses evaluating CI versus STI of various time-dependent antimicrobials have failed to convincingly demonstrate improved clinical outcomes on mortality and clinical cure (1–6). It is noteworthy, however, that in the majority of studies included in these meta-analyses, the total daily dose of antimicrobials was lower for patients treated with EI or CI (1, 2, 5). In a subset of randomized controlled trials (RCTs) in which the total daily dose was equivalent in the two intervention arms, the clinical failure rate was lower for patients treated with CI (1).
Inferences about the dosing regimens of antimicrobials are commonly based on plasma pharmacokinetics-pharmacodynamics (PK-PD) indices, despite the fact that the majority of bacterial pathogens reside in the interstitial space of solid tissues. However, incomplete and uneven tissue distribution was eventually demonstrated in a number of studies for different combinations of drug and tissue (7–13). As the gap between steady-state plasma concentrations and MICs may be rather limited using CI, incomplete tissue penetration may partly explain why improved clinical outcomes for CI have been difficult to demonstrate, particularly when the total daily dose is reduced.
Deep-seated orthopedic infections, like osteomyelitis and implant-associated infections (IAI), are difficult to treat, often requiring extensive surgical debridement and long-lasting antimicrobial therapy (14). In a recent porcine study, we demonstrated substantially impaired bone and subcutaneous tissue (SCT) penetration of cefuroxime (15). The pharmacokinetic profiles suggested that EI or CI of the drug might attain increased time with tissue concentrations above the MIC (T>MIC) for relevant microorganisms.
Ultimately, the dosing regimens of antimicrobials should be based on results of RCTs for a specific combination of drug, bug, and disease. However, in order to increase the probability of obtaining useful information from such trials, the selection of dosing regimens should be guided by the results from tissue pharmacokinetic studies.
In the present study, we used the microdialysis (MD) technique to obtain the pharmacokinetic parameters of cefuroxime in the SCT and bone of pigs receiving 1,500 mg of cefuroxime as either traditional STI or CI. The primary endpoint of this randomized trial was the T>MIC, which is the key PK-PD index for cephalosporins (16).
MATERIALS AND METHODS
This study was conducted at the Institute of Clinical Medicine, Aarhus University Hospital, Denmark. Chemical analyses were performed at the Department of Biochemistry, Aarhus University Hospital. The study was approved by the Danish Animal Experiments Inspectorate and carried out in accordance with existing laws.
Animals, anesthesia, and surgical procedures.
Twelve female pigs were included in the study (Danish Landrace breed, weighing 73 to 79 kg). Anesthesia was maintained during the entire study period using a combination of propofol (200 to 550 mg/h, continuous infusion) and fentanyl (0.4 to 0.85 mg/h, continuous infusion). Body temperature was kept within the range of 37.5 to 39.0°C. Normal kidney function, assessed by plasma creatinine level, was confirmed for all pigs before inclusion in the study. pH was monitored during the entire study using arterial gas analysis and was kept within a range of 7.36 to 7.54 by regulating tidal volume and respiratory frequency. The surgical procedures were initiated immediately after the induction of anesthesia. Using two distinct anteromedial approaches, MD catheters were placed in drill holes in the cortical bone of the anterior margin of the tibia and in cancellous bone within the tibial condyles. The depths of the drill holes were 14.5 ± 0.5 mm and 20 ± 1 mm for the cortical and cancellous drill holes, respectively. A 2-mm drill was used for both sites. Drilling was stopped every few seconds in order not to overheat the bone. Before wound closure, the catheters were fixed to the skin with a single suture. At the end of each experiment, it was verified by autopsy that the catheters had not been displaced from the drill holes. The intracortical location of the cortical drill holes was assessed by postmortem computed tomography (CT) scans of the tibia.
In addition to the two bone catheters, a reference catheter was placed in the SCT of the abdomen, according to the guidelines of the manufacturer.
Microdialysis and sampling procedures.
The principles of MD have been described in detail elsewhere (17–19). Briefly, MD is a minimally invasive probe-based technique that allows for continuous sampling of small unbound water-soluble molecules in the interstitial spaces of virtually all tissues (10, 20–24). The diffusion of solutes takes place across a semipermeable membrane at the tip of the probe along the concentration gradient. As the probe is continuously perfused, equilibrium will never occur, and the concentration in the dialysate will represent only a fraction of the actual concentration in the tissue. This fraction is referred to as relative recovery (RR). Consequently, a calibration procedure, in which the RR is determined, is imperative if absolute tissue concentrations are to be determined.
The MD system in the present study consisted of CMA 63 catheters (membrane length, 10 mm; molecular cutoff, 20 kDa) and CMA 107 precision pumps (M Dialysis AB, Stockholm, Sweden). Following implantation, the catheters were perfused with 0.9% NaCl containing 5 μg/ml cefuroxime. The perfusion rate was 2 μl/min. When surgery was completed, a 30-min tissue equilibration period was allowed. The probes were then calibrated using the retrodialysis method (25) by collecting a sample over a 30-min interval. The RR was calculated using the following equation:
| (1) |
where Cin is the cefuroxime concentration in the perfusate and Cout is the concentration in the dialysate. Individual in vivo calibration was performed for all catheters.
Following calibration, the perfusate was changed to blank 0.9% NaCl, and a 105-min washout period was allowed. A dialysate was collected during the last 20 min of this period in order to assess the efficacy of washout. The animals were then randomly assigned to receive 1,500 mg of cefuroxime (Fresenius Kabi AB, Sweden) as either STI (over 15 min) or CI (500 mg as a loading dose over 5 min, followed by CI of the remaining 1,000 mg over 7 h 55 min). Fifteen hundred milligrams was chosen because it is the standard dose for orthopedic procedures in Denmark, and because the weight of the animals resembled that of an average human being. In both groups, the dialysates were collected every 30 min for 8 h, starting at the beginning of the infusions. For the subsequent data analysis, the cefuroxime concentration in the dialysates was attributed to the midpoint of the sampling interval. The absolute tissue concentrations (Ctissue) were obtained by correcting for RR using the following equation:
| (2) |
Blood samples were drawn from a central venous catheter halfway through every dialysate sampling interval.
Handling of samples.
The dialysates were immediately frozen and stored at −80°C until analysis. The venous blood samples were stored at 5°C for a maximum of 20 h before being centrifuged at 3,000 × g for 10 min. The plasma aliquots were then frozen and stored at −80°C until analysis.
Quantification of cefuroxime concentrations.
The dialysate and free plasma concentrations of cefuroxime were quantified using a validated ultrahigh-performance liquid chromatography assay (reference 15 and M. Tøttrup and T. F. Hardlei, unpublished data). The intrarun (interrun) imprecisions (in percent coefficients of variation [%CVs]) were 5.6% (6.8%) at 0.25 μg/ml, 4.3% (4.7%) at 2.5 μg/ml, and 2.6% (2.8%) at 10 μg/ml for the dialysates. For the free plasma concentration, the intrarun (interrun) imprecision rates were 1.8% (6.5%) at 9.2 μg/ml and 1.6% (6.2%) at 38 μg/ml. The lowest limit of quantification was defined as the lowest concentration to be measured with an intrarun %CV of <20% and was found to be 0.06 μg/ml for both dialysates and the free cefuroxime concentration in plasma.
Pharmacokinetic analysis and statistics. (i) Population PK modeling.
We explored one- and two-compartment models with zero- and first-order kinetics in order to find the best description of the drug concentration in each tissue. A two-compartment model with zero-order appearance and first-order clearance was found to provide the best description of the cefuroxime concentrations in SCT, cancellous bone, and cortical bone. For the free plasma concentrations, an ordinary two-compartment model with first-order kinetics provided the best description of the drug concentration. For CI, the drug concentrations in SCT, cancellous bone, and cortical bone are given using the following equation:
| (3) |
where k1 is the appearance rate, k3 is the clearance rate, t is time, I is the continuous infusion rate, and x0 is the plasma concentration at time zero. For plasma, the drug concentration (Cplasma) is given by:
| (4) |
The drug concentration in the case of STI is obtained from the expressions above by putting I equal to zero.
From these expressions, it is possible to determine the T>MIC, the area under the concentration-time curve (AUC), peak drug concentration (Cmax), time to Cmax (Tmax), and in the case of STI half-life (t1/2) (calculated by log2/k3), though in the case of the T>MIC for plasma, this has to be done numerically.
(ii) Statistical analysis.
The two-compartment model was fitted to the drug concentration data separately for the different tissues using a nonlinear mixed-effects regression model with a random animal effect for each of the parameters k1, k3, x0, and I. The washout concentrations were low and as such were neglected in the analysis. Monte Carlo simulation was used to determine 95% confidence intervals for the T>MIC, AUC, Cmax, Tmax, t1/2, and the ratio between the AUCs, as well as the test for no difference between bolus and continuous infusions with regard to these quantities. More specifically, this was done by simulating 50,000 curves from the joint asymptotic normal distribution of the parameter estimates, calculating the derived quantities for each set of parameters, and then determining the 95% confidence intervals from the empirical distribution of these. The confidence intervals for the ratio between the AUCs were derived under the additional assumption that parameter estimates corresponding to the different tissues were independent. The data were analyzed using R version 3.0.2 (R Core Team, Vienna, Austria) with the package nlme.
RESULTS
All 12 experiments were completed, and no MD-related problems were encountered. In one of the pigs receiving CI, the postmortem CT scan revealed that the cortical drill hole penetrated to the bone marrow. Thus, the measurements obtained from this hole were excluded from the analysis. The mean ± standard deviation [SD] in vivo RRs were 15.4% ± 6.5%, 20.9% ± 10.4%, and 13.6% ± 5.8% for cortical bone, cancellous bone, and SCT, respectively. The mean ± SD concentrations in the washout samples were 0.09 ± 0.07 μg/ml, 0.02 ± 0.02 μg/ml, and 0.03 ± 0.03 μg/ml for the same anatomical sites, respectively.
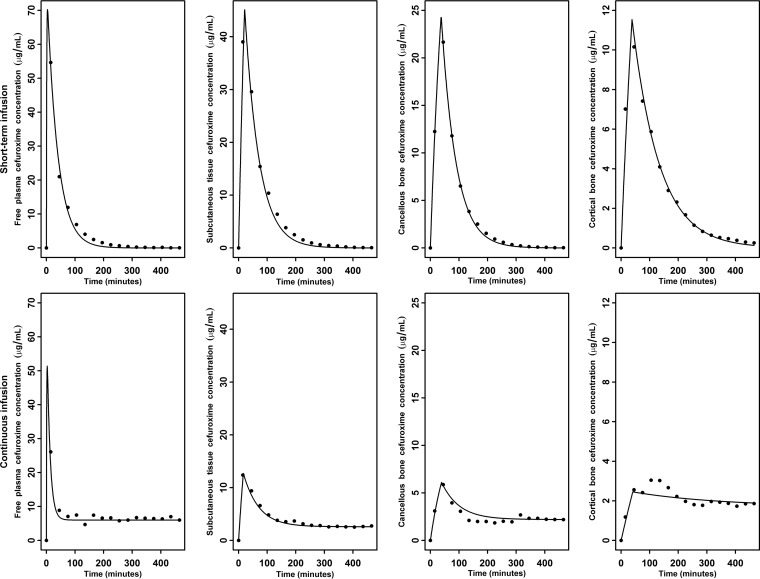
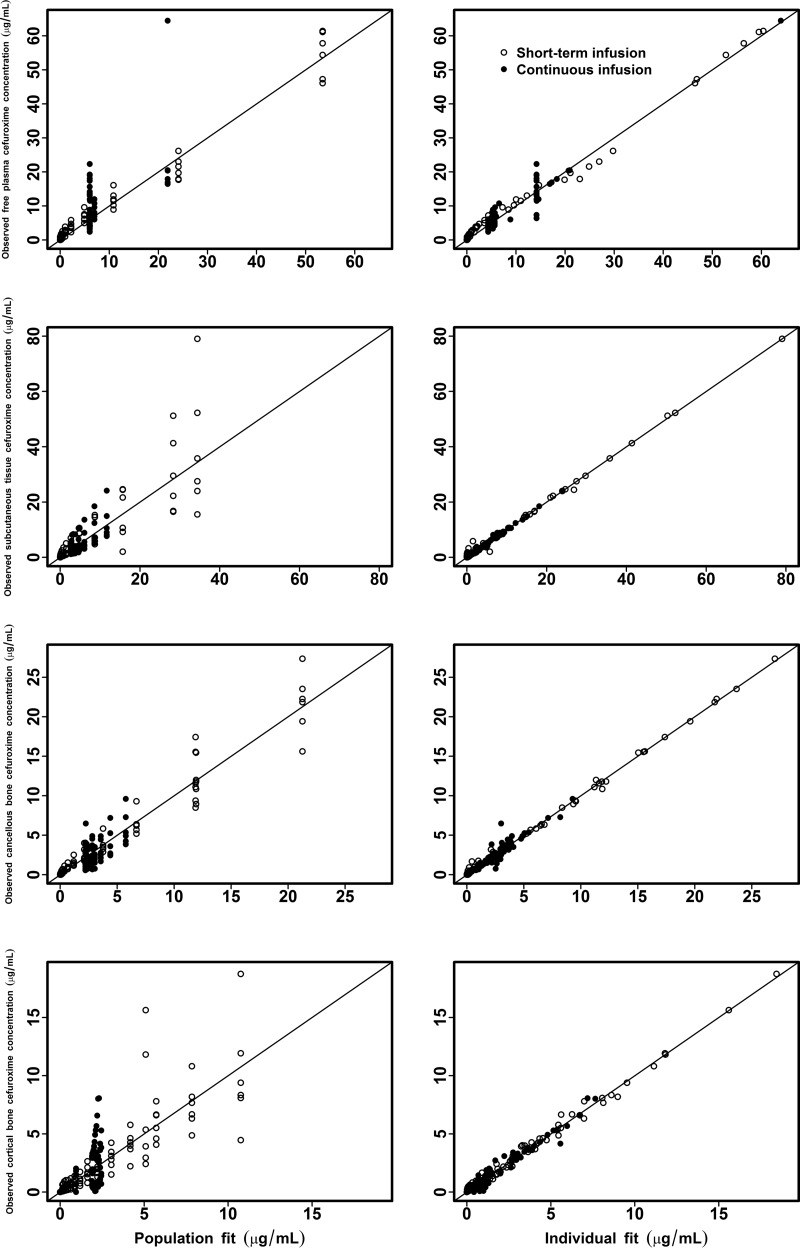
The mean observed concentrations and population fitted concentration-time profiles are depicted in Fig. 1. The observed versus fitted cefuroxime concentrations are shown in Fig. 2.
FIG 1.
Mean observed concentrations (dots) and population-fitted concentration-time profiles (lines) for short-term infusion (top) and continuous infusion (bottom).
FIG 2.
Observed versus simulated individual and population cefuroxime concentrations for free plasma, SCT, cortical bone, and cancellous bone.
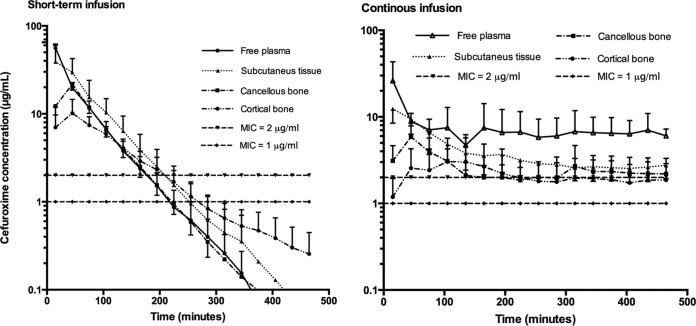
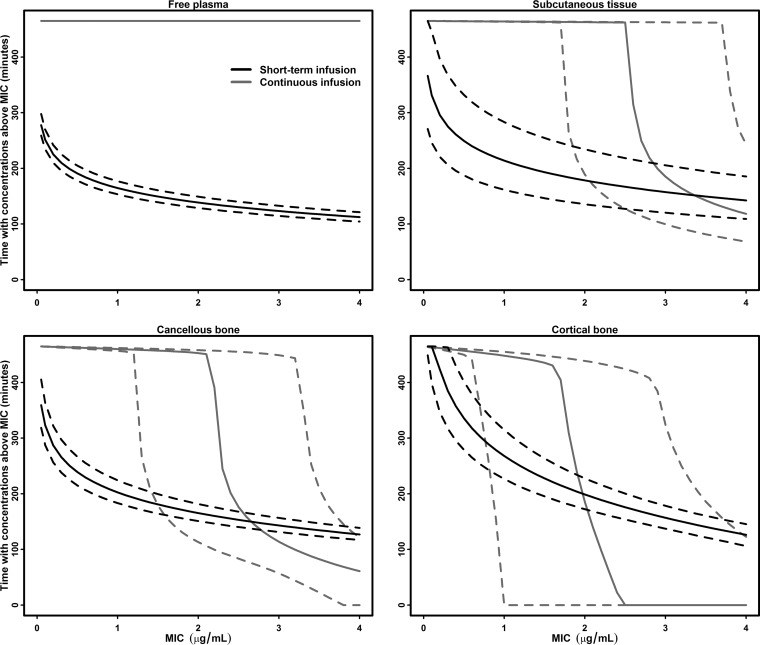
Estimates of the key standard pharmacokinetic parameters for free plasma, SCT, cancellous bone, and cortical bone are shown in Tables 1 and 2. The corresponding mean concentration-time profiles are displayed in Fig. 3. Comparisons of the AUC, tissue penetration ratios, and T>MIC between the STI and CI group can be found in Tables 3 and 4, respectively. In Fig. 4, the relationship between the T>MIC and MIC is depicted for free plasma, SCT, cancellous bone, and cortical bone.
TABLE 1.
Key STI pharmacokinetic parameters for free plasma, subcutaneous tissue, cancellous bone, and cortical bone
| Pharmacokinetic parametera | Mean (95% confidence interval) values in: |
|||
|---|---|---|---|---|
| Free plasma | SCTb | Cancellous bone | Cortical bone | |
| AUC0–last (min · μg/ml) | 2,919 (2,615–3,263) | 2,820 (1,986–3,986) | 1,786 (1,557–2,049) | 1,319 (1,096–1,586) |
| Cmax (μg/ml) | 70.2 (64.5–76.4) | 45.4 (31.7–64.7) | 24.4 (20.7–28.8) | 11.5 (8.1–16.4) |
| Tmax (min) | 3.7 (3.7–3.8) | 21.0 (16.6–26.5) | 37.7 (34.0–41.8) | 38.3 (26.8–54.7) |
| t1/2 (min) | 26.1 (24.3–28.0) | 35.1 (25.0–49.2) | 36.0 (31.0–41.8) | 65.9 (50.6–85.9) |
| fAUCtissue/fAUCplasma | 0.97 (0.67–1.39) | 0.61 (0.51–0.73) | 0.45 (0.36–0.56) | |
AUC0–last, area under the concentration-time curve from 0 to the last measured value; Cmax, peak drug concentration; Tmax, time to Cmax; t1/2, half-life; fAUCtissue/fAUCplasma, tissue penetration expressed as the ratio of free AUC tissue to free AUC plasma.
SCT, subcutaneous tissue.
TABLE 2.
Key CI pharmacokinetic parameters for free plasma, subcutaneous tissue, cancellous bone, and cortical bone
| Pharmacokinetic parametera | Mean (95% confidence interval) values in: |
|||
|---|---|---|---|---|
| Free plasma | SCTb | Cancellous bone | Cortical bone | |
| AUC0–last (min · μg/ml) | 3,437 (2,586–4,578) | 1,809 (1,240–2,636) | 1,296 (859–1,759) | 919 (471–1,545) |
| Cmax (μg/ml) | 51.4 (28.0–94.2) | 12.7 (9.0–17.8) | 6.1 (3.8–8.3) | 2.5 (0.9–5.8) |
| Tmax (min) | 2.7 (2.4–3.1) | 16.6 (14.3–19.4) | 39.9 (33.2–48.9) | 52.1 (31.4–95.8) |
| fAUCtissue/fAUCplasma | 0.53 (0.33–0.84) | 0.38 (0.23–0.57) | 0.27 (0.13–0.48) | |
AUC0–last, area under the concentration-time curve from 0 to the last measured value; Cmax, peak drug concentration; Tmax, time to Cmax; fAUCtissue/fAUCplasma, tissue penetration expressed as the ratio of free AUC tissue to free AUC plasma.
SCT, subcutaneous tissue.
FIG 3.
Mean concentration-time profiles for short-term and continuous infusion of cefuroxime for free plasma, SCT, cancellous bone, and cortical bone. The error bars represent standard deviations.
TABLE 3.
Comparison of AUC and tissue penetration
| Parametera | Mean (95% confidence interval) for: |
P value | |
|---|---|---|---|
| STI | CI | ||
| AUC0–last (min · μg/ml) for: | |||
| Free plasma | 2,919 (2,615–3,263) | 3,437 (2,586–4,578) | 0.33 |
| SCT | 2,820 (1,986–3,986) | 1,809 (1,240–2,636) | 0.1 |
| Cancellous bone | 1,786 (1,557–2,049) | 1,296 (859–1,759) | 0.06 |
| Cortical bone | 1,319 (1,096–1,586) | 919 (471–1545) | 0.18 |
| fAUCtissue/fAUCplasma for: | |||
| SCT | 0.97 (0.67–1.39) | 0.53 (0.33–0.84) | 0.05 |
| Cancellous bone | 0.61 (0.51–0.73) | 0.38 (0.23–0.57) | 0.02 |
| Cortical bone | 0.45 (0.36–0.56) | 0.27 (0.13–0.48) | 0.07 |
AUC0–last, area under the concentration-time curve from 0 to the last measured value; fAUCtissue/fAUCplasma, tissue penetration expressed as the ratio of free AUC tissue to free AUC plasma.
FIG 4.
Time with concentrations above MIC-MIC profiles for free plasma, SCT, cancellous bone, and cortical bone. The dotted lines represent 95% confidence intervals.
Except for SCT in the STI group, tissue penetration was incomplete for all tissues. Both the tissue AUCs and tissue penetration ratios were generally found to be lowest in the CI group. For cancellous bone, the tissue penetration ratio for CI was significantly lower than that of STI, whereas for SCT and cortical bone, this ratio only just failed to be significantly lower for CI. Nevertheless, a significantly longer T>MIC was found for CI up to MICs of 4 μg/ml, 2 μg/ml, 2 μg/ml, and 0.5 μg/ml for plasma, SCT, cancellous bone, and cortical bone, respectively. The same is true for lower MICs for all tissues, but with increasing MIC, the differences in T>MIC between STI and CI leveled out, with T>MIC eventually becoming higher for STI than that for CI for high MICs in the solid tissues (Fig. 4 and Table 4).
TABLE 4.
Comparison of time above the MIC
| T>MIC for tissues by concn (μg/ml) | Mean (95% confidence interval) for (min): |
P value | |
|---|---|---|---|
| STI | CI | ||
| 0.5 | |||
| Plasma | 190 (178–205) | 465 (465–465) | <0.001 |
| SCT | 249 (187–332) | 464 (464–465) | <0.001 |
| Cancellous bone | 239 (215–266) | 463 (461–464) | <0.001 |
| Cortical bone | 335 (279–401) | 457 (450–460) | <0.001 |
| 1 | |||
| Plasma | 164 (153–177) | 465 (465–465) | <0.001 |
| SCT | 214 (162–283) | 464 (463–464) | <0.001 |
| Cancellous bone | 202 (183–224) | 460 (457–462) | <0.001 |
| Cortical bone | 268 (227–315) | 448 (0–455) | 0.12 |
| 1.5 | |||
| Plasma | 149 (139–160) | 465 (465–465) | <0.001 |
| SCT | 193 (147–255) | 463 (463–464) | <0.001 |
| Cancellous bone | 180 (165–199) | 457 (179–460) | <0.001 |
| Cortical bone | 227 (195–263) | 435 (0–448) | 0.07 |
| 2 | |||
| Plasma | 139 (129–149) | 465 (465–465) | <0.001 |
| SCT | 178 (136–234) | 463 (189–463) | <0.001 |
| Cancellous bone | 165 (151–182) | 453 (113–458) | 0.001 |
| Cortical bone | 199 (172–228) | 185 (0–439) | 0.9 |
| 4 | |||
| Plasma | 113 (104–121) | 465 (465–465) | <0.001 |
| SCT | 142 (109–185) | 118 (68–243) | 0.6 |
| Cancellous bone | 127 (117–139) | 61 (0–121) | 0.03 |
| Cortical bone | 126 (106–145) | 0 (0–122) | <0.001 |
DISCUSSION
This is the first article to report concurrent pharmacokinetics of a β-lactam antibiotic in plasma, SCT, and bone administered as STI and CI. The main finding is that a longer T>MIC can be achieved using CI rather than STI of a drug with a short half-life, like cefuroxime. Nevertheless, the data also clearly indicate that with a CI approach, the gap between tissue concentrations and MIC may be limited or even inversed, depending on the tissue type and MIC. A similar relationship has been found for piperacillin (26). From this point of view, it is not surprising that convincing evidence for the superiority of CI over STI has been difficult to establish, despite the apparent theoretical advantages. Accordingly, the practice of lowering the total daily dose for CI seems unsafe.
In recent years, therapeutic drug monitoring (TDM) has become increasingly available as a routine analysis method in the daily clinical setting. As the pharmacokinetics of β-lactams has been shown to be unpredictable and display considerable interindividual variation, particularly in critically ill patients (27, 28), TDM is expected to optimize PK-PD target attainment and thus ultimately improve the treatment of serious infections (29–31). Our data suggest that care should be taken when adjusting antimicrobial dosing that is based merely upon the plasma concentrations and MICs of isolated pathogens, as incomplete tissue penetration may result in subtherapeutic tissue concentrations at the site of the infection. CI obviously has the potential to improve target attainment. However, if tissue penetration is substantially incomplete, CI may result in subtherapeutic concentrations at the site of infection for the entire dosing interval. On the other hand, STI may provide therapeutic concentrations but only for a limited part of the dosing interval. Clearly, tissue concentrations cannot be measured in the individual patient, but the available data on tissue pharmacokinetics for the specific combination of drug and infection should be integrated in the clinical decision making in order to prevent treatment failure. It was recently argued that an aggressive target of obtaining free plasma concentrations of 4 to 5 times the MIC for the entire dosing interval may be more predictive of a successful clinical outcome (31). At least for critically ill patients, in whom heterogeneous tissue distribution has been well documented (10, 11), our findings for CI support this aggressive approach.
A key finding of this study is the heterogeneous tissue distribution of cefuroxime, which was present regardless of the type of administration of the drug. In agreement with our previous study, the poorest tissue penetration was found for bone (15). Somewhat surprisingly, bone penetration in the CI group was poorer than in the STI group, but this seems partly compensated for by higher free plasma AUCs. Based on the present data, it can be speculated that plasma-tissue equilibrium may be concentration dependent in a dynamic manner. However, this hypothesis obviously needs further investigation.
Our finding of bone penetration ratios of approximately 1:3 to 2:3 suggests that incomplete tissue penetration may partly explain the prolonged antimicrobial treatment needed for osteomyelitis and IAIs. Accordingly, in terms of T>MIC, a standard target of remaining at >2 μg/ml for 50% of the dosing interval was achieved in neither cancellous nor cortical bone using traditional STI. The majority of isolated Staphylococcus aureus exhibits MICs of 1 μg/ml (http://mic.eucast.org/Eucast2/SearchController/search.jsp?action=performSearch&BeginIndex=0&Micdif=mic&NumberIndex=50&Antib=46&Specium=-1). Based on the T>MIC values for CI, it appears that increased T>MIC can be achieved using CI but only for low MICs. Nevertheless, in order to remain above the MIC for the entire dosing interval for pathogens with higher MICs, the total daily dose needs to be increased, which also seems reasonable for serious infections.
Although pigs resemble humans in terms of physiology and anatomy (32), the major limitation of this study is obviously that it is not a clinical study. Consequently, the findings cannot readily be extrapolated to humans or to pathological conditions. Moreover, the pigs had to be kept under general anesthesia during the entire study period, which is known to cause physiological alterations that may affect pharmacokinetics. This also precludes the opportunity to conduct measurements after the administration of multiple doses of cefuroxime. Nonetheless, it seems rational to use this large-animal model to explore the basic concepts of CI versus STI, focusing on the role of antimicrobial tissue penetration. This approach provides a sound foundation for future clinical studies, while attention is drawn to the possible pitfalls of CI and incomplete tissue penetration.
Over the last decade, MD has become the method of choice for obtaining antimicrobial tissue pharmacokinetics, including the particular case of bone (7, 10, 18, 20–24, 26, 33–37). Due to mandatory correction for RR in pharmacokinetic studies, a magnification of the variations associated with the preanalytical sample handling and chemical assay is inherent to the MD approach. These variations will increase exponentially with decreasing recovery (17). Consequently, MD studies should always be interpreted with this possible limitation in mind. Our finding of the comparable variations of the pharmacokinetic parameters in plasma and solid tissues suggests that our setup was adequately reliable in terms of precision and that a significant part of the variation can be regarded as biological.
In conclusion, the findings in the present study indicate that CI of β-lactams with short half-lives may be favorable compared to STI, if dosed appropriately. These animal data cannot be applied uncritically in a clinical setting, but incomplete tissue penetration of antimicrobials should be considered when planning CI and using TDM. For bone, the tissue penetration was substantially incomplete. The high rates of treatment failure for osteomyelitis and IAIs may therefore partly be attributable to incomplete target site penetration of the antimicrobials. MD seems to be a valuable and reliable tool for investigating these matters, and as such, clinical studies with similar methodological setups are warranted.
ACKNOWLEDGMENTS
We thank Line Jensen (Research Unit, Horsens Regional Hospital) for meticulous language editing.
The study was supported by grants from the Familien Hede Nielsens Foundation and The Korning Foundation.
REFERENCES
- 1.Kasiakou SK, Sermaides GJ, Michalopoulos A, Soteriades ES, Falagas ME. 2005. Continuous versus intermittent intravenous administration of antibiotics: a meta-analysis of randomised controlled trials. Lancet Infect Dis 5:581–589. doi: 10.1016/S1473-3099(05)70218-8. [DOI] [PubMed] [Google Scholar]
- 2.Korbila IP, Tansarli GS, Karageorgopoulos DE, Vardakas KZ, Falagas ME. 2013. Extended or continuous versus short-term intravenous infusion of cephalosporins: a meta-analysis. Expert Rev Anti Infect Ther 11:585–595. doi: 10.1586/eri.13.44. [DOI] [PubMed] [Google Scholar]
- 3.Shiu J, Wang E, Tejani AM, Wasdell M. 2013. Continuous versus intermittent infusions of antibiotics for the treatment of severe acute infections. Cochrane Database Syst Rev 3:CD008481. doi: 10.1002/14651858.CD008481.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ. 2013. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: a systematic review and meta-analysis. Clin Infect Dis 56:272–282. doi: 10.1093/cid/cis857. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JA, Webb S, Paterson D, Ho KM, Lipman J. 2009. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit Care Med 37:2071–2078. doi: 10.1097/CCM.0b013e3181a0054d. [DOI] [PubMed] [Google Scholar]
- 6.Tamma PD, Putcha N, Suh YD, Van Arendonk KJ, Rinke ML. 2011. Does prolonged beta-lactam infusions improve clinical outcomes compared to intermittent infusions? A meta-analysis and systematic review of randomized, controlled trials. BMC Infect Dis 11:181. doi: 10.1186/1471-2334-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreas D, Zeitlinger M, Hoeferl M, Jaeger W, Zimpfer D, Hiesmayr JM, Laufer G, Hutschala D. 2013. Internal mammary artery harvesting influences antibiotic penetration into presternal tissue. Ann Thorac Surg 95:1323–1329, discussion 1329–1330. doi: 10.1016/j.athoracsur.2012.10.088. [DOI] [PubMed] [Google Scholar]
- 8.Brill MJ, Houwink AP, Schmidt S, Van Dongen EP, Hazebroek EJ, van Ramshorst B, Deneer VH, Mouton JW, Knibbe CA. 2013. Reduced subcutaneous tissue distribution of cefazolin in morbidly obese versus non-obese patients determined using clinical microdialysis. J Antimicrob Chemother 69:715–723. doi: 10.1093/jac/dkt444. [DOI] [PubMed] [Google Scholar]
- 9.Brunner M, Pernerstorfer T, Mayer BX, Eichler HG, Müller M. 2000. Surgery and intensive care procedures affect the target site distribution of piperacillin. Crit Care Med 28:1754–1759. doi: 10.1097/00003246-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Joukhadar C, Frossard M, Mayer BX, Brunner M, Klein N, Siostrzonek P, Eichler HG, Müller M. 2001. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit Care Med 29:385–391. doi: 10.1097/00003246-200102000-00030. [DOI] [PubMed] [Google Scholar]
- 11.Tegeder I, Schmidtko A, Bräutigam L, Kirschbaum A, Geisslinger G, Lötsch J. 2002. Tissue distribution of imipenem in critically ill patients. Clin Pharmacol Ther 71:325–333. doi: 10.1067/mcp.2002.122526. [DOI] [PubMed] [Google Scholar]
- 12.Barbour A, Schmidt S, Rout WR, Ben-David K, Burkhardt O, Derendorf H. 2009. Soft tissue penetration of cefuroxime determined by clinical microdialysis in morbidly obese patients undergoing abdominal surgery. Int J Antimicrob Agents 34:231–235. doi: 10.1016/j.ijantimicag.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 13.De La Peña A, Dalla Costa T, Talton JD, Rehak E, Gross J, Thyroff-Friesinger U, Webb AI, Müller M, Derendorf H. 2001. Penetration of cefaclor into the interstitial space fluid of skeletal muscle and lung tissue in rats. Pharm Res 18:1310–1314. doi: 10.1023/A:1013042128791. [DOI] [PubMed] [Google Scholar]
- 14.Lew DP, Waldvogel FA. 1997. Osteomyelitis. N Engl J Med 336:999–1007. doi: 10.1056/NEJM199704033361406. [DOI] [PubMed] [Google Scholar]
- 15.Tøttrup M, Hardlei TF, Bendtsen M, Bue M, Brock B, Fuursted K, Søballe K, Birke-Sørensen H. 2014. Pharmacokinetics of cefuroxime in porcine cortical and cancellous bone determined by microdialysis. Antimicrob Agents Chemother 58:3200–3205. doi: 10.1128/AAC.02438-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10, quiz 11–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 17.Chaurasia CS, Müller M, Bashaw ED, Benfeldt E, Bolinder J, Bullock R, Bungay PM, DeLange ECM, Derendorf H, Elmquist WF, Hammarlund-Udenaes M, Joukhadar C, Kellogg DL, Lunte CE, Nordstrom CH, Rollema H, Sawchuk RJ, Cheung BWY, Shah VP, Stahle L, Ungerstedt U, Welty DF, Yeo H. 2007. AAPS-FDA workshop white paper: microdialysis principles, application, and regulatory perspectives report from the Joint AAPS-FDA Workshop, November 4–5, 2005, Nashville, TN. AAPS J 9:47–59. doi: 10.1208/aapsj0901006. [DOI] [Google Scholar]
- 18.Joukhadar C, Müller M. 2005. Microdialysis: current applications in clinical pharmacokinetic studies and its potential role in the future. Clin Pharmacokinet 44:895–913. doi: 10.2165/00003088-200544090-00002. [DOI] [PubMed] [Google Scholar]
- 19.Müller M, Schmid R, Georgopoulos A, Buxbaum A, Wasicek C, Eichler HG. 1995. Application of microdialysis to clinical pharmacokinetics in humans. Clin Pharmacol Ther 57:371–380. doi: 10.1016/0009-9236(95)90205-8. [DOI] [PubMed] [Google Scholar]
- 20.Hutschala D, Skhirtladze K, Kinstner C, Zeitlinger M, Wisser W, Jaeger W, Hoeferl M, Müller M, Tschernko E. 2013. Effect of cardiopulmonary bypass on regional antibiotic penetration into lung tissue. Antimicrob Agents Chemother 57:2996–3002. doi: 10.1128/AAC.02627-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schintler MV, Traunmüller F, Metzler J, Kreuzwirt G, Spendel S, Mauric O, Popovic M, Scharnagl E, Joukhadar C. 2009. High fosfomycin concentrations in bone and peripheral soft tissue in diabetic patients presenting with bacterial foot infection. J Antimicrob Chemother 64:574–578. doi: 10.1093/jac/dkp230. [DOI] [PubMed] [Google Scholar]
- 22.Stolle LB, Arpi M, Holmberg-Jørgensen P, Riegels-Nielsen P, Keller J. 2004. Application of microdialysis to cancellous bone tissue for measurement of gentamicin levels. J Antimicrob Chemother 54:263–265. doi: 10.1093/jac/dkh291. [DOI] [PubMed] [Google Scholar]
- 23.Traunmüller F, Schintler MV, Spendel S, Popovic M, Mauric O, Scharnagl E, Joukhadar C. 2010. Linezolid concentrations in infected soft tissue and bone following repetitive doses in diabetic patients with bacterial foot infections. Int J Antimicrob Agents 36:84–86. doi: 10.1016/j.ijantimicag.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Shukla C, Patel V, Juluru R, Stagni G. 2009. Quantification and prediction of skin pharmacokinetics of amoxicillin and cefuroxime. Biopharm Drug Dispos 30:281–293. doi: 10.1002/bdd.658. [DOI] [PubMed] [Google Scholar]
- 25.Ståhle L, Arner P, Ungerstedt U. 1991. Drug distribution studies with microdialysis. III: extracellular concentration of caffeine in adipose tissue in man. Life Sci 49:1853–1858. [DOI] [PubMed] [Google Scholar]
- 26.Roberts JA, Roberts MS, Robertson TA, Dalley AJ, Lipman J. 2009. Piperacillin penetration into tissue of critically ill patients with sepsis–bolus versus continuous administration? Crit Care Med 37:926–933. doi: 10.1097/CCM.0b013e3181968e44. [DOI] [PubMed] [Google Scholar]
- 27.Gonçalves-Pereira J, Póvoa P. 2011. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of β-lactams. Crit Care 15:R206. doi: 10.1186/cc10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sime FB, Roberts MS, Peake SL, Lipman J, Roberts JA. 2012. Does Beta-lactam pharmacokinetic variability in critically ill patients justify therapeutic drug monitoring? A systematic review. Ann Intensive Care 2:35. doi: 10.1186/2110-5820-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi Y, Lipman J, Udy AA, Ng M, McWhinney B, Ungerer J, Lust K, Roberts JA. 2013. β-Lactam therapeutic drug monitoring in the critically ill: optimising drug exposure in patients with fluctuating renal function and hypoalbuminaemia. Int J Antimicrob Agents 41:162–166. doi: 10.1016/j.ijantimicag.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Hites M, Taccone FS, Wolff F, Cotton F, Beumier M, De Backer D, Roisin S, Lorent S, Surin R, Seyler L, Vincent JL, Jacobs F. 2013. Case-control study of drug monitoring of β-lactams in obese critically ill patients. Antimicrob Agents Chemother 57:708–715. doi: 10.1128/AAC.01083-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts JA, Ulldemolins M, Roberts MS, McWhinney B, Ungerer J, Paterson DL, Lipman J. 2010. Therapeutic drug monitoring of beta-lactams in critically ill patients: proof of concept. Int J Antimicrob Agents 36:332–339. doi: 10.1016/j.ijantimicag.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Swindle MM, Makin A, Herron AJ, Clubb FJ Jr, Frazier KS. 2012. Swine as models in biomedical research and toxicology testing. Vet Pathol 49:344–356. doi: 10.1177/0300985811402846. [DOI] [PubMed] [Google Scholar]
- 33.Barbour A, Schmidt S, Sabarinath SN, Grant M, Seubert C, Skee D, Murthy B, Derendorf H. 2009. Soft-tissue penetration of ceftobiprole in healthy volunteers determined by in vivo microdialysis. Antimicrob Agents Chemother 53:2773–2776. doi: 10.1128/AAC.01409-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buerger C, Plock N, Dehghanyar P, Joukhadar C, Kloft C. 2006. Pharmacokinetics of unbound linezolid in plasma and tissue interstitium of critically ill patients after multiple dosing using microdialysis. Antimicrob Agents Chemother 50:2455–2463. doi: 10.1128/AAC.01468-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stolle L, Arpi M, H-Jørgensen P, Riegels-Nielsen P, Keller J. 2005. Distribution of gentamicin from a Gentacoll sponge measured by in vivo microdialysis. Scand J Infect Dis 37:284–287. doi: 10.1080/00365540410021108-1. [DOI] [PubMed] [Google Scholar]
- 36.Stolle LB, Plock N, Joukhadar C, Arpi M, Emmertsen KJ, Buerger C, Riegels-Nielsen P, Kloft C. 2008. Pharmacokinetics of linezolid in bone tissue investigated by in vivo microdialysis. Scand J Infect Dis 40:24–29. doi: 10.1080/00365540701509873. [DOI] [PubMed] [Google Scholar]
- 37.Stolle LB, Arpi M, Jørgensen PH, Riegels-Nielsen P, Keller J. 2003. In situ gentamicin concentrations in cortical bone: an experimental study using microdialysis in bone. Acta Orthop Scand 74:611–616. doi: 10.1080/00016470310018045. [DOI] [PubMed] [Google Scholar]