Abstract
Antibiotics disrupt the intestinal microbiota, rendering patients vulnerable to colonization by exogenous pathogens. Intermicrobial interactions may attenuate this effect. Incubation with ceftriaxone-resistant, ccrA-positive, β-lactamase-producing Bacteroides strains raised the minimum bactericidal concentration of ceftriaxone required to kill a susceptible Escherichia coli strain (mean change, <0.25 to 29 mg/liter; P = 0.009); incubation with ceftriaxone-resistant but non-β-lactamase-producing Bacteroides strains had no effect. The production of β-lactamase by common members of the intestinal microbiota (Bacteroides) can protect susceptible fellow commensals from β-lactams.
TEXT
The indigenous anaerobic microbiota of the lower intestinal tract remains a crucial mammalian host defense against colonization by exogenous, potentially pathogenic microorganisms (1–3). This defense mechanism is termed colonization resistance and may be abolished in hospitalized patients by the administration of antibiotic therapy. Antibiotics, including β-lactam antibiotics, may disrupt the intestinal microbiota, rendering patients susceptible to colonization or infection with nosocomial pathogens.
We previously demonstrated in β-lactam-treated mice that oral recombinant proteolysis-resistant β-lactamase enzymes that inactivate β-lactams can preserve gut colonization resistance against multiple nosocomial pathogens, including vancomycin-resistant Enterococcus (VRE), extended-spectrum β-lactamase-producing Klebsiella pneumoniae, and Clostridium difficile (4–6), via intraintestinal degradation of excreted antibiotic by intraluminal β-lactamases. This strategy holds promise for the prevention of pathogen colonization in patients treated with parenteral antibiotics, as antibiotic degradation within the colonic lumen does not impact systemic concentrations (7). We also demonstrated that, despite their receipt of parenteral β-lactams, mice intestinally colonized with a β-lactamase-producing member of the commensal microbiota (Bacteroides thetaiotaomicron) preserved colonization resistance against VRE and C. difficile (8). However, the genetics of β-lactam resistance in the protective Bacteroides species in this study were not known, and a comparator anaerobe was not studied. Here, we hypothesized that ceftriaxone-resistant Bacteroides species producing the broad-spectrum metallo-β-lactamase CcrA would protect β-lactam-susceptible members of the microbiota from the β-lactam ceftriaxone in vitro, while ceftriaxone-resistant (but non-β-lactamase-producing) Bacteroides species would not.
(Portions of this study were previously presented in abstract form at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 17 to 20 September 2011.)
Bacterial strains.
To test the protection of a clinical strain of ceftriaxone-susceptible Escherichia coli (chosen as a representative member of the human gut microbiome that can be grown on a medium different than that for Bacteroides) from ceftriaxone, four highly cephalosporin-resistant clinical Bacteroides fragilis group microorganisms were used (strain 1, B. fragilis; strain 2, Bacteroides distasonis; strain 3, B. fragilis; strain 4, B. thetaiotaomicron; all strains are part of a collection that was a kind gift from D. W. Hecht, Maywood, IL, and selection is described below). The MICs of ceftriaxone for the four Bacteroides strains were 500, 500, 250, and 500 mg/liter, respectively. The MIC of ceftriaxone for the E. coli strain was <0.25 mg/liter.
Genetic and phenotypic characterization of β-lactamases.
Ceftriaxone-resistant clinical Bacteroides group organisms were assessed by PCR for the presence of the four common β-lactamase (bla) genes found in this genus (cblA, cepA, cfxA, and cfiA/ccrA). Primer sequences for the target genes were chosen based on either previously published primers or, if such were not available, flanking the entire gene sequence for the enzyme as reported in the literature (9–12). PCR was then performed according to the methods described by Hujer et al. (13).
The production of β-lactamase in all the isolates was also characterized phenotypically according to our previous methods (8) using a qualitative assay with the chromogenic cephalosporin substrate nitrocefin, which changes color within 1 min of undergoing hydrolysis of the amide bond by β-lactamase enzymes (14). As a result of these examinations, four Bacteroides strains were selected for the mixing studies (described below).
Minimum bactericidal concentrations.
We obtained the broth microdilution minimum bactericidal concentrations (MBCs) of ceftriaxone for all five organisms according to standard microbiologic methods (15, 16). We used supplemented brucella broth (Becton-Dickinson, Cockeysville, MD) for determinations of Bacteroides susceptibilities and Mueller-Hinton broth (Becton-Dickinson) to determine the E. coli strain susceptibilities. Subsequently, in vitro mixing studies, and the measurement of broth microdilution MBCs with E. coli in the presence of each of the 4 Bacteroides isolates, were performed in brucella broth. We obtained MBCs, rather than MICs, as the visual inspection of antibiotic-containing microtiter plates does not indicate whether it is the ceftriaxone-susceptible or the ceftriaxone-resistant organism that has survived. For the mixing studies, standard concentrations (1:100 dilution of the 0.5 McFarland standard) of the five organisms were prepared. We prepared antibiotic dilutions of ceftriaxone according to CLSI methods (15) for MBC determinations in microtiter plates. We then inoculated ceftriaxone-containing wells of the microtiter plates first with the appropriate concentration of each specified Bacteroides strain and then with the McFarland dilution of E. coli. After they were incubated overnight at 37°C in an anaerobic chamber (Coy Laboratories, Grass Lake, MI), MBCs for the susceptible E. coli strain were determined by plating samples from all of the wells onto agar selective for facultative Gram-negative bacteria (MacConkey, Becton-Dickinson, Sparks, MD). The plates were subsequently incubated aerobically at 37°C overnight to determine E. coli growth. We defined bactericidal activity as a ≥3-log10 reduction in the initial inoculum (99.9% killing) after 24 h of incubation (17).
Statistical analysis.
The paired t test was used to compare differences in the effects of ceftriaxone on the MBCs for the E. coli strain that had been incubated with ceftriaxone-resistant ccrA-positive versus that incubated with ccrA-negative Bacteroides strains.
Bacteroides strain choice.
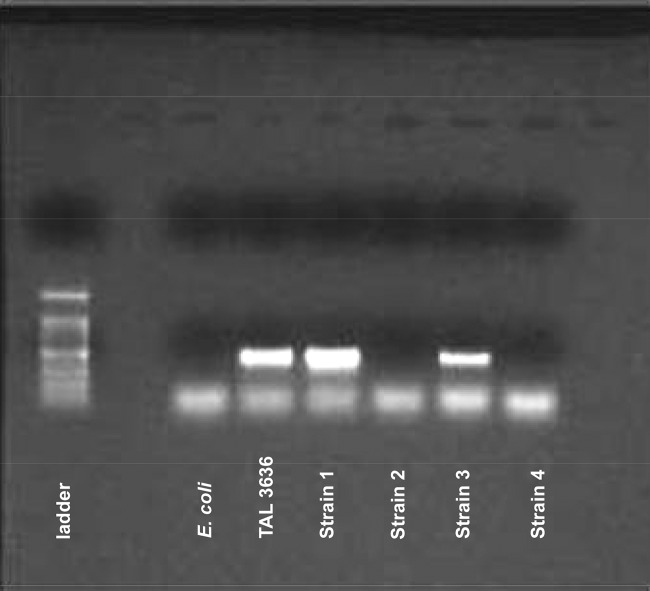
After assaying for known Bacteroides β-lactamase genes among our collection, four Bacteroides strains were chosen to test our hypothesis. PCR for ccrA (a broad-spectrum Ambler class B metallo-β-lactamase that hydrolyzes carbapenems and cephalosporins [18]) in 4 cephalosporin-resistant Bacteroides strains showed that strains 1 and 3 possessed this gene, while strains 2 and 4 did not (Fig. 1). All four strains were PCR negative for the other known Bacteroides β-lactamase genes (cblA, cepA, and cfxA [other gels not shown]). Additionally, strains 1 and 3 exhibited very rapid hydrolysis of nitrocefin, while strains 2 and 4 did not hydrolyze nitrocefin (i.e., their mechanism of resistance to ceftriaxone did not appear to be related to the production of a cephalosporinase).
FIG 1.
ccrA β-lactamase background in the four Bacteroides sp. PCRs for the ccrA metallo-β-lactamase gene in 4 carbapenem-resistant clinical Bacteroides strains show that isolates 1 and 3 possess this gene, while isolates 2 and 4 do not. Isolates 1 and 3 also displayed good hydrolytic activity against the chromogenic cephalosporin substrate nitrocefin, while 2 and 4 did not. E. coli was run as a negative control that does not possess the ccrA gene. TAL3636 was used as the positive control as it is a Bacteroides sp. known to possess the ccrA gene (18).
MBCs.
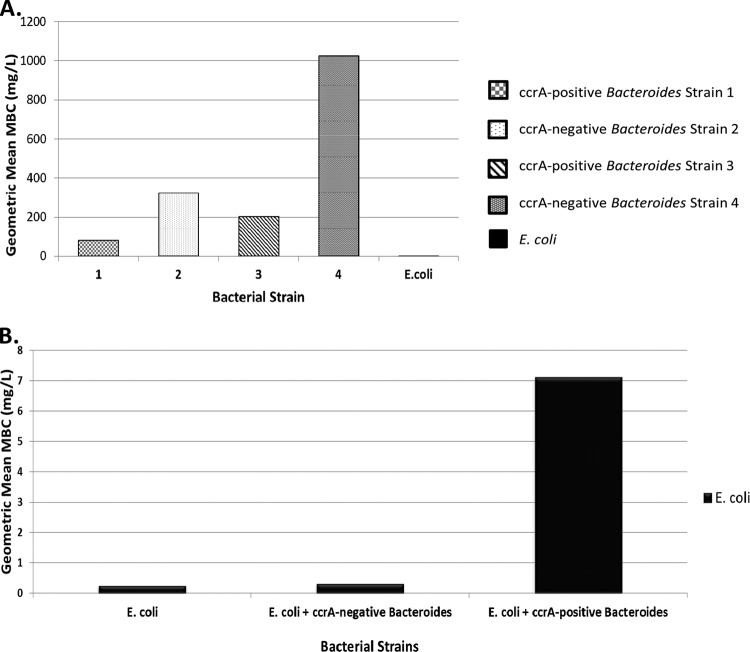
With regard to the determinations of individual MBCs, all 4 strains of Bacteroides were resistant to the commonly used β-lactam antibiotic ceftriaxone, while the E. coli strain used in these experiments displayed good susceptibility to ceftriaxone, with MBCs in the <0.25-mg/liter range (Fig. 2A).
FIG 2.
MBC determinations. (A) Geometric mean MBCs of ceftriaxone for each of the five bacterial species. All 4 strains of Bacteroides were resistant to the commonly used β-lactam antibiotic ceftriaxone. The E. coli strain used in these experiments, however, displayed good susceptibility to ceftriaxone, with MBCs in the <0.25-mg/liter range. (B) Geometric mean MBCs of ceftriaxone for the E. coli strain when it was grown alone, in the presence of ccrA-negative non-β-lactamase-producing Bacteroides spp., or in the presence of ccrA-positive β-lactamase-producing Bacteroides spp. All MBC determinations were made at least in triplicate.
Mixing study results.
Results of the mixing studies demonstrated that, in repeated broth microdilution MBC testing by standard methods, the geometric mean MBC of ceftriaxone for E. coli was raised when it was determined in the presence of ccrA-positive Bacteroides strains expressing rapid hydrolytic activity against nitrocefin but not when determined in the presence of cephalosporin-resistant but ccrA-negative Bacteroides strains displaying poor nitrocefin hydrolysis (Fig. 2B). Incubation with Bacteroides strains 1 or 3 raised the MBC of ceftriaxone required to kill a susceptible E. coli strain from <0.25 mg/liter to a geometric mean value of 7.12 mg/liter or a pooled average MBC of 29 mg/liter (P = 0.009), whereas incubation with Bacteroides strains 2 or 4 had no significant effects (geometric mean MBC change from <0.25 mg/liter to 0.31 mg/liter or pooled average MBC change to 0.33 mg/liter; P = 0.17). Survival of the E. coli isolates in the presence of Bacteroides strains 1 and 3 was not secondary to the development of resistance, as repeat MBC testing on recovered E. coli isolates from the mixing studies with Bacteroides still showed it to be susceptible to ceftriaxone (MBC, <0.25 mg/liter).
In a previous series of animal experiments, we showed that β-lactamase production by a B. thetaiotaomicron isolate could protect susceptible organisms within the same microbiota from β-lactam antibiotics (8). In the present study, we demonstrated that the magnitude of this effect is correlated with the genetic and phenotypic characteristics of the β-lactamase background in the protecting anaerobes. Cephalosporinase-producing anaerobes that possess a broad-spectrum β-lactamase gene allowed survival of a susceptible E. coli isolate despite high concentrations of ceftriaxone, while cephalosporin-resistant anaerobes without cephalosporinase activity did not.
These results highlight a potential mechanism by which antimicrobial resistance in bacteria—usually an undesirable phenomenon—may one day be leveraged to benefit hospitalized patients. According to recent estimates, Bacteroides group organisms (B. fragilis, B. distasonis, Bacteroides ovatus, B. thetaiotaomicron, and Bacteroides vulgatus) may comprise up to one-fourth of the indigenous intestinal microbiota in humans (19); our results suggest that β-lactam resistance in these microorganisms may not always be a bad thing. From an ecological perspective, such findings may also represent part of the explanation for why only some β-lactam-treated patients conserve their microbiome in the hospital setting and resist colonization by nosocomial pathogens.
Our study has some limitations. It is an in vitro examination of microbial interactions that usually take place within a complex ecosystem, and as such, it serves as a model. Second, it would be of interest to characterize the mechanism of resistance to β-lactam antibiotics in the non-β-lactamase-producing Bacteroides isolates, and it may have been of interest to measure the amount of ceftriaxone present in the wells by high-pressure liquid chromatography (HPLC) or other methods after the mixed incubation of multiple organisms. Third, many hospitalized patients receive more than one class of antibiotic. Any pharmaceutical, probiotic, or ecologic strategy attempting to capitalize on these findings would have to be accompanied by effective antimicrobial stewardship, as β-lactamases produced by gut anaerobes would not protect against non-β-lactam classes of antibiotics that are secreted into the intestinal tract.
In summary, we have shown that the degree of protection from β-lactams conferred by common anaerobes on other members of the gut microbiome in vitro appears to be associated with their bla genetic and phenotypic profiles and not simply with the presence of β-lactam resistance in the protecting anaerobes. Further investigations to determine the importance of this mechanism of protection in hospitalized patients are indicated, as these results may inspire novel approaches to prevent pathogen colonization.
ACKNOWLEDGMENT
This project was supported by an Advanced Research Career Development Award (CDA-2) from the Department of Veterans Affairs (to U.S.).
REFERENCES
- 1.Vollaard EJ, Clasener HAL. 1994. Colonization resistance. Antimicrob Agents Chemother 38:409–414. doi: 10.1128/AAC.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan A, Edlund C, Nord CE. 2001. Effect of antimicrobial agents on the ecological balance of human microbiota. Lancet Infect Dis 1:101–114. doi: 10.1016/S1473-3099(01)00066-4. [DOI] [PubMed] [Google Scholar]
- 3.Donskey CJ, Chowdhry TK, Hecker MT, Hoyen CK, Hanrahan JA, Hujer AM, Hutton-Thomas RA, Whalen CC, Bonomo RA, Rice LB. 2000. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 343:1925–1932. doi: 10.1056/NEJM200012283432604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stiefel U, Pultz NJ, Harmoinen J, Koski P, Lindevall K, Helfand MS, Donskey CJ. 2003. Oral administration of β-lactamase preserves colonization resistance of piperacillin-treated mice. J Infect Dis 188:1605–1609. doi: 10.1086/379153. [DOI] [PubMed] [Google Scholar]
- 5.Stiefel U, Harmoinen J, Koski P, Kaariainen S, Wickstrand B, Lindevall K, Pultz NJ, Bonomo RA, Helfand MS, Donskey CJ. 2005. Orally administered recombinant metallo-β-lactamase preserves colonization resistance of piperacillin-tazobactam-treated mice. Antimicrob Agents Chemother 49:5190–5191. doi: 10.1128/AAC.49.12.5190-5191.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stiefel U, Nerandzic MM, Koski P, Donskey CJ. 2008. Orally administered β-lactamase enzymes represent a novel strategy to prevent colonization by Clostridium difficile. J Antimicrob Chemother 62:1105–1108. doi: 10.1093/jac/dkn298. [DOI] [PubMed] [Google Scholar]
- 7.Harmoinen J, Vaali K, Koski P, Syrjänen K, Laitinen O, Lindevall K, Westermarck E. 2003. Enzymic degradation of a beta-lactam antibiotic, ampicillin, in the gut: a novel treatment modality. J Antimicrob Chemother 51:361–365. doi: 10.1093/jac/dkg095. [DOI] [PubMed] [Google Scholar]
- 8.Stiefel U, Tima MA, Nerandzic MM. 2014. Gastrointestinal colonization with a cephalosporinase-producing Bacteroides species preserves colonization resistance against vancomycin-resistant Enterococcus and Clostridium difficile in cephalosporin-treated mice. Antimicrob Agents Chemother 58:4535–4542. doi: 10.1128/AAC.02782-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers MB, Parker AC, Smith CJ. 1993. Cloning and characterization of the endogenous cephalosporinase gene, cepA, from Bacteroides fragilis reveals a new subgroup of Ambler class A β-lactamases. Antimicrob Agents Chemother 37:2391–2400. doi: 10.1128/AAC.37.11.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith CJ, Bennett TK, Parker AC. 1994. Molecular and genetic analysis of the Bacteroides uniformis cephalosporinase gene, cblA, encoding the species-specific β-lactamase. Antimicrob Agents Chemother 38:1711–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giraud-Morin C, Madinier I, Fosse T. 2003. Sequence analysis of cfxA2-like β-lactamases in Prevotella species. J Antimicrob Chemother 51:1293–1296. doi: 10.1093/jac/dkg221. [DOI] [PubMed] [Google Scholar]
- 12.Thompson JS, Malamy MH. 1990. Sequencing the gene for an imipenem-cefoxitin-hydrolyzing enzyme (cfiA) from Bacteroides fragilis TAL2480 reveals strong similarity between cfiA and Bacillus cereus β-lactamase II. J Bacteriol 172:2584–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hujer KM, Hujer AM, Hulten E, Bajaksouzian S, Adams JM, Donskey CJ, Ecker DJ, Massire C, Eshoo MW, Sampath R, Thomson JM, Rather PN, Craft DW, Fishbain JT, Ewell AJ, Jacobs MR, Paterson DL, Bonomo RA. 2006. Analysis of antibiotic resistance genes in multidrug resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother 50:4114–4123. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Callaghan CH, Morris A, Kirby SM. 1972. Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother 1:283–288. doi: 10.1128/AAC.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. 2012. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 8th ed. NCCLS document M11-A8. National Committee for Clinical Laboratory Standards, Wayne, PA. [Google Scholar]
- 16.Summanen P, Sutter VL. 1993. Wadsworth anaerobic bacteriology manual, 5th ed. Star Publishing Co, Belmont, CA. [Google Scholar]
- 17.Sader HS, Fritsche TR, Jones RN. 2006. Daptomycin bactericidal activity and correlation between disk and broth microdilution method results in testing of Staphylococcus aureus strains with decreased susceptibility to vancomycin. Antimicrob Agents Chemother 50:2330–2336. doi: 10.1128/AAC.01491-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Rasmussen BA, Bush K. 1992. Biochemical characterization of the metallo-β-lactamase CcrA from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother 36:1155–1157. doi: 10.1128/AAC.36.5.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wexler H. 2007. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev 20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]