Abstract
wALADin1 benzimidazoles are specific inhibitors of δ-aminolevulinic acid dehydratase from Wolbachia endobacteria of filarial nematodes. We report that wALADin1 and two derivatives killed blood stage Plasmodium falciparum in vitro (50% inhibitory concentrations, 39, 7.7, and 12.8 μM, respectively). One of these derivatives inhibited gliding motility of Plasmodium berghei ANKA infectious sporozoites with nanomolar affinity and blocked invasion into hepatocytes but did not affect intrahepatocytic replication. Hence, wALADin1 benzimidazoles are tools to study gliding motility and potential antiplasmodial drug candidates.
TEXT
wALADin benzimidazoles are a recently described class of species-specific inhibitors of the heme biosynthesis enzyme δ-aminolevulinic acid dehydratase (ALAD; porphobilinogen synthase) of Wolbachia endobacteria of pathogenic filarial worms (1). The compounds killed filarial nematodes ex vivo in a Wolbachia-dependent manner with no cytotoxicity below 500 µM, thus representing drug lead candidates (1).
The malaria parasite Plasmodium falciparum is able to synthesize heme de novo, suggesting that it is a potential target pathway for new antimalarial drugs (2). P. falciparum uses two forms of ALAD for heme biosynthesis: one is host-derived ALAD (Homo sapiens ALAD [HsALAD]) imported from infected erythrocytes (3), and the second is an endogenous form (PfALAD) (4) targeted to the apicoplast (5). PfALAD accounts for ∼10% of total parasite-derived ALAD activity but may be indispensable during certain life cycle stages or for apicoplast homeostasis (5). In contrast to HsALAD, PfALAD possesses a binding site for allosteric Mg2+ (4) similar to the Wolbachia protein, for which a functional competition with the activation by Mg2+ is part of the inhibitory mechanism (1). We therefore reasoned that wALADins might be effective inhibitors of PfALAD and hence possess antimalarial activity.
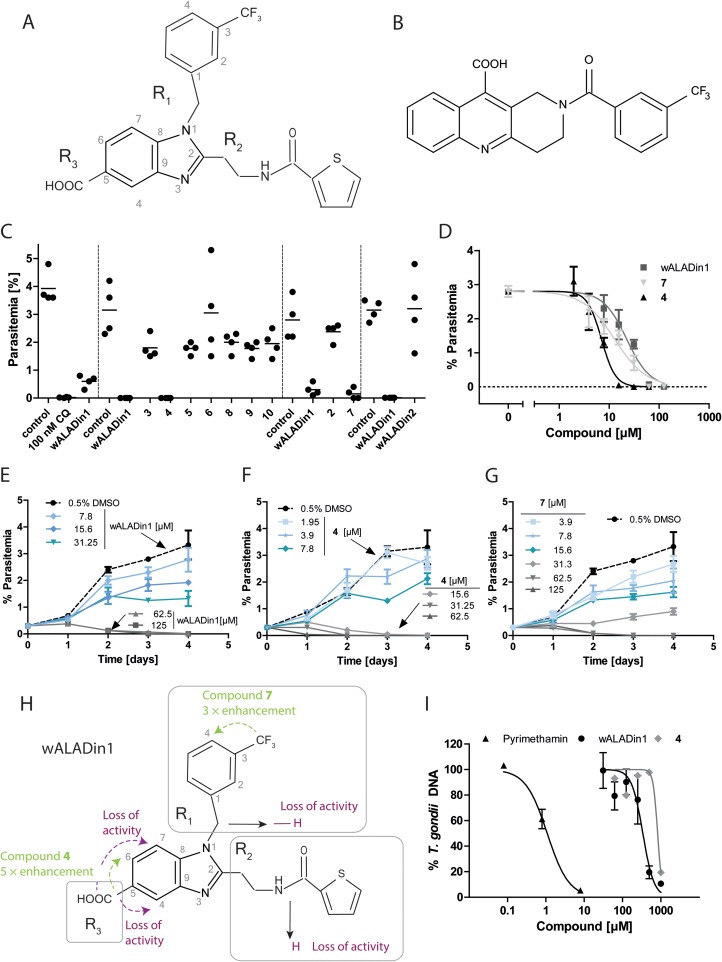
To determine the antiplasmodial activity of wALADins, P. falciparum strain 3D7 parasites were cultivated in human blood type A+ erythrocytes under a defined gaseous atmosphere (92% CO2, 3% O2, 5% CO2) at 37°C as published previously (6). Parasitemia was adjusted to 0.2 to 0.5% before the parasites were exposed to 62.5 μM wALADin1 (Fig. 1A, Table 1), the derivatives, or 0.625% dimethyl sulfoxide (DMSO) for 72 h. Compound 1 (wALADin1) and derivatives 4 and 7 reduced parasitemia to nearly 0%, while parasitemia of the DMSO control parasites increased to ∼3% (Fig. 1C). None of the other derivatives had antiplasmodial activity (Fig. 1C). wALADin2, a nonbenzimidazole compound (Fig. 1B) with similar inhibitory activity and mechanism against Wolbachia ALAD (7), was inactive against blood stage P. falciparum (Fig. 1C). Dose-response curves for effective derivatives were very steep (Fig. 1D), and time curve experiments revealed similar progressions of the antiplasmodial effect for all active compounds (Fig. 1E to G). Parasites were killed within 24 to 72 h of exposure to >10 μM compound 4. At lower concentrations, parasite growth was retarded. The corresponding activity profile of wALADin benzimidazoles is shown in Fig. 1H and Table 1. wALADin1 had the weakest activity (50% inhibitory concentration [IC50], 39.3 ± 11.7 μM), while replacement of the R1-3-CF3-benzyl with 4-CF3-benzyl in compound 7 led to an ∼3-fold improvement (IC50, 12.8 ± 0.02 μM). The most potent antiplasmodial compound was compound 4 (IC50, 7.7 ± 1.7 μM), which differs from wALADin1 by repositioning of the R3-COOH from position 5 to 6 of the benzimidazole core. In contrast, derivatives with R3-COOH at position 4 or 7 were inactive. Replacement of either the R1 or R2 moiety with a hydrogen atom abrogated the antiplasmodial activity.
FIG 1.
(A and B) Chemical structures of wALADin1 (A) and wALADin2 (B). (C) wALADin1 and derivatives were tested at 62.5 μM for 72 h in a P. falciparum erythrocyte culture. Parasitemia was determined by counting erythrocytes in blood smears. Chloroquine at 100 nM (CQ) was used as a positive control. Dashed vertical lines separate different experiments. (D) Dose-response curves for wALADin compounds 1, 4, and 7. Curves were fit to a sigmoidal (four-parameter) equation with GraphPad Prism 5.0 [log(inhibitor versus normalized response – variable slope); bottom = 0; top = shared by all data sets]. (E to G) Time course of parasitemia in P. falciparum culture in the presence of wALADin1 (E), compound 4 (F), and compound 7 (G) for 4 days. (H) Structure-activity profile of wALADin1 benzimidazoles in P. falciparum culture. The substituents R1, R2, and R3 are highlighted in gray boxes. Dashed arrows indicate positional changes of substituent groups; continuous arrows indicate replacement with a different chemical group. Arrow or font color indicates an enhancement in antiplasmodial activity (green) or a loss of activity (purple). (I) wALADin1 and compound 4 inhibited replication of T. gondii in LLC-MK2 cells, as determined by real-time PCR, with IC50s 1 order of magnitude (wALADin1) or several orders of magnitude (compound 4) higher than for P. falciparum, indicating specificity of the antiplasmodial effect (D). The graph shows means ± standard errors of the means of results for ≥2 experiments for pyrimethamine and wALADin1 and as means of results for a single experiment for compound 4.
TABLE 1.
Antiplasmodial activity of wALADin1 benzimidazoles is not correlated with inhibitory activity against PfALAD
| Compound | R1 residue | R2 residue | Position of R3 residue | P. falciparum erythrocyte culture LD50 (μM)a | PfALAD IC50 (μM)b |
|---|---|---|---|---|---|
| 1 (wALADin1) | 3-CF3-benzyl | 2-[(2-thienylcarbonyl)amino]ethyl | C-5 | 39.3 ± 11.7 (n = 4) | ∼568 |
| 2 | H | 2-[(2-thienylcarbonyl)amino]ethyl | C-5 | NA | ND |
| 3 | 3-CF3-benzyl | H | C-5 | NA | NI |
| 4 | 3-CF3-benzyl | 2-[(2-thienylcarbonyl)amino]ethyl | C-6 | 7.7 ± 1.7 (n = 3) | NI |
| 5 | 3-CF3-benzyl | 2-[(2-thienylcarbonyl)amino]ethyl | C-4 | NA | ND |
| 6 | 3-CF3-benzyl | 2-[(2-thienylcarbonyl)amino]ethyl | C-7 | NA | ∼625 |
| 7 (ALPin1) | 4-CF3-benzyl | 2-[(2-thienylcarbonyl)amino]ethyl | C-5 | 12.8 ± 0.02 (n = 2) | NI |
| 8 | 4-CF3-benzyl | H | C-5 | NA | ND |
| 9 | CH3 | H | C-5 | NA | ND |
| 10 | H | H | C-5 | NA | ND |
| 11 (wALADin2) | Tricyclic quinoline derivative | NA | NI |
NA, no antiplasmodial activity at 62.5 μM.
IC50 concentrations were determined by nonlinear regression analysis of data derived from several independent experiments. NI, no in vitro activity against PfALAD enzymatic activity (highest concentration tested, 533 μM). ND, not determined.
To elucidate whether the antiplasmodial effect was due to inhibition of PfALAD, we tested selected derivatives against recombinant PfALAD protein (5) (expression plasmid kindly provided by V. Arun Nagaraj). Enzymatic assays were performed as described previously for orthologs using 250 nM PfALAD in 100 mM Tris-HCl (pH 8.0), 1 mM MgCl2, 200 μM 5-ALA for 60 min (1). wALADin1 and compound 9 showed marginal inhibitory activity at the highest concentration tested (533 μM), whereas neither compound 4 nor 7 was inhibitory (Table 1). Although it may not be ruled out that under physiological conditions the PfALAD may be more susceptible to inhibition, it is likely that the antiplasmodial activity elicited in the one-digit (compound 4) or low two-digit (wALADin1, compound 7) micromolar range is mediated via an alternative target than ALAD.
The specificity of the antiplasmodial effect was tested by determining the activity of wALADins against the related apicomplexan parasite Toxoplasma gondii for which neither wALADin1 nor compound 4 has inhibitory activity against its ALAD ortholog (8). Briefly, LLC-MK2 cells were infected with T. gondii RH strain tachyzoites (multiplicity of infection [MOI], 5). After 2 h, nonadherent parasites were removed by washing and cells were exposed to wALADin1 or compound 4 for 48 h. After DNA extraction from infected cells, tachyzoite numbers were determined by real-time PCR of the T. gondii repeat DNA sequence (GenBank accession no. AF146527.1) normalized to the number of host cells determined by Macaca mulatta β-actin PCR (GenBank Gene ID 574285) (Fig. 1I; Table 2). wALADin1 inhibited T. gondii replication with an IC50 of ∼340 μM; compound 4 had an IC50 between 500 and 1,000 μM. This minor anti-Toxoplasma activity is likely identical to the weak antiproliferative effect on eukaryotic cells previously reported for LLC-MK2 and HEK cells at concentrations of ≥500 μM (1). Thus, the selectivity window of the antiplasmodial effect of compound 4 (IC50, 7.7 μM) over T. gondii and other mammalian cells is ∼100-fold, and the antiplasmodial potency of this compound even exceeds the antifilarial potency of wALADin1 by >10-fold (7).
TABLE 2.
PCR conditions for T. gondii repeat DNA and M. mulatta β-actin PCRa
| Parameter | T. gondii repeat DNA PCR | M. mulatta β-actin PCR |
|---|---|---|
| Forward primer | 5′-GATATCAGGACTGTAGATGAAGG-3′ (300 nM) | 5′-GATGAGATTGGCTTTA-3′ (300 nM) |
| Reverse primer | 5′-GCGTCGTCTCGTCTAGATC-3′ (300 nM) | 5′-AACCGACTGCTGTCACCTTC-3′ (300 nM) |
| Hybridization probe | 5′-6-FAM-AAGCGACGAGAGTCGGAGAGGGAG-3′-BHQ-1 (50 nM, TaqMan) | 1× SYBR green dye |
| Conditions | 0.5 U HotStar Taq; 50 μM dNTPs; 4 mM MgCl2 | 0.5 U HotStar Taq; 50 μM dNTPs; 3 mM MgCl2 |
| Cycling protocol | 15 min at 95°C followed by 45 cycles of 10 s at 95°C and 30 s at 60°C (fluorescence acquisition) | 15 min at 95°C followed by 45 cycles of 15 s at 94°C, 20 s at 58°C, and 20 s at 72°C (fluorescence acquisition) |
6-FAM, 6-carboxyfluorescein; BHQ-1, black hole quencher 1; dNTPs, deoxynucleoside triphosphates.
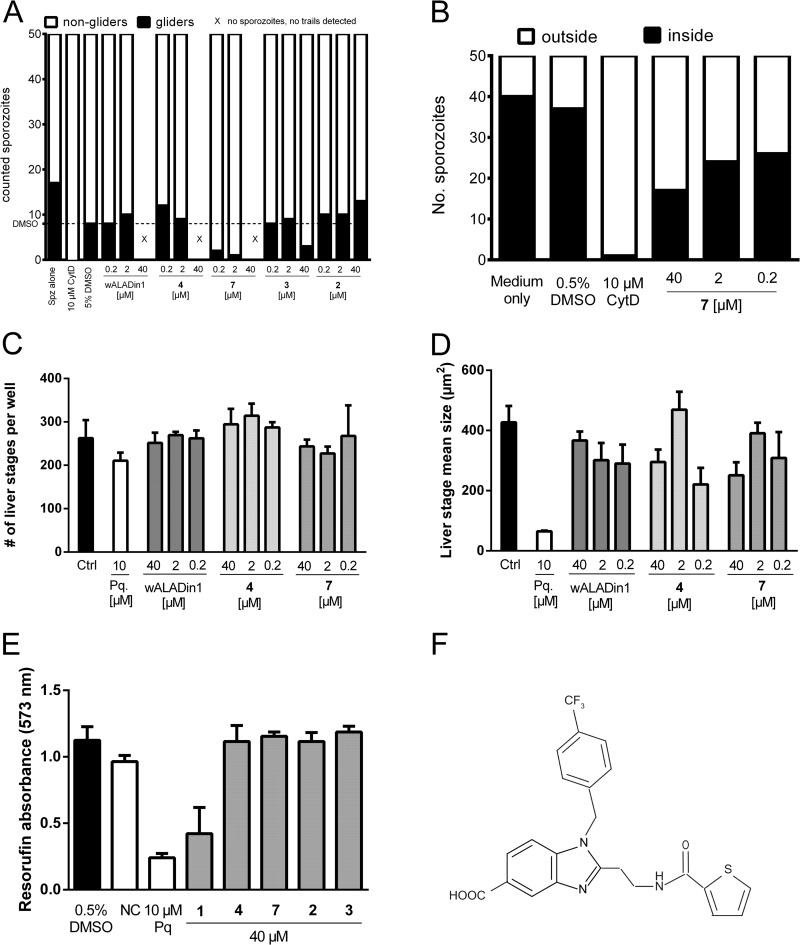
To delineate the activity of wALADins against other malarial parasite life cycle stages (sporozoites and liver stages), we used the rodent parasite Plasmodium berghei ANKA. To assess gliding motility, 10,000 infective sporozoites were preincubated with compounds for 15 min on ice. After 30 min of gliding on bovine serum albumin (BSA)-coated glass slides, samples were fixed and gliders and nongliders were differentiated according to the presence or absence of a trail of the major sporozoite surface protein, circumsporozoite protein (CSP), released from the apical end of gliding sporozoites (9). CSP was detected by immunofluorescence microscopy using a Zeiss Axiovert 100M microscope and Zeiss Image Examiner software. Compound 7 dramatically reduced the gliding motility of P. berghei ANKA sporozoites (75% reduction at 200 nM) and completely prevented sporozoites from adhering to glass slides at 40 μM. wALADin1 and compound 4 also prevented sporozoite adhesion at 40 μM but showed no effect on gliding motility at lower concentrations (Fig. 2A). Control compounds 2 and 3 had no effect on sporozoite motility. As gliding motility is required for successful invasion of hepatocytes, we tested whether compound 7 was able to inhibit this important infection process. Sporozoites were preincubated with compound 7 as described above and allowed to invade immortalized human hepatoma cells (Huh7) for 90 min before samples were fixed and stained according to the two-color host cell invasion method (10). Compound 7 blocked sporozoite invasion into hepatoma cells (Fig. 2 B), presumably as a result of suppressed gliding motility. In contrast to invasion into hepatocytes, intrahepatocytic replication was not affected by wALADins at concentrations up to 40 μM (Fig. 2 C and D). alamarBlue cytotoxicity assays with a concentration of 40 μM compound revealed a moderate antiproliferative effect of wALADin1 against Huh7 cells but not of compound 4 or 7, confirming the specificity of the latter compounds. Due to its potent effect on sporozoite gliding motility, we named compound 7 “wALADin-derived inhibitor of Plasmodium motility and invasion1 (ALPin1)” (the chemical structure is shown in Fig. 2E). As a small-molecule tool, ALPin1 may help identify unknown components of the actin-myosin motor-dependent gliding machinery (11, 12).
FIG 2.
P. berghei ANKA sporozoites were extracted from the salivary glands of infected Anopheles stephensi mosquitoes on days 19 to 23 postinfection, taken up in RPMI medium supplemented with 3% BSA, and used on the same day. (A) Compound 7 potently inhibited the gliding motility of P. berghei ANKA sporozoites. A total of 10,000 sporozoites were added to glass slides precoated with 3% BSA–RPMI medium and allowed to glide for 30 min before fixation with 4% paraformaldehyde–phosphate-buffered saline. Gliding sporozoites were differentiated from adhesive but nongliding sporozoites by a trail of circumsporozoite protein (CSP) detected on the slide by immunofluorescence microsocopy after staining with monoclonal anti-CSP antibody and anti-mouse Alexa Fluor 488 (both at 1:300 in 10% fetal calf serum-PBS) (6). “X” indicates the absence of both sporozoites and CSP trails on the slide. (B) Compound 7 (ALPin1) inhibited the invasion of sporozoites into Huh7 cells. A total of 25,000 cells were seeded onto Lab-Tek 8-well chamber slides 1 day before infection with sporozoites for 90 min (MOI, 0.4). Parasite numbers were determined by fixation and staining using the two-color host cell invasion method. (C and D) For extraerythocytic stage development assays, Huh7 cells were infected with sporozoites for 90 min before free parasites were washed off and compounds were added. Cells were fixed after 24 h, 40 h, or 60 h with ice-cold methanol, followed by staining of liver stage parasites with monoclonal anti-P. berghei HSP70 antibody and goat anti-mouse Alexa Fluor 488 secondary antibody (13). None of the tested wALADins had any effect (up to 40 μM) on the number (C) or the size (D) of liver stage parasites. (E) A total of 6,000 Huh7 cells were seeded onto a 96-well plate before incubation with a 40 μM concentration of the indicated compound or with 0.5% DMSO for 72 h at 37°C and 5% CO2 in triplicate experiments. Neither compound 4 nor compound 7 affected Huh7 cell viability or replication as assessed with alamarBlue reagent. The inhibitor of actin polymerization cytochalasin D (CytD) served as a positive-control inhibitor for motility and invasion studies; primaquine (Pq) was used for intrahepatic stages. NC, negative control. (F) Chemical structure of compound 7 (ALPin1).
In addition to the specificity of the biological effect, the druglike chemical properties of wALADin benzimidazoles, their easy synthetic accessibility, and their steep antiplasmodial activity profile (Fig. 1H) render a future detailed assessment of this compound class as antiplasmodial agents attractive. Validation of these compounds as new antimalarial agents will be completed by target identification using cross-linkable chemical probes based on the chemical scaffold of ALPin1 (compound 7) to identify the molecular target.
ACKNOWLEDGMENTS
We thank V. Arun Nagaran (Department of Biochemistry, Indian Institute of Science, Bangalore, India) for providing the pRSETa-PfALAD expression plasmid and protocol. We thank I. Reiter-Owona and H. Neufeld (Institute for Medical Microbiology, Immunology and Parasitology, University Hospital of Bonn) for supplying T. gondii RH strain tachyzoites.
This work was supported through a grant from the Liverpool School of Tropical Medicine to A.H. and K.M.P as part of the A-WOL Consortium funded by the Bill and Melinda Gates Foundation and through funding from the Deutsche Forschungsgemeinschaft to A.H. and M.F. (SFB 704) and to K.M.P. and A.H. (PF 673/3-2; FOR 854). A.H. is a member of the Cluster of Excellence ImmunoSensation (EXC 1023). J.M.S. is a recipient of the Rahel-Goitein-Strauss-Program of the University Hospital Heidelberg.
REFERENCES
- 1.Lentz CS, Halls V, Hannam JS, Niebel B, Strubing U, Mayer G, Hoerauf A, Famulok M, Pfarr KM. 2013. A selective inhibitor of heme biosynthesis in endosymbiotic bacteria elicits antifilarial activity in vitro. Chem Biol 20:177–187. doi: 10.1016/j.chembiol.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Surolia N, Padmanaban G. 1992. De novo biosynthesis of heme offers a new chemotherapeutic target in the human malarial parasite. Biochem Biophys Res Commun 187:744–750. doi: 10.1016/0006-291X(92)91258-R. [DOI] [PubMed] [Google Scholar]
- 3.Bonday ZQ, Taketani S, Gupta PD, Padmanaban G. 1997. Heme biosynthesis by the malarial parasite. Import of delta-aminolevulinate dehydrase from the host red cell. J Biol Chem 272:21839–21846. [DOI] [PubMed] [Google Scholar]
- 4.Sato S, Wilson RJ. 2002. The genome of Plasmodium falciparum encodes an active delta-aminolevulinic acid dehydratase. Curr Genet 40:391–398. doi: 10.1007/s00294-002-0273-3. [DOI] [PubMed] [Google Scholar]
- 5.Dhanasekaran S, Chandra NR, Chandrasekhar Sagar BK, Rangarajan PN, Padmanaban G. 2004. Delta-aminolevulinic acid dehydratase from Plasmodium falciparum: indigenous versus imported. J Biol Chem 279:6934–6942. doi: 10.1074/jbc.M311409200. [DOI] [PubMed] [Google Scholar]
- 6.Potocnjak P, Yoshida N, Nussenzweig RS, Nussenzweig V. 1980. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med 151:1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lentz CS, Stumpfe D, Bajorath J, Famulok M, Hoerauf A, Pfarr KM. 2013. New chemotypes for wALADin1-like inhibitors of delta-aminolevulinic acid dehydratase from Wolbachia endobacteria. Bioorg Med Chem Lett 23:5558–5562. doi: 10.1016/j.bmcl.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 8.Lentz CS, Halls VS, Hannam JS, Strassel S, Lawrence SH, Jaffe EK, Famulok M, Hoerauf A, Pfarr KM. 2014. wALADin benzimidazoles differentially modulate the function of porphobilinogen synthase orthologs. J Med Chem 57:2498–2510. doi: 10.1021/jm401785n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart MJ, Vanderberg JP. 1991. Malaria sporozoites release circumsporozoite protein from their apical end and translocate it along their surface. J Protozool 38:411–421. doi: 10.1111/j.1550-7408.1991.tb01379.x. [DOI] [PubMed] [Google Scholar]
- 10.Renia L, Miltgen F, Charoenvit Y, Ponnudurai T, Verhave JP, Collins WE, Mazier D. 1988. Malaria sporozoite penetration. A new approach by double staining. J Immunol Methods 112:201–205. [DOI] [PubMed] [Google Scholar]
- 11.Kappe SH, Buscaglia CA, Bergman LW, Coppens I, Nussenzweig V. 2004. Apicomplexan gliding motility and host cell invasion: overhauling the motor model. Trends Parasitol 20:13–16. doi: 10.1016/j.pt.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Munter S, Sabass B, Selhuber-Unkel C, Kudryashev M, Hegge S, Engel U, Spatz JP, Matuschewski K, Schwarz US, Frischknecht F. 2009. Plasmodium sporozoite motility is modulated by the turnover of discrete adhesion sites. Cell Host Microbe 6:551–562. doi: 10.1016/j.chom.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji M, Mattei D, Nussenzweig RS, Eichinger D, Zavala F. 1994. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol Res 80:16–21. doi: 10.1007/BF00932618. [DOI] [PubMed] [Google Scholar]