Abstract
The global consequence of drug efflux gene overexpression in bacteria has not been specifically analyzed because strains showing high-level expression typically have mutations in genes encoding regulatory proteins that control other genes. Results from a transcriptional profiling study performed with a strain of Neisseria gonorrhoeae that is capable of high-level transcription of the mtrCDE efflux pump operon independently of control by cognate regulatory proteins revealed that its overexpression has ramifications for systems other than drug efflux.
TEXT
Bacteria use efflux pumps to resist the action of antibiotics during treatment of infection or to survive in the presence of antimicrobials in their environment (1). The level of expression of efflux pump-encoding genes is modulated by a complex regulatory system of repressors and activators, but derepression due to mutation in repressor-encoding genes or their promoters can significantly decrease bacterial susceptibility to antimicrobials (2). As an example, mutation in the mtrR repressor gene of Neisseria gonorrhoeae enhanced transcription of the mtrCDE-encoded efflux pump and resulted in both increased resistance of gonococci to structurally diverse antimicrobials (3–5) and greater in vivo fitness in an experimental mouse model of lower genital tract infection (6, 7). MtrR also has more global action, however, as it directly or indirectly impacts expression of >65 genes involved in diverse systems such as peptidoglycan synthesis, pilin secretion, the general stress response, and glutamine metabolism (8–10).
The question asked in this study was this: does overexpression of a drug efflux pump per se have a potential impact on bacteria? Heretofore, this question could not be addressed because bacteria that overexpress efflux pump genes typically contain mutations that impact cognate gene regulators (e.g., MtrR), which would make interpretation of results difficult. Taking advantage of a unique promoter that drives high-level expression of mtrCDE independently of MtrR control (11) (as well as activation by MtrA [12]), we performed a transcriptional profiling study. Here, we provide data that support the hypothesis that overexpression of a bacterial efflux system may have unexpected global ramifications with respect to microbial physiology.
Bacterial strains, culture conditions, and antimicrobial testing.
N. gonorrhoeae strains FA19 and FA19mtr120 were the main strains used (Table 1). “mtr120” signifies a single nucleotide change (C to T) 120 nucleotides upstream of mtrCDE (7) that generates a new promoter for mtrCDE transcription (11) and increases gonococcal resistance to diverse antimicrobials (Table 1), including host defense antimicrobials (7, 13). Transformants of these strains bearing an insertionally inactivated ccpR gene due to the presence of the nonpolar aphA-3 cassette were constructed by previously described methods (12). Gonococci were routinely grown as nonpiliated, opacity-negative variants on gonococcal medium base (GCB) agar or in broth, each with defined supplements I and II, as previously described (4). The MIC of antimicrobials recognized by the MtrCDE efflux pump was determined by agar dilution (4). To test gonococcal susceptibility to peroxides, a microtiter plate assay was used. Briefly, this involved exposing 105 gonococci in GCB broth to various concentrations of hydrogen peroxide (H2O2) or tert-butylhydroperoxide (tBuOOH) at 37°C for 45 min before spotting 5 μl onto GCB agar plates for assessment of viability; H2O2 and tBuOOH are not substrates of the MtrCDE pump (unpublished observations).
TABLE 1.
Transcriptional response of gonococci to overexpression of mtrCDE efflux pump operon
| Gene and category | Common name | Fold change | Functional classificationa |
|---|---|---|---|
| Upregulated in the presence of the mtr120 mutation | |||
| NGO1363 | mtrE | 4.44 | Mtr efflux pump protein component: outer membrane channel protein (MtrE) |
| NGO1364 | mtrD | 5.63 | Mtr efflux pump protein component: RND family transporter (MtrD) |
| NGO1365 | mtrC | 5.42 | Mtr efflux pump protein component: periplasmic fusion protein (MtrC) |
| NGO1769 | ccpR | 2.52 | Cytochrome c peroxidase |
| Downregulated in the presence of the mtr120 mutation | |||
| NGO0218 | NGO0218 | −15.02 | Hypothetical |
| NGO0585 | NGO0585 | −2.02 | Hypothetical integral membrane protein |
| NGO0593 | clpP | −2.03 | ATP-dependent Clp protease subunit |
| NGO0618 | NGO0618 | −2.14 | Hypothetical |
| NGO0650 | NGO0650 | −2.05 | ATP-dependent RNA helicase |
| NGO1058 | surE | −2.14 | Stationary-phase-survival protein |
| NGO1246 | sohB | −2.72 | Periplasmic peptidase, S49 family |
| NGO1248 | NGO1248 | −5.23 | Hypothetical |
| NGO1360 | NGO1360 | −3.56 | FadR/GntR family transcriptional regulator |
| NGO1368 | mtrF | −2.67 | Mtr efflux pump accessory protein |
| NGO1481 | NGO1481 | −4.59 | Putative SAM-dependent methyltransferase |
| NGO1857 | secE | −4.83 | Protein translocase channel subunit |
| NGO1917 | terC | −3.06 | Transmembrane transporter, tellurium resistance |
RND, resistance-nodulation-division; SAM, S-adenosylmethionine.
RNA-seq and qRT-PCR studies.
A transcriptional-profiling comparison study using transcriptome sequencing (RNA-seq) and three independent RNA samples prepared from broth-grown, late-logarithmic cultures of isogenic strains FA19 and FA19mtr120 was performed using previously described methods (14). We defined differentially expressed genes as having (i) a fold change value of ≥2, (ii) a total read number larger than 5, and (iii) a Bonferroni-corrected P value of ≤0.05. For quantitative reverse transcriptase PCR (qRT-PCR) analysis of gene expression, RNA samples from RNA-seq experiments were used to synthesize cDNA using random hexamers. Transcripts of the genes of interest (ccpR and rpS15) were quantified using qRT-PCR and oligonucleotide primers (see Table S1 in the supplemental material) essentially as described previously (14); rpS15 was used as a reference for normalization of the results.
Global gene expression consequences of overexpression of mtrCDE.
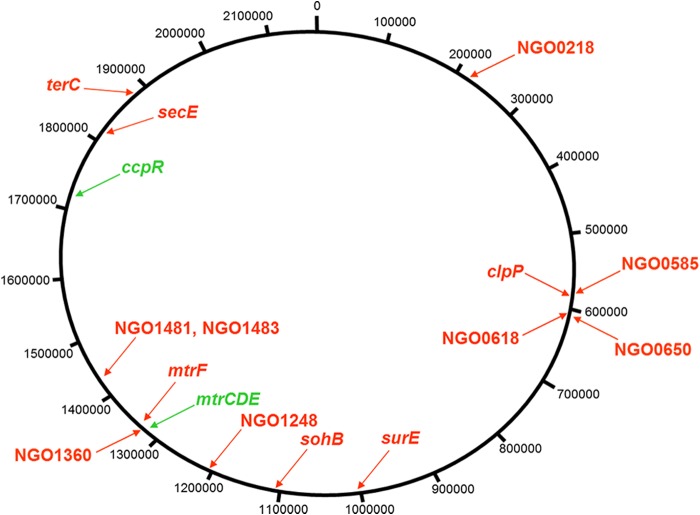
As expected from previous studies (7, 11), RNA-seq analysis revealed that expression of mtrC, mtrD, and mtrE was highly upregulated in strain FA19mtr120 compared to wild-type strain FA19, which, based on earlier reports (4, 5, 9), served as an internal control for the results of the transcriptome comparison (Table 1); this upregulation was confirmed by qRT-PCR (see Table S1 in the supplemental material). Importantly, analysis of the RNA-seq results revealed changes in the expression levels of 13 non-mtr genes which were widely distributed across the genome (Fig. 1) and represented a variety of functional classes (Table 1) based on annotation of the FA1090 genome (www.genome.ou.edu). Only one of these non-mtr genes (ccpR) was overexpressed in strain FA19mtr120. Proteins encoded by the 13 underexpressed genes included four hypothetical proteins; a protease (encoded by clpP); a periplasmic peptidase (sohB); a stationary-phase-associated survival protein (surE); a transcriptional regulator (NGO1360) involved in modulating expression of glutamate metabolism genes in meningococci (15) and located just downstream of the upregulated mtrCDE operon; an ATP-RNA helicase (NGO0650); a component of the protein translocating system (secE); a tellurium resistance-associated protein (terC); and a putative methyltransferase (NGO1481).
FIG 1.
Shown are chromosomal-map positions of genes differentially expressed in strain FA19mtr120 compared to wild-type strain FA19 as identified by RNA-seq analysis (Table 1). The circular map is from the annotated FA1090 genome (www.genome.ou.edu). Upregulated genes are shown in green, while downregulated genes are shown in red.
We selected ccpR, which encodes cytochrome C peroxidase (16), as a model gene to further test if overexpression of mtrCDE could have distal effects on gonococcal gene expression; ccpR was also chosen as it may contribute to gonococcal resistance to oxidative stresses (16, 17). By qRT-PCR analysis, we confirmed enhanced expression of ccpR in strain FA19mtr120 compared to wild-type strain FA19 (see Table S1 in the supplemental material). We next examined our test strains with or without a ccpR::kan mutation for differences in susceptibility to MtrCDE-substrate antimicrobials and two peroxides (H2O2 and tBuOOH), since the peroxidase activity of CcpR would likely influence levels of gonococcal susceptibility to peroxides but not MtrR substrates. In three independent experiments, strain FA19mtr120 showed an increase in resistance to efflux pump substrates (Table 2) and resistance to H2O2 and tBuOOH at levels 2-fold- and 4-fold greater, respectively, than those seen with strain FA19; peroxide resistance levels were independent of the presence of a functional MtrCDE efflux pump (data not presented). While the susceptibility of strain FA19mtr120 ccpR::kan to pump substrates was unchanged from that seen with FA19mtr120, strain FA19mtr120 ccpR::kan was reproducibly 2-fold more sensitive to tBuOOH, which was not unexpected given the multiple ways gonococci resist peroxides (16, 17).
TABLE 2.
Sensitivity of isogenic gonococci to antimicrobials
| Strain | Level of susceptibility to antimicrobialsa |
|||||
|---|---|---|---|---|---|---|
| Em | CV | TX-100 | PxB | H2O2 | tBuOOH | |
| FA19 | 0.25 | 0.31 | 125 | 100 | 0.002 | 0.004 |
| FA19ccpR::kan | 0.25 | 0.31 | 125 | 100 | 0.002 | 0.004 |
| FA19mtr120 | 2 | 1.25 | >16,000 | 400 | 0.004 | 0.016 |
| FA19mtr120 ccpR::kan | 2 | 1.25 | >16,000 | 400 | 0.002 | 0.008 |
Em, erythromycin; CV, crystal violet; TX-100, Triton X-100; PxB, polymyxin B; H2O2, hydrogen peroxide; tBuOOH, tert-butylhydroperoxide. For efflux pump substrates Erm, CV, TX-100, and PxB, the numbers refer to MICs (μg/ml), while the numbers for H2O2 and tBuOOH are minimal bactericidal concentrations (MBCs) in percent (vol/vol). All determinations were performed in triplicate.
We propose that overexpression of a drug efflux pump has unexpected secondary effects on bacterial gene expression and associated metabolic processes. The mechanism(s) by which these expression changes occur is unclear but should be considered in drug efflux studies.
Nucleotide sequence accession numbers.
The complete data set can be accessed through GEO accession number GSE47048 and SRA accession number SRA079863.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jacqueline Balthazar for excellent technical assistance and Yaramah Zalucki for critical reading of early versions of the paper.
This work was supported by NIH grants AI021150-29 (W.M.S.), AI42053 (A.E.J.), AI096788 (T.D.R., D. Dean, and R. Selden), and AI031496-22 (to P. F. Sparling, University of North Carolina) and a VA Merit Review Grant to W.M.S. W.M.S. is the recipient of a Senior Research Career Scientist Award from the VA Medical Research Service.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04148-14.
REFERENCES
- 1.Nikaido H. 1996. Multidrug efflux pumps of Gram-negative bacteria. J Bacteriol 178:5853–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zalucki YM, Mercante AD, Cloward JM, Ohneck EA, Kandler JL, Goytia M, Johnson PJT, Shafer WM. 2013. Function and regulation of Neisseria gonorrhoeae efflux pumps, p 207–221. In Yu EW. (ed), Microbial efflux pumps: current research. Horizon Press, Inc., Pittsburgh, PA. [Google Scholar]
- 3.Pan W, Spratt BG. 1994. Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol Microbiol 11:769–775. doi: 10.1111/j.1365-2958.1994.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 4.Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM. 1995. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141:611–622. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- 5.Hagman KE, Shafer WM. 1995. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J Bacteriol 177:4162–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warner DM, Folster JP, Shafer WM, Jerse AE. 2007. Regulation of the MtrC-MtrD-MtrE efflux-pump system modulates the in vivo fitness of Neisseria gonorrhoeae. J Infect Dis 196:1804–1812. doi: 10.1086/522964. [DOI] [PubMed] [Google Scholar]
- 7.Warner DM, Shafer WM, Jerse AE. 2008. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol Microbiol 70:462–478. doi: 10.1111/j.1365-2958.2008.06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folster JP, Dhulipali V, Nicholas RA, Shafer WM. 2007. Differential regulation of ponA and pilMNOPQ expression by the Neisseria gonorrhoeae MtrR transcriptional regulatory protein. J Bacteriol 189:4569–4577. doi: 10.1128/JB.00286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folster JP, Johnson PJT, Jackson L, Dhulipali V, Dyer DW, Shafer WM. 2009. MtrR modulates rpoH expression and levels of antimicrobial resistance in Neisseria gonorrhoeae. J Bacteriol 191:287–297. doi: 10.1128/JB.01165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson PJT, Stringer VA, Shafer WM. 2011. Off-target gene regulation mediated by transcriptional repressors of antimicrobial efflux pump genes in Neisseria gonorrhoeae. Antimicrob Agents Chemother 55:2559–2565. doi: 10.1128/AAC.00010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohneck EA, Zalucki YM, Johnson PJT, Dhulipala V, Golparian D, Unemo M, Jerse AE, Shafer WM. 2011. A novel mechanism of high-level, broad-spectrum antibiotic resistance caused by a single base pair change in Neisseria gonorrhoeae. mBio 2:e00187–11. doi: 10.1128/mBio.00187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouquette C, Harmon JB, Shafer WM. 1999. Induction of the mtrCDE-encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC-like protein. Mol Microbiol 33:651–658. doi: 10.1046/j.1365-2958.1999.01517.x. [DOI] [PubMed] [Google Scholar]
- 13.Shafer WM, Qu X-D, Waring AJ, Lehrer RI. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci U S A 95:1829–1833. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vélez Acevedo RN, Ronpirin C, Kandler JL, Shafer WM, Cornelissen CN. 2014. Identification of regulatory elements that control expression of the tbpBA operon in Neisseria gonorrhoeae. J Bacteriol 196:2762–2774. doi: 10.1128/JB.01693-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagliarulo C, Salvatore P, De Vitis LR, Colicchio R, Monaco C, Tredici M, Tala A, Bardaro M, Lavitola A, Bruni CB, Alifano P. 2004. Regulation and differential expression of gdhA encoding NADP-specific glutamate dehydrogenase in Neisseria meningitidis clinical isolates. Mol Microbiol 51:1757–1772. doi: 10.1111/j.1365-2958.2003.03947.x. [DOI] [PubMed] [Google Scholar]
- 16.Turner S, Reid E, Smith H, Cole J. 2003. A novel cytochrome c peroxidase from Neisseria gonorrhoeae: a lipoprotein from a Gram-negative bacterium. Biochem J 373:865–873. doi: 10.1042/BJ20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falsetta ML, Steichen CT, McEwan AG, Cho C, Ketterer M, Shao J, Hunt J, Jennings MP, Apicella MA. 2011. The composition and metabolic phenotype of Neisseria gonorrhoeae biofilms. Front Microbiol 2:75. doi: 10.3389/fmicb.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.