Abstract
Burkholderia cepacia complex and Stenotrophomonas maltophilia infections are associated with poor clinical outcomes in persons with cystic fibrosis (CF). The MIC50 based on planktonic growth and the biofilm concentration at which 50% of the isolates tested are inhibited (BIC50) of tobramycin were measured for 180 B. cepacia complex and 101 S. maltophilia CF isolates and were 100 μg/ml for both species. New inhalation devices that deliver high tobramycin levels to the lung may be able to exceed these MICs.
TEXT
As individuals with cystic fibrosis (CF) age, they are increasingly infected in their lungs with multidrug-resistant Gram-negative organisms such as Burkholderia cepacia complex and Stenotrophomonas maltophilia, which are associated with poor clinical outcomes (1–5). Treatment of these infections is difficult (6–9) due to their numerous mechanisms of antimicrobial resistance, including efflux pumps, chromosomally encoded β-lactamases, decreased outer membrane permeability, intracellular survival, and biofilm formation (10–12). However, newer inhalational antibiotic therapies have the ability to deliver very high concentrations of drug to the lung, which may be able to overcome some of these mechanisms. One of the new inhalational antibiotics available is tobramycin inhalation powder (TIP), delivered by the Podhaler device, which can achieve up to 1.5- to 2-fold higher sputum tobramycin concentrations (up to 2,000 μg/g) than tobramycin inhalation solution (TIS) (13). It is not known whether these higher tobramycin concentrations can overwhelm the efficient efflux pumps known to be present in B. cepacia complex and S. maltophilia (14–16).
In order to determine whether the known pulmonary concentrations of inhaled high-dose tobramycin powder can overcome these inhibitory concentrations, the aim of this study was to measure the inhibitory concentrations of tobramycin for a large collection of B. cepacia complex and S. maltophilia isolates, grown planktonically and in a biofilm, from pediatric and adult CF patients.
B. cepacia complex isolates (n = 180) were prospectively collected from sputum samples from CF patients from four study sites, The Hospital for Sick Children (n = 10), St. Michael's Hospital (n = 36), the Cystic Fibrosis Foundation Burkholderia cepacia Research Repository at the University of Michigan (n = 16), and the Canadian Burkholderia cepacia Complex Research and Referral Repository at the University of British Columbia, Vancouver (n = 118). S. maltophilia isolates (n = 101) were obtained from pediatric CF patients at The Hospital for Sick Children in Toronto (n = 67) and from adult CF patients (n = 34) at St. Michael's Hospital in Toronto. All the isolates used in this study were independent strains (1 isolate/patient). Antimicrobial susceptibility testing was performed on isolates grown planktonically by broth microdilution using Clinical and Laboratory Standards Institute (CLSI) guidelines (17). Antimicrobial susceptibility testing was also performed on isolates grown as a biofilm using a modified form of the Calgary biofilm technique (18, 19). The antibiotic panels contained tobramycin at concentrations of 0, 10, 100, 200, 400, 800, 1,600, and 3,200 μg/ml. The MIC based on planktonic growth and the biofilm inhibitory concentration (BIC) of tobramycin for each isolate were determined by visually assessing the turbidity of each well (see Supplementary Methods in the supplemental material for more detail).
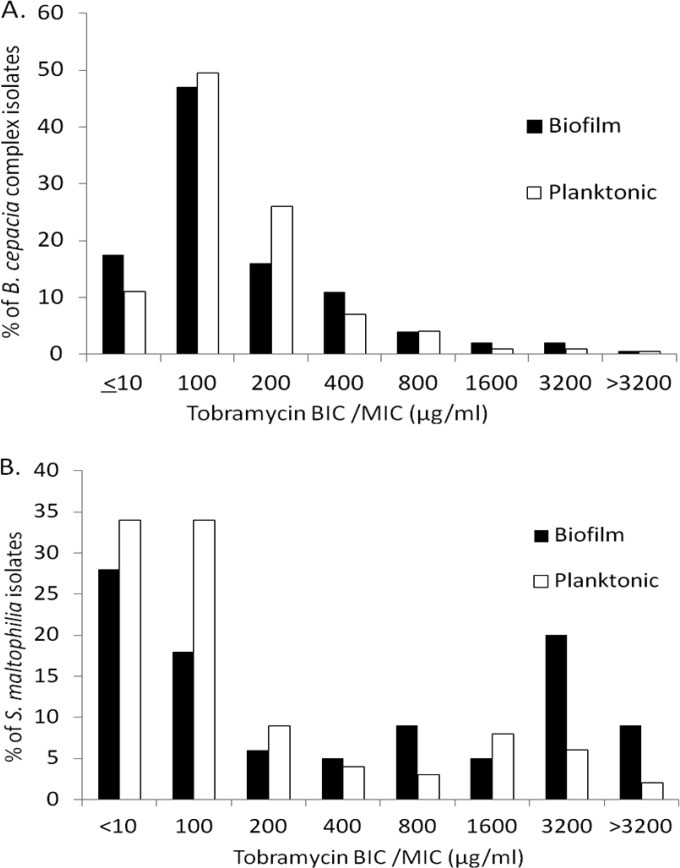
The tobramycin MIC50 and BIC50 (the BIC at which 50% of isolates were susceptible) were 100 μg/ml for a large collection of CF B. cepacia complex isolates (Table 1), largely consistent across most species of the B. cepacia complex. Burkholderia vietnamiensis, previously shown to be more susceptible to aminoglycosides (15), had an MIC50 of 10 μg/ml. Burkholderia dolosa isolates, responsible for an outbreak at a U.S. CF care center (20), demonstrated a higher MIC50 of 200 μg/ml. Similarly, the tobramycin MIC50 and BIC50 for S. maltophilia were 100 μg/ml. The distribution of the tobramycin MICs and BICs for B. cepacia complex and S. maltophilia is shown in Fig. 1. A significant proportion of B. cepacia complex isolates had tobramycin MICs (n = 20/180, 11%) and BICs (n = 32/180, 18%) that were ≤10 μg/ml, as did S. maltophilia isolates, with 34% (n = 34/101) of MICs and 29% of BICs (n = 29/101) that were ≤10 μg/ml. Conversely, the MIC90 and BIC90 for B. cepacia complex isolates were 400 μg/ml and for S. maltophilia isolates were 1,600 μg/ml and 3,200 μg/ml (Table 1), respectively, suggesting that in these cases, TIP administration may not be capable of exceeding these high inhibitory concentrations.
TABLE 1.
Tobramycin MICs and BICs for Burkholderia cepacia complex and Stenotrophomonas maltophilia CF isolates
| Organism (no. of isolates) | MIC50 (μg/ml) | BIC50 (μg/ml) | MIC90 (μg/ml) | BIC90 (μg/ml) |
|---|---|---|---|---|
| B. cepacia complex (180) | 100 | 100 | 400 | 400 |
| B. cenocepacia (83) | 100 | 100 | 800 | 800 |
| B. multivorans (41) | 100 | 100 | 400 | 400 |
| B. stabilis (16) | 100 | 100 | 100 | 400 |
| B. vietnamiensis (19) | 10 | 10 | 100 | 100 |
| B. dolosa (14) | 200 | 200 | 200 | 400 |
| B. cepacia (7) | 100 | 100 | 800 | 400 |
| S. maltophilia (101) | 100 | 100 | 1,600 | 3,200 |
FIG 1.
Distribution of tobramycin biofilm inhibitory concentrations (BICs) measured by biofilm antimicrobial susceptibility testing and MICs measured by planktonic antimicrobial susceptibility testing for Burkholderia cepacia complex (A) and Stenotrophomonas maltophilia (B) cystic fibrosis isolates.
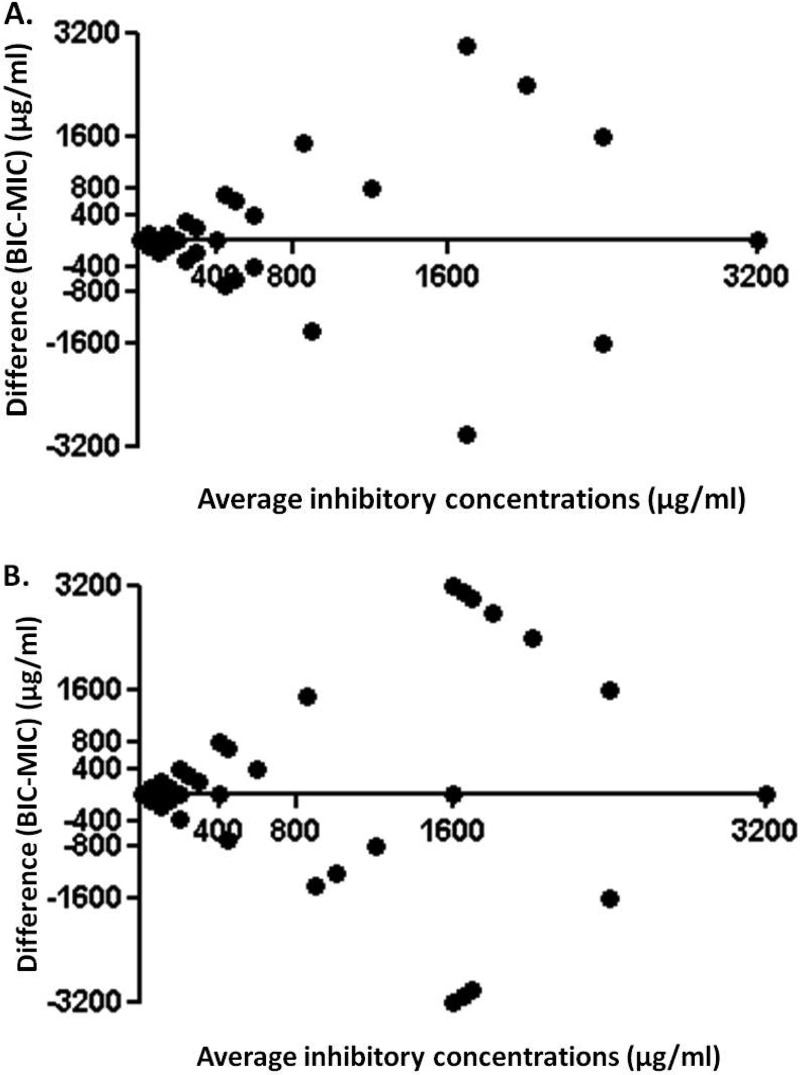
The correlations between the two methods (planktonic and biofilm) of antimicrobial susceptibility testing was calculated using the Spearman correlation coefficient and were found to be statistically significant for B. cepacia complex isolates (r = 0.5549, P < 0.0001) and for S. maltophilia isolates (r = 0.3638, P = 0.0002), suggesting that tobramycin can function well against organisms grown in a biofilm state, as expected in the CF lung. The agreement between the two methods of antimicrobial susceptibility testing is illustrated in a Bland-Altman plot for B. cepacia complex (Fig. 2A) and S. maltophilia (Fig. 2B) isolates.
FIG 2.
Bland-Altman plots of average inhibitory concentrations versus difference between biofilm inhibitory concentrations (BICs) and MICs for Burkholderia cepacia complex (A) and Stenotrophomonas maltophilia (B) cystic fibrosis isolates, with points on the x axis (y = 0) indicating complete agreement.
To date, this is the largest in vitro study of a contemporary collection of clinical CF isolates to determine the tobramycin concentrations required to inhibit the planktonic and biofilm growth of B. cepacia complex and S. maltophilia. Although traditionally considered to be intrinsically resistant to systemically attainable aminoglycoside concentrations based on CLSI breakpoints (17), our data suggest that TIP treatment can achieve a maximal drug concentration (Cmax)/MIC ratio of up to 20-fold for the majority of B. cepacia complex and S. maltophilia isolates from CF patients. It is unknown what Cmax/MIC ratio is required to successfully suppress bacterial growth in the CF lung, but there is a relationship between the Cmax and the MIC required to inhibit Pseudomonas aeruginosa growth, with higher ratios associated with greater reduction in bacterial density (21).
We also demonstrated that tobramycin inhibitory concentrations were similar regardless of whether the organisms were grown planktonically or as a biofilm, suggesting that at these high levels, tobramycin may be effective in the CF lung environment. Different classes of antimicrobials have various degrees of efficacy against dense slow-growing matrix-enveloped bacterial communities based on their ability to penetrate biofilms and their mechanism of action (22). Aztreonam, for example, is not as effective as tobramycin at reducing P. aeruginosa biofilm mass on airway epithelial cells, and tolerance to aztreonam may develop secondary to biofilm exopolysaccharide production (23). In our study, however, high-dose tobramycin overcame mechanisms of biofilm resistance and inhibited bacterial protein synthesis in stationary-phase organisms.
Despite these results, however, in vitro susceptibility testing, whether by the planktonic or biofilm method of growth, does not necessarily predict clinical response in CF patients, and it is unclear whether TIP, which delivers a sputum tobramycin concentration 1.5- to 2-fold higher than TIS, will translate into improved efficacy in the treatment of these infections. Clinical trials of TIP therapy in this patient population are under way to assess this question (ClinicalTrials.gov identifier NCT02212587).
In conclusion, TIP administration may deliver pulmonary drug concentrations in excess of what is required to inhibit the majority of B. cepacia complex and S. maltophilia CF isolates, even when grown as a biofilm. This offers a potential therapeutic option to a CF population for whom there is no effective chronic suppressive antimicrobial treatment.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the work of Danuta Kovach and Carlos Costano in the laboratory and all the staff at the microbiology laboratories involved in this study. We also thank CF Canada for funding the Canadian Burkholderia cepacia Complex Research and Referral Repository and the U.S. CF Foundation for supporting the Cystic Fibrosis Foundation Burkholderia cepacia Research Repository.
This study was funded through an unrestricted investigator-initiated grant from Novartis Pharmaceutical Canada, Inc. A.R. was supported by a summer studentship award from Cystic Fibrosis Canada. D.P.S. is supported by a grant from CF Canada. Neither Novartis nor CF Canada had any involvement in the study design, interpretation of the data, writing of the manuscript, or the decision to submit the manuscript for publication.
E.T. has received consultancy and speaking fees from Novartis. All other authors declare no confict of interests.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04123-14.
REFERENCES
- 1.Alexander BD, Petzold EW, Reller LB, Palmer SM, Davis RD, Woods CW, Lipuma JJ. 2008. Survival after lung transplantation of cystic fibrosis patients infected with Burkholderia cepacia complex. Am J Transplant 8:1025–1030. doi: 10.1111/j.1600-6143.2008.02186.x. [DOI] [PubMed] [Google Scholar]
- 2.Corey M, Farewell V. 1996. Determinants of mortality from cystic fibrosis in Canada, 1970–1989. Am J Epidemiol 143:1007–1017. doi: 10.1093/oxfordjournals.aje.a008664. [DOI] [PubMed] [Google Scholar]
- 3.Tablan OC, Chorba TL, Schidlow DV, White JW, Hardy KA, Gilligan PH, Morgan WM, Carson LA, Martone WJ, Jason JM, Jarvis WR. 1985. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J Pediatr 107:382–387. doi: 10.1016/S0022-3476(85)80511-4. [DOI] [PubMed] [Google Scholar]
- 4.Waters V, Atenafu EG, Lu A, Yau Y, Tullis E, Ratjen F. 2013. Chronic Stenotrophomonas maltophilia infection and mortality or lung transplantation in cystic fibrosis patients. J Cyst Fibros 12:482–486. doi: 10.1016/j.jcf.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Waters V, Yau Y, Prasad S, Lu A, Atenafu E, Crandall I, Tom S, Tullis E, Ratjen F. 2011. Stenotrophomonas maltophilia in cystic fibrosis: serologic response and effect on lung disease. Am J Respir Crit Care Med 183:635–640. doi: 10.1164/rccm.201009-1392OC. [DOI] [PubMed] [Google Scholar]
- 6.Horsley A, Jones AM. 2012. Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation. Cochrane Database Syst Rev 10:CD009529. doi: 10.1002/14651858.CD009529.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Horsley A, Webb K, Bright-Thomas R, Govan J, Jones A. 2011. Can early Burkholderia cepacia complex infection in cystic fibrosis be eradicated with antibiotic therapy? Front Cell Infect Microbiol 1:18. doi: 10.3389/fcimb.2011.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilchrist FJ, Webb AK, Bright-Thomas RJ, Jones AM. 2012. Successful treatment of cepacia syndrome with a combination of intravenous cyclosporin, antibiotics and oral corticosteroids. J Cyst Fibros 11:458–460. doi: 10.1016/j.jcf.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Grimwood K, Kidd TJ, Tweed M. 2009. Successful treatment of cepacia syndrome. J Cyst Fibros 8:291–293. doi: 10.1016/j.jcf.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Andrade A, Valvano MA. 2014. A Burkholderia cenocepacia gene encoding a non-functional tyrosine phosphatase is required for the delayed maturation of the bacteria-containing vacuoles in macrophages. Microbiology 160:1332–1345. doi: 10.1099/mic.0.077206-0. [DOI] [PubMed] [Google Scholar]
- 11.Nzula S, Vandamme P, Govan JR. 2002. Influence of taxonomic status on the in vitro antimicrobial susceptibility of the Burkholderia cepacia complex. J Antimicrob Chemother 50:265–269. doi: 10.1093/jac/dkf137. [DOI] [PubMed] [Google Scholar]
- 12.Waters V. 2012. New treatments for emerging cystic fibrosis pathogens other than Pseudomonas. Curr Pharm Des 18:696–725. doi: 10.2174/138161212799315939. [DOI] [PubMed] [Google Scholar]
- 13.Konstan MW, Flume PA, Kappler M, Chiron R, Higgins M, Brockhaus F, Zhang J, Angyalosi G, He E, Geller DE. 2011. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: the EAGER trial. J Cyst Fibros 10:54–61. doi: 10.1016/j.jcf.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buroni S, Pasca MR, Flannagan RS, Bazzini S, Milano A, Bertani I, Venturi V, Valvano MA, Riccardi G. 2009. Assessment of three resistance-nodulation-cell division drug efflux transporters of Burkholderia cenocepacia in intrinsic antibiotic resistance. BMC Microbiol 9:200. doi: 10.1186/1471-2180-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jassem AN, Zlosnik JE, Henry DA, Hancock RE, Ernst RK, Speert DP. 2011. In vitro susceptibility of Burkholderia vietnamiensis to aminoglycosides. Antimicrob Agents Chemother 55:2256–2264. doi: 10.1128/AAC.01434-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooke JS. 2012. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41. doi: 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing: 22nd informational supplement, M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 18.Wu K, Yau YC, Matukas L, Waters V. 2013. Biofilm compared to conventional antimicrobial susceptibility for Stenotrophomonas maltophilia isolates from cystic fibrosis patients. Antimicrob Agents Chemother 57:1546–1548. doi: 10.1128/AAC.02215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. 1999. The Calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 37:1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biddick R, Spilker T, Martin A, LiPuma JJ. 2003. Evidence of transmission of Burkholderia cepacia, Burkholderia multivorans and Burkholderia dolosa among persons with cystic fibrosis. FEMS Microbiol Lett 228:57–62. doi: 10.1016/S0378-1097(03)00724-9. [DOI] [PubMed] [Google Scholar]
- 21.LiPuma JJ. 2001. Microbiological and immunologic considerations with aerosolized drug delivery. Chest 120:118S–123S. doi: 10.1378/chest.120.3_suppl.118S [DOI] [PubMed] [Google Scholar]
- 22.Bjarnsholt T, Ciofu O, Molin S, Givskov M, Hoiby N. 2013. Applying insights from biofilm biology to drug development—can a new approach be developed? Nat Rev Drug Discov 12:791–808. doi: 10.1038/nrd4000. [DOI] [PubMed] [Google Scholar]
- 23.Yu Q, Griffin EF, Moreau-Marquis S, Schwartzman JD, Stanton BA, O'Toole GA. 2012. In vitro evaluation of tobramycin and aztreonam versus Pseudomonas aeruginosa biofilms on cystic fibrosis-derived human airway epithelial cells. J Antimicrob Chemother 67:2673–2681. doi: 10.1093/jac/dks296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.