Abstract
Chronic constipation is a very common disease in children. Successful treatment of constipation can be achieved not only with medication but also with lifestyle changes, including a proper diet. Diets including fruits, fluids, and probiotics are good for constipation. Some dietary components are helpful for constipation, and some are harmful. In this study, we present diets related to constipation from the literature, and propose some perspectives regarding diets related to constipation.
Keywords: Constipation, Diet, Fluid, Child
INTRODUCTION
The outcome of a disease treatment generally depends on patient or guardian cooperation, particularly in pediatric patients. In children with functional constipation, the better the patients eat, the better their bowel movements. This phenomenon is observed more frequently in young patients with a defecation diary. This study focuses on diets associated with constipation, such as the 3Fs: fluid (water, juice, and veberage, etc.), fiber, fruits, probiotics, and milk products. Fruits include bananas, persimmons, kiwifruit, plum, (prune), etc.
FIBER
The amount of total fiber ingested per day includes that from dietary and functional fiber sources. Dietary fiber is nondigestible carbohydrate and lignin that are intrinsic and intact in plants. Functional fiber includes isolated, nondigestible carbohydrate that has beneficial physiological effects in humans. The recommended daily total fiber consumption for children >1 year is expressed as "age plus 5-(10) g" or "0.5 g/kg" [1,2,3]. Common fiber-rich foods include cereals (rice, corn), bread, vegetables, fruits, potatoes (with peel), and whole grains. The mechanism of action of fiber on constipation includes: 1) Fiber increases stool bulk and accelerates colon transit; 2) fermenting fiber produces short-chain fatty acids (butyrate, propionate, acetate, etc.), which increase osmotic load and accelerate colon transit; 3) short-chain fatty acids change the intraluminal microbiome (mass) directly or indirectly by decreasing luminal pH, which accelerates colon transit; and 4) fiber contains water. All these improve stool consistency and amount [4,5,6].
Classically, fiber is classified into water-soluble and water-insoluble fiber. Water-insoluble fiber includes cellulose, hemicellulose, methylcelluose, lignin, and synthetic fibers (calcium polycarbophil). Water-soluble fiber includes gums (fenugreek gum, guar gum, tara gum, locust bean gum, or carob gum), pectin, mucilage, psyllium, and glucomannan. However, this classification is too theoretical to apply to actual treatment because every component of fiber exists in nature. A more complex classification for naturally occurring fiber has been proposed. According to this classification, 1) short-chain carbohydrates or fiber are oligosaccharides. 2) Long-chain carbohydrates: ① Soluble, highly fermentable non-starch polysaccharide fiber includes resistant starch, pectin, inulin, and guar gum. ② Intermediate soluble and fermentable fiber includes psyllium, ispaghula and oats. ③ Insoluble, slowly fermentable fiber includes wheat bran, lignin (flax), and fruits and vegetables. ④ Insoluble, non-fermentable fiberincludes cellulose, sterculia, and methylcellulose [7]. Generally, it is well-known that water-insoluble fiber is helpful for constipation. However, some authors insist that intermediate soluble and fermentable fiber is helpful for constipation [7]. The Food and Drug Administration approved methylcelluose, psyllium, and polycarbophill for constipation as of 2014. Higher dietary fiber intake (fruits, legumes, and vegetables) is associated with a lower incidence of constipation in some studies [8,9]. In terms of treatment, water-insoluble fiber with wheat bran and rye bread improves bowel movement frequency and defecation difficulty significantly [10,11]. However, the water-soluble fiber (psyllium and glucomannan) results are conflicting [12,13,14]. As of 2014, North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) do not support the use of fiber supplements in the treatment of functional constipation [15].
For perspective, it is warranted to verify that for which children and at what point should fiber treatment for constipation begin? How should the fiber source be selected? Which constipation subtypes should be considered?
FLUIDS
About 10 L of fluid per day, including about 2 L from the diet, are loaded into the adult gastrointestinal tract, and 8.5 L are absorbed by the small intestine. Another 1.3-1.4 L is absorbed in the colon, and 0.1-0.2 L is excreted in stool. About 5 L are loaded into the gastrointestinal tract of a small child per day. The absorptive capacity of the small bowel and colon can increase within a wide range to meet needs [16]. However, considering toddler's diarrhea [17], the absorptive capacity of the small bowel and colon may not reach maturity at least until 5-6 years of age.
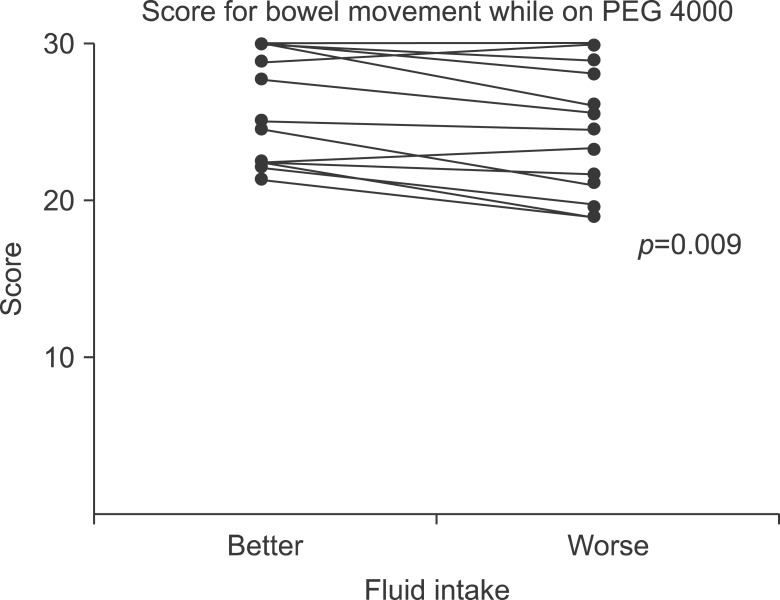
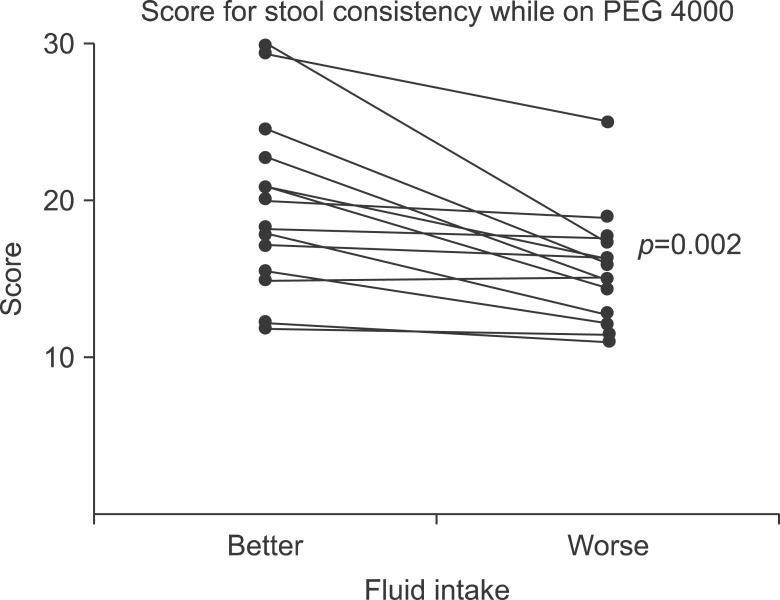
Against common sense that drinking water is helpful for constipation, many well-known studies, including the 2014 ESPGHAN and ESPGHAN paper, insist that no evidence supports the use of extra fluid intake to treat functional constipation [15]. However, additional fluid during treatment for chronic functional constipation led to better outcomes in terms of bowel movement frequency and stool consistency in children who were administered polyethylene glycol (PEG 4000, Forlax; Beaufour Ipsen Pharma., Paris, France) (Fig. 1 and 2) [18]. Considering the mechanism of action of osmotic and fiber agents, sufficient fluid intake is helpful for treating constipation.
Fig. 1.
Bowel movement scores for patients on polyethylene glycol 4000 (PEG 4000, Forlax; Beaufour Ipsen Pharma., Paris, France). Scores for the better fluid intake periods were significantly better than those for the worse fluid intake periods (median, 27.78; range, 21.43-30.00 vs. median, 25.13; range, 19.00-30.00; p=0.009).
Fig. 2.
Stool consistency scores for patients on polyethylene glycol 4000 (PEG 4000, Forlax; Beaufour Ipsen Pharma., Paris, France). Scores for the better fluid intake periods were significantly better than those for the worse fluid intake period (median, 20.00; range, 11.79-20.00; vs. median, 15.91; range, 11.00-25.00; p=0.002).
Fruit juices contain sorbitol, fructose, and phytochemicals as well as water. Some juices have a fiber component. Fruits juices are generally helpful for constipation, particularly in young children, whose intestinal function has not fully matured. Apple, prune, and pear juices are usually recommended for constipation.
Lubiprostone (type-2 chloride channels) and linaclotide (cystic fibrosis transmembrane conductance regulator) are recently introduced drugs for treatment of adult constipation that act on intestinal epithelial ion channels and increase intestinal luminal water content [19,20]. We believe that lifestyle changes are important to treat constipation and prevent a relapse. Thus, increasing fluid intake is recommended rather than using luminal fluid-increasing agents in terms of safety and long-term efficacy.
PROBIOTICS
Probiotics are live microorganisms that confer a health benefit on the host when administered in adequate amounts. Dysbiosis of intestinal flora, high frequencies of Clostridium and Enterobacteriaceae species, which are rarely isolated in healthy children, have been reported in children with constipation [21]. The proposed probiotic mechanism for constipation involves 1) Bifidobacteria and lactobacilli produce lactic acid, acetic acid, and other acids, which lower pH in the colon and enhance peristalsis. 2) Probiotics may exert anti-inflammatory effect and immunomodulation effect, which may improve certain mechanism of dysmotility. 3) Metabolic functions of the altered microbiota may affect intestinal luminal content. For example, methane gas can slow gut transit, and probiotics may improve this effect. 4) Particular probiotic strains stimulate motility and peristalsis, which is particularly helpful to treat slow transit constipation [22].
Lactobacillus reuteri has a positive effect on bowel frequency in infants with functional chronic constipation but no improvements in stool consistency are observed [23]. Bifidobacteria (B. bifidum, B. infantis, and B. longum) and Lactobacillus (L. casei, L. plantarum, and L. rhamnosus) increase bowel movement frequency, decrease fecal incontinence, and reduce abdominal pain in children 4-16 years of age; however, they have no effect on stool consistency [24]. In one study, Lactobacillus GG was ineffective as an adjunct to lactulose for treating constipation in children [25]. As of 2014, NASPGHAN and ESPGHAN do not support the use of pre- or probiotics in the treatment of childhood constipation [15]. Some probiotics are helpful for diarrhea, and some are useful for constipation. Further studies are warranted for probiotic specificity for diarrhea or constipation. Is there strain specificity for constipation or diarrhea within the same Lactobacillus species?
MILK AND MILK PRODUCTS
Breast-milk-fed infants are less frequently constipated than those who are formula fed. Several mechanisms were proposed for this. 1) Large amounts of prebiotic oligosaccharides in human milk provide substrates for gut bacteria and this improves osmotic balance and stool consistency. 2) The fat composition of human milk may help create softer stools. 3) Breast milk contains non-digestible oligosaccharides, which act like dietary fiber, stimulate the growth of beneficial bacteria, and promote maturation of the gastrointestinal tract. 4) Breast milk also has the optimal whey protein composition and a low phosphorous content. 5) Increased levels of gastric inhibitory peptide, neurotensin, and vasoactive intestinal peptide are observed in formula-fed infants compared with those in breast-fed infants [26]. A food allergy to cow milk protein can also cause constipation. A crossover dietary trial demonstrated an association between chronic functional constipation and cow milk consumption [27]. In one study, type IV allergies developed frequently and lymphocyte stimulation test values were related to constipation. Symptoms improved in the majority of infants after eliminating the cow milk antigen [28]. Colon peristalsis in infants with constipation is abolished after ingesting cow's milk but recovers after stopping cow's milk. Colonic stenosis due to a cow milk protein allergy mimicking Hirschsprung's disease is well known [29]. As of 2014, NASPGHAN and ESPGHAN state that evidence is conflicting for allergy testing to diagnose a cow milk allergy in children with functional constipation [15]. Investigations into the immunological or biochemical mechanisms occurring during chronic functional constipation are required, including investigations of intolerance reactions.
FRUITS
Fruits contain water, sorbitol, fructose, fiber, and phytochemicals. Fruits thought to be useful for treating constipation are pear, grape, plump, and apple with peel, which are rich in fiber. Here, some fruits available in Korea will be considered, including green kiwifruit, prune (plum), banana, and persimmon.
Green kiwifruit
Green kiwifruit significantly increases defecation frequency, stool volume, softness of bowel motion, and ease of defecation in adult clinical studies. The proposed mechanisms for this are as follow: 1) Green kiwifruit contains 2-3 g of dietary fiber per 100 gm. Fiber plays a physicochemical role during constipation. 2) Actinidine, a protease enzyme in green kiwifruit, stimulates upper gastrointestinal tract motility. Possible induction of activity in the colon remains to be clarified. 3) Kissper, a peptide in green kiwifruit, has been characterized by anion selectivity and ion channeling. 4) Phytochemicals occurring naturally in the fruit may have biological significance [30].
Prune
A prune is a dried plum, and these fruits are beneficial for constipation. They contain high levels of fiber (6.1 g/100 g), fructose (fructan), and sorbitol (14.7 g/100 g). Large amounts of phenolic compounds (184 mg/100 g), mainly as neochlorogenic and chlorogenic acids, may aid in the laxative effect [12]. Prune juice contains less sorbitol and fiber than that of prunes. Japanese apricot (Prunus mume; Mae-sil) increases defecation frequency and contraction of the rat colon [31].
Persimmon
Persimmons are not usually eaten in western countries; however, they are one of the most popular fruits in eastern Asia. Several types of persimmon are available. However, we will classify them into non-astringent varieties (sweet persimmon), which have a lower tannin acid concentration, and astringent varieties (mellowed persimmon and dried persimmon), which have a higher tannin acid concentration. Astringency of persimmons depends on the quantity of soluble tannins in the fruit flesh. As persimmons mature, soluble tannins become insoluble, which makes the fruit much less bitter. The total quantity of tannins decreases as the persimmon increases in size [32]. However, healthy individuals experience painful defecation when even ripe persimmons are eaten. Tannin acid reduced small intestinal secretions and inhibits peristalsis. Thus, any forms of persimmons should be avoided in children with constipation.
Banana
Several types of banana are available worldwide. However, we will consider only the species of banana that is normally available in markets. Unripe bananas contain 100-250 mg tannins/100 g and have high amylase-resistant starch content. Thus, they can cause or aggravate pre-existing constipation. This property has been used in the BRAT (banana, rice, apple sauce, and toast) diet for diarrhea. As bananas ripen, the quantities of tannins and amylase-resistant starch decrease, while soluble sugars accumulate. Ripe bananas contain 3 g fiber/120 g, mostly in the form of soluble fiber. They also contain amylase-resistant starch and tannins [33]. We recommend not feeding banana to a constipated child, as many other good sources of fiber are available.
CONCLUSION
Constipation cannot be managed using medication alone. Better short- and long-term outcomes are achieved with lifestyle changes, including a proper diet. Pediatricians treating a child with constipation should pay more attention to these changes.
References
- 1.Williams CL. Importance of dietary fiber in childhood. J Am Diet Assoc. 1995;95:1140–1146. doi: 10.1016/S0002-8223(95)00307-X. [DOI] [PubMed] [Google Scholar]
- 2.Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for energy, carbohydrates, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 3.Committee on Nutrition, American Academy of Pediatrics. Pediatric Nutrition Handbook. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 1998. [Google Scholar]
- 4.McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998;12:491–497. doi: 10.1046/j.1365-2036.1998.00336.x. [DOI] [PubMed] [Google Scholar]
- 5.Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991;70:443–459. doi: 10.1111/j.1365-2672.1991.tb02739.x. [DOI] [PubMed] [Google Scholar]
- 6.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and non-starch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 7.Eswaran S, Muir J, Chey WD. Fiber and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:718–727. doi: 10.1038/ajg.2013.63. [DOI] [PubMed] [Google Scholar]
- 8.Lee WT, Ip KS, Chan JS, Lui NW, Young BW. Increased prevalence of constipation in pre-school children is attributable to under-consumption of plant foods: a community-based study. J Paediatr Child Health. 2008;44:170–175. doi: 10.1111/j.1440-1754.2007.01212.x. [DOI] [PubMed] [Google Scholar]
- 9.Morais MB, Vítolo MR, Aguirre AN, Fagundes-Neto U. Measurement of low dietary fiber intake as a risk factor for chronic constipation in children. J Pediatr Gastroenterol Nutr. 1999;29:132–135. doi: 10.1097/00005176-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Hongisto SM, Paajanen L, Saxelin M, Korpela R. A combination of fibre-rich rye bread and yoghurt containing Lactobacillus GG improves bowel function in women with self-reported constipation. Eur J Clin Nutr. 2006;60:319–324. doi: 10.1038/sj.ejcn.1602317. [DOI] [PubMed] [Google Scholar]
- 11.Badiali D, Corazziari E, Habib FI, Tomei E, Bausano G, Magrini P, et al. Effect of wheat bran in treatment of chronic nonorganic constipation. A double-blind controlled trial. Dig Dis Sci. 1995;40:349–356. doi: 10.1007/BF02065421. [DOI] [PubMed] [Google Scholar]
- 12.Attaluri A, Donahoe R, Valestin J, Brown K, Rao SS. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther. 2011;33:822–828. doi: 10.1111/j.1365-2036.2011.04594.x. [DOI] [PubMed] [Google Scholar]
- 13.Loening-Baucke V, Miele E, Staiano A. Fiber (glucomannan) is beneficial in the treatment of childhood constipation. Pediatrics. 2004;113:e259–e264. doi: 10.1542/peds.113.3.e259. [DOI] [PubMed] [Google Scholar]
- 14.Chmielewska A, Horvath A, Dziechciarz P, Szajewska H. Glucomannan is not effective for the treatment of functional constipation in children: a double-blind, placebo-controlled, randomized trial. Clin Nutr. 2011;30:462–468. doi: 10.1016/j.clnu.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Tabbers MM, DiLorenzo C, Berger MY, Faure C, Langendam MW, Nurko S, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58:258–274. doi: 10.1097/MPG.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 16.Harrell LE, Chang EB. Intestinal water and electrolyte transport. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran's gastrointestinal and liver disease. 8th ed. Philadelphia: Saunders Elservier Co.; 2006. pp. 2127–2146. [Google Scholar]
- 17.Kneepkens CM, Hoekstra JH. Chronic nonspecific diarrhea of childhood: pathophysiology and management. Pediatr Clin North Am. 1996;43:375–390. doi: 10.1016/s0031-3955(05)70411-9. [DOI] [PubMed] [Google Scholar]
- 18.Bae SH, Son JS, Lee R. Effect of fluid intake on the outcome of constipation in children: PEG 4000 versus lactulose. Pediatr Int. 2010;52:594–597. doi: 10.1111/j.1442-200X.2009.03017.x. [DOI] [PubMed] [Google Scholar]
- 19.Lacy BE, Levy LC. Lubiprostone: a chloride channel activator. J Clin Gastroenterol. 2007;41:345–351. doi: 10.1097/01.mcg.0000225665.68920.df. [DOI] [PubMed] [Google Scholar]
- 20.Blackshaw LA, Brierley SM. Emerging receptor target in the pharmacotherapy of irritable bowel syndrome with constipation. Expert Rev Gastroenterol Hepatol. 2013;7(5 Suppl 1):15–19. doi: 10.1586/17474124.2013.820045. [DOI] [PubMed] [Google Scholar]
- 21.Zoppi G, Cinquetti M, Luciano A, Benini A, Muner A, Bertazzoni Minelli E. The intestinal ecosystem in chronic functional constipation. Acta Paediatr. 1998;87:836–841. doi: 10.1080/080352598750013590. [DOI] [PubMed] [Google Scholar]
- 22.Quigley EM. Probiotics in the management of functional bowel disorders: promise fulfilled? Gastroenterol Clin North Am. 2012;41:805–819. doi: 10.1016/j.gtc.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Coccorullo P, Strisciuglio C, Martinelli M, Miele E, Greco L, Staiano A. Lactobacillus reuteri (DSM 17938) in infants with functional chronic constipation: a double-blind, randomized, placebo-controlled study. J Pediatr. 2010;157:598–602. doi: 10.1016/j.jpeds.2010.04.066. [DOI] [PubMed] [Google Scholar]
- 24.Bekkali NL, Bongers ME, Van den Berg MM, Liem O, Benninga MA. The role of a probiotics mixture in the treatment of childhood constipation: a pilot study. Nutr J. 2007;6:17. doi: 10.1186/1475-2891-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banaszkiewicz A, Szajewska H. Ineffectiveness of Lactobacillus GG as an adjunct to lactulose for the treatment of constipation in children: a double-blind, placebo-controlled randomized trial. J Pediatr. 2005;146:364–369. doi: 10.1016/j.jpeds.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Quinlan PT, Lockton S, Irwin J, Lucas AL. The relationship between stool hardness and stool composition in breast- and formula-fed infants. J Pediatr Gastroenterol Nutr. 1995;20:81–90. doi: 10.1097/00005176-199501000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Crowley ET, Williams LT, Roberts TK, Dunstan RH, Jones PD. Does milk cause constipation? A crossover dietary trial. Nutrients. 2013;5:253–266. doi: 10.3390/nu5010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ida S, Ikeda K, Etani Y, Shouji Y, Yamada H, Kawahara H, et al. Chronic constipation as a symptom of cow's milk allergy in infants and children in Japan [abstract] Taipei: The 4th World Congress of Pediatric Gastroenterology, Hepatology and Nutrition; 2012. p. 152. [Google Scholar]
- 29.Lee JH, Choe YH, Lee SK, Seo JM, Kim JH, Suh YL. Allergic proctitis and abdominal distention mimicking Hirschsprung's disease in infants. Acta Paediatr. 2007;96:1784–1789. doi: 10.1111/j.1651-2227.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 30.Drummond L, Gearry RB. Kiwifruit modulation of gastrointestinal motility. Adv Food Nutr Res. 2013;68:219–232. doi: 10.1016/B978-0-12-394294-4.00012-2. [DOI] [PubMed] [Google Scholar]
- 31.Na JR, Oh KN, Park SU, Bae D, Choi EJ, Jung MA, et al. The laxative effects of Maesil (Prunus mume Siebold & Zucc.) on constipation induced by a low-fibre diet in a rat model. Int J Food Sci Nutr. 2013;64:333–345. doi: 10.3109/09637486.2012.738648. [DOI] [PubMed] [Google Scholar]
- 32.Bubba MD, Giordani E, Pippucci L, Cincinelli A, Checchini L, Galvan P. Changes in tannins, ascorbic acid and sugar content in astringent persimmons during on-tree growth and ripening and in response to different postharvest treatments. J Food Compos Anal. 2009;22:668–677. [Google Scholar]
- 33.Shiga TM, Soares CA, Nascimento JR, Purgatto E, Lajolo FM, Cordenunsi BR. Ripening-associated changes in the amounts of starch and non-starch polysaccharides and their contributions to fruit softening in three banana cultivars. J Sci Food Agric. 2011;91:1511–1516. doi: 10.1002/jsfa.4342. [DOI] [PubMed] [Google Scholar]