Abstract
Purpose
The method of percutaneous endoscopic gastrostomy (PEG) tube placement can be divided into the pull and introducer techniques. We compared short-term complications and prognosis between patients who underwent the pull technique and two other types of introducer techniques, the trocar introducer technique and T-fastener gastropexy technique.
Methods
Twenty-six patients who underwent PEG were enrolled in this study. We retrospectively investigated the age, sex, body weight, weight-for-age Z-score, underlying diseases, PEG indications, complications, duration of NPO (nil per os), pain control frequency, and duration of antibiotic therapy. The patients were classified into three groups according to the PEG technique. The occurrence of complications was monitored for 10 weeks after the procedure.
Results
The age, sex, body weight, and weight-for-age Z-score were not significantly between the three groups. Most patients had cerebral palsy and seizure disorders. Dysphagia was the most common indication for PEG. Major complications occurred in 5 (50%), 4 (66.7%), and 0 (0%) patients in group I, II, and III, respectively (p=0.005). Further, peristomal infection requiring systemic antibiotic therapy occurred in 2 (20%), 3 (50%), and 0 (0%) patients in group I, II, and III, respectively (p=0.04). There was no significant difference between the groups with respect to minor complications, duration of NPO, pain control frequency, and duration of antibiotic therapy.
Conclusion
The results indicate that the T-fastener gastropexy technique was associated with the lowest rate of major complications.
Keywords: Gastrostomy, Complications
INTRODUCTION
Percutaneous endoscopic gastrostomy (PEG) is a procedure of placing a nutrition tube into the stomach through the abdominal wall using an endoscope. PEG is used to deliver nutrition to patients who have normal digestive function but who cannot maintain oral intake for prolonged periods [1]. This procedure was first described by Gauderer and colleagues [2] in 1980; they used the "pull technique", wherein the gastrostomy tube is placed into the stomach through the oral cavity under endoscopic guidance, and pulled to the outside. Since then, it has been widely used as an alternative for surgical PEG [2,3]. Gauderer's pull technique is simple and easy to perform, but may be associated with a number of complications, occurring at various rates, including peristomal infection, peristomal leakage, granulation tissue, gastric tissue outgrowth, hemorrhage, ileus, pneumoperitoneum, colon injury, aspiration pneumonia, buried bumper syndrome, abscess, and gastrocutaneous fistula [4,5,6].
In 1984, Russell et al. [7] introduced a new PEG placement technique, the "push technique", where the gastric wall is fixed to the abdominal wall under endoscopic guidance. The gastrostomy tube is passed into the stomach through the abdominal wall from the outside, bypassing the oral cavity. Since then, various techniques that differ in the method of fixing the stomach wall to the abdominal wall, or in the method of penetrating the stomach wall, such as the introducer (Russell) and Versa (T-fastener) techniques, have been introduced [8]. Such introducer methods allow bypassing of the oral cavity and reduce the risk of infection. However, these techniques increase the risk of pneumoperitoneum with repeated inflation of stomach, which can cause leakage of air from the stomach into the abdominal cavity [9,10].
PEG-associated complications can vary not only between the push and pull techniques but also between the different methods of puncturing the gastric wall and fixing the stomach to the abdominal wall in the push techniques. Most studies on the complications of PEG have compared the pull technique with surgical methods, or the pull technique with the introducer technique [11,12,13,14]. There is no study comparing the three groups including the pull technique and different types of introducer techniques. In the present study, we compared short-term complications and prognosis between patients who underwent the pull technique and two different types of introducer techniques, the large-caliber trocar introducer technique and T-fastener gastropexy.
MATERIALS AND METHODS
Study design
This study enrolled 26 patients who underwent PEG at the Department of Pediatrics, Chungnam National University Hospital, between September 2005 and June 2014. All patients provided written informed consent before the PEG procedure. These patients underwent one of the three types of PEG techniques during the study period. Patients underwent the Ponsky-Gauderer pull technique (group I) from December 2005 to July 2010; large-caliber trocar introducer PEG (group II) from August 2010 to June 2012; and T-fastener gastropexy (group III) from July 2012 to June 2014 (Table 1). At our hospital, we revised our method of PEG placement to implement better procedural techniques when new PEG placement techniques had been introduced in Korea.
Table 1.
Period Using the Technique, Procedure Place, Gastrostomy Tube Size
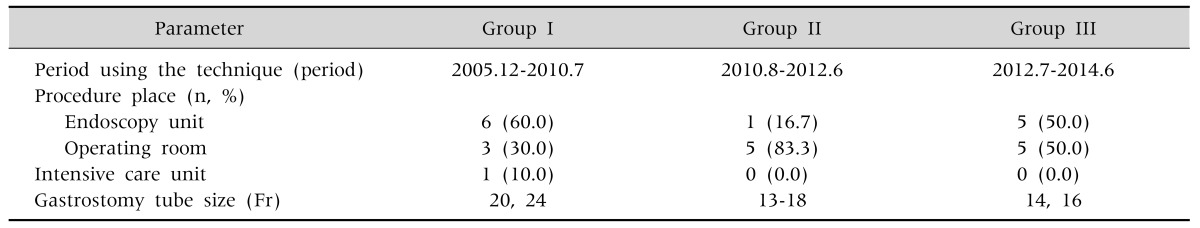
Group I: patients underwent the Ponsky-Gauderer pull technique from December 2005 to July 2010, group II: patients underwent large-caliber trocar introducer percutaneous endoscopic gastrostomy from August 2010 to June 2012, group III: patients underwent T-fastener gastropexy from July 2012 to June 2014.
The medical records of these subjects were reviewed retrospectively, and we analyzed the differences in the patient's age, sex, weight, weight-for-age Z score, underlying diseases, PEG indications, complications, post-procedural fasting period, pain control frequency, and the duration of antibiotics if required. Short-term complications were monitored for 10 weeks after the PEG procedure. The study protocol was approved by the institutional review board of Chungnam National University Hospital (IRB No. 2014-08-15).
Percutaneous endoscopic gastrostomy techniques
The PEG procedure was performed either in the endoscopy room or in the operating room, under the guidance of endoscopes with external diameters of 9.2 mm (GIF-Q260; Olympus Optical Co., Ltd., Tokyo, Japan) or 6.5 mm (GIF-XP260; Olympus Optical Co., Ltd.). We used the Cook PEG Kit (Wilson-Cook Medical Inc., Winston-Salem, NC, USA) for the pull technique, and either the Cliny PEG Kit (Create Medic, Yokohama, Japan) or the Kimberly-Clark MIC G Introducer Kit (Vygon Ltd., Cirencester, UK) for the introducer techniques (Table 1).
1. Ponsky-Gauderer pull technique
The oral cavity was sterilized using povidone-iodine solution on the day of the procedure and immediately before the procedure. An endoscope equipped with a snare was inserted into the stomach, and after inflating the stomach with air, the site where the gastric wall was closest to the abdominal wall was selected and marked. A skin incision of a length similar to that of the external diameter of the gastrostomy tube was made in the marked area. A Medicut cannula was quickly inserted. A guide wire was placed into the stomach through the cannula, which was grasped with the snare. While holding the guide wire in place, the endoscope was removed through the oral cavity, and the gastrostomy tube was fixed on the guide wire. The gastrostomy tube attached to the guide wire was pulled outside through the abdominal wall and fixed [15].
2. Large-caliber trocar introducer percutaneous endoscopic gastrostomy
The site for tube insertion was selected under endoscopic guidance as described above. Two double-lumen gastropexy devices were used to fix areas of the gastric wall to the abdominal wall. A 5-mm incision was made between the two fixed areas. Then, the plastic tube containing the trocar was inserted into the stomach through the incision. The trocar was removed, and the gastrostomy tube was stationed within the stomach through the plastic tube. The balloon was inflated using 5 mL of sterile water, and the plastic tube was broken to remove it, following which the gastrostomy tube was fixed [16].
3. T-fastener gastropexy
The site for gastrostomy tube insertion was chosen and a T-fastener was inserted into the stomach through the abdominal wall for 3-point fixation, in the shape of a triangle. At the center of the triangle, a small skin incision was made, through which an 18-gauge BD Angiocath catheter (Becton Dickinson Infusion Therapy Systems Inc., Sandy, UT, USA) was inserted into the stomach. Then, a guide wire was placed into the stomach through the Jelco. The Jelco was removed, and a peel-away sheath dilator was placed systemically through the guide wire until the fourth positioned in the stomach. The first three dilators (excluding the fourth dilator) were removed, and the gastrostomy tube was put into the stomach through the dilator and fixed [17].
For all three techniques, those who underwent the procedure in the endoscopy room or the intensive care unit received midazolam and ketamine or midazolam, ketamine, and demerol together for sedation. During the procedure, their heart rates and oxygen saturation levels were monitored. The skin incision site was locally anesthetized using lidocaine. Those who received the procedure in the operating room underwent general anesthesia by a board-qualified anesthesiologist. The patients were fasted for 8 hours before the procedure and received prophylactic antibiotics 30 minutes before the procedure.
Complications
The complications were divided into major and minor categories. Major complications included those that required systemic antibiotic treatment or surgical intervention due to problems with the gastrostomy site. Other complications belonged to minor categories. These included stomal leakage, peristomal infection, granulation tissue formation, and pneumoperitoneum. Peristomal infection associated with erythema and small amounts of exudate, which did not require antibiotic treatment, was categorized as a minor complication. Those that required systemic antibiotic therapy, because they involved exudate or pus with positive cultures or fever of greater than 38℃, were categorized as major complications.
Statistical analysis
The age, weight, and weight-for-age Z-scores were recorded as mean and standard deviation. The fasting period, pain control frequency, and the duration of antibiotic therapy were recorded as median (range). Other variables were recorded as the number of cases (percentage). Continuous variables were analyzed using Kruskal-Wallis validation, and other non-continuous variables, including the complications, were compared between the three groups using the Fisher's exact test. All statistical analyses were performed using IBM SPSS Statistics version 21.0 software (IBM Co., Armonk, NY, USA), and p-values less than 0.05 were considered statistically significant.
RESULTS
Patient characteristics
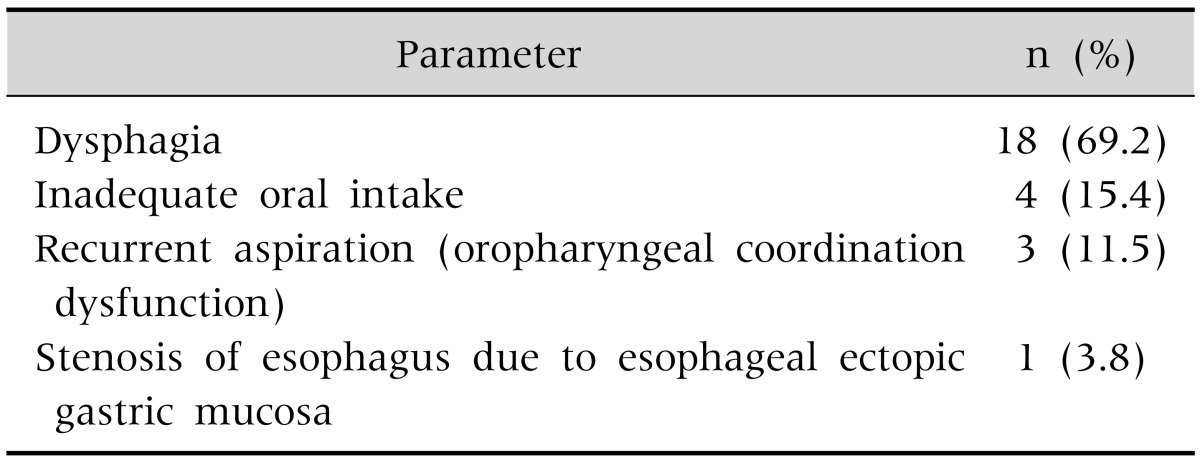
Of the 26 patients, 14 patients (53.8%) were male and 12 (46.2%) were female. The patient ages were 10.7±8.0, 6.0±5.1, and 10.9±7.9 years for those in group I, II, and III, respectively, with no statistically significant differences between the three groups (Table 2). The three most common underlying diseases were cerebral palsy (69.2%), epileptic disease (61.5%), hypoxemic ischemic brain injury (19.2%), and chromosomal abnormality (11.5%), with no significant difference between the three groups (Table 3). The indications for PEG were dysphagia in 18 patients (69.2%), prolonged insufficient oral intake in 4 patients (15.4%), and frequent aspiration in 3 patients (11.5%) (Table 4).
Table 2.
Comparison of Baseline Characteristics between the Three Groups
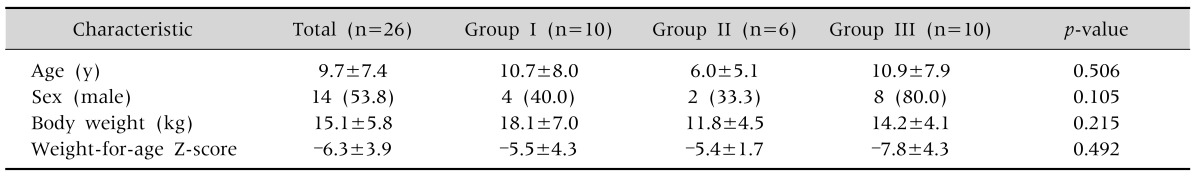
Values are presented as mean±standard deviation or number (%).
Group I: patients underwent the Ponsky-Gauderer pull technique from December 2005 to July 2010, group II: patients underwent large-caliber trocar introducer percutaneous endoscopic gastrostomy from August 2010 to June 2012, group III: patients underwent T-fastener gastropexy from July 2012 to June 2014.
Table 3.
Underlying Conditions of the 26 Patients
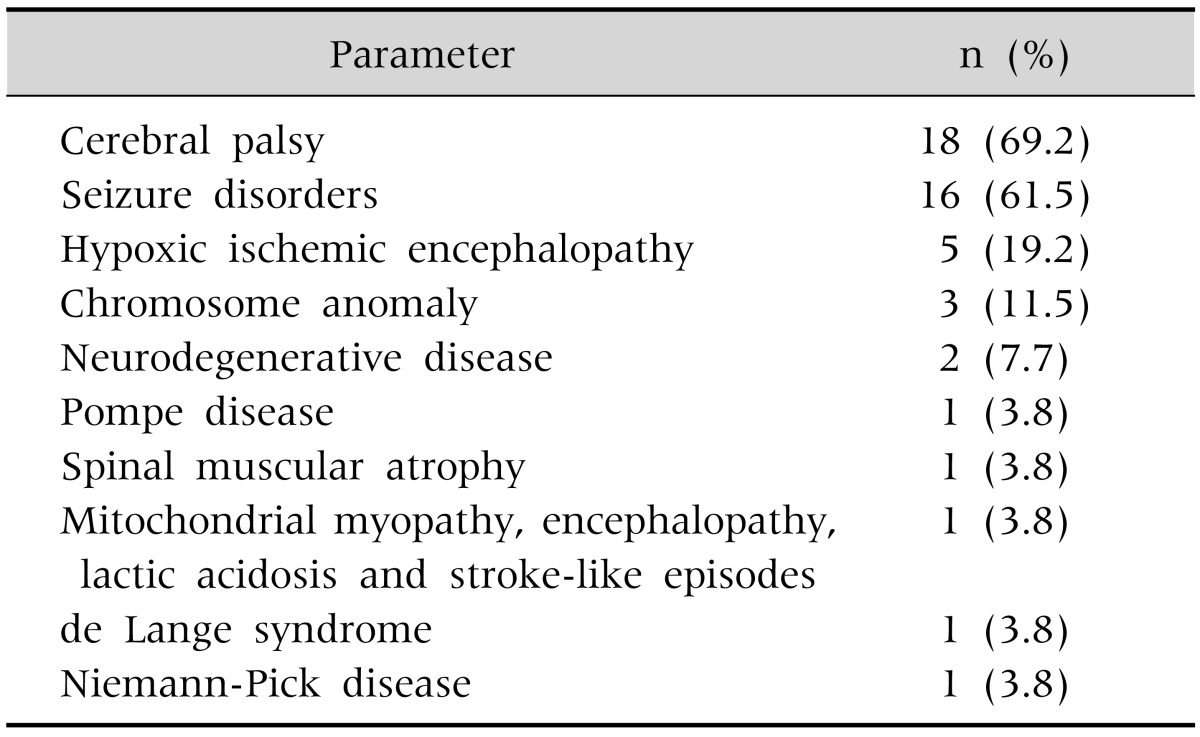
Table 4.
Indications for Percutaneous Endoscopic Gastrostomy (n=26)
Post-procedural complications
Complications developed in 22 of the 26 patients (84.6%), with no significant difference between the three groups. Of the 22 patients who had complications, 9 had major complications (34.6%). The incidence of major complications was 50.0%, 66.7%, and 0% in groups I, II, and III, respectively (p=0.005, Table 5). The incidence of peristomal infection was 20%, 50% and 0% in groups I, II, and III, respectively (p=0.04, Table 5). The main organisms that were cultured from the wounds were methicillin-resistant Staphylococcus aureus in 1 patient and Pseudomonas aeruginosa in 4 patients (as well as Klebsiella pneumonia in one of them). For the major complications, 1 patient in group I developed peristomal infection and buried bumper syndrome, and 1 patient in group II experienced peristomal infection and granulation tissue formation. The most common minor complications were stomal leakage, granulation tissue, peristomal infection, pneumoperitoneum, and ileus in order of frequency, with no significant difference in incidence between the three groups (Table 6). All patients who developed major complications also had other minor complications. Multiple complications occurred in 13 patients (50.0%), which consisted of 2 patients (7.7%) with 2 major complications, 9 patients (34.6%) with a major and minor complication, and 11 patients (42.3%) with 2 minor complications.
Table 5.
Incidence of Major Complications Related to Percutaneous Endoscopic Gastrostomy
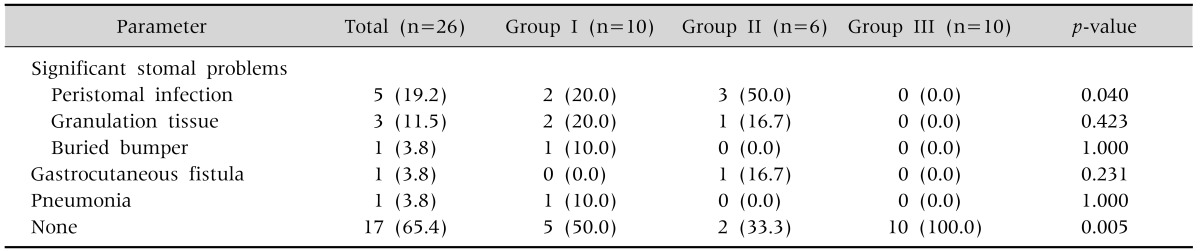
Values are presented as number (%).
Group I: patients underwent the Ponsky-Gauderer pull technique from December 2005 to July 2010, group II: patients underwent large-caliber trocar introducer percutaneous endoscopic gastrostomy from August 2010 to June 2012, group III: patients underwent T-fastener gastropexy from July 2012 to June 2014.
Table 6.
Incidence of Minor Complications Related to Percutaneous Endoscopic Gastrostomy
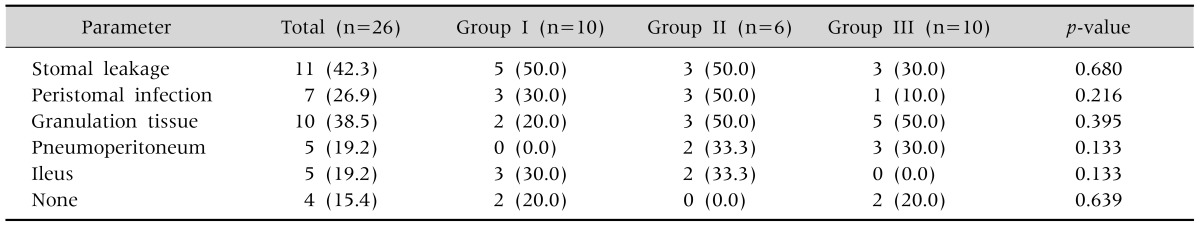
Values are presented as number (%).
Group I: patients underwent the Ponsky-Gauderer pull technique from December 2005 to July 2010, group II: patients underwent large-caliber trocar introducer percutaneous endoscopic gastrostomy from August 2010 to June 2012, group III: patients underwent T-fastener gastropexy from July 2012 to June 2014.
Post-procedural fasting period, pain control frequency, and duration of intravenous antibiotic therapy
The mean fasting period after the procedure was the longest in group II, at 63.8 hours (20-90 hours), but there was no significant difference between the three groups (Table 7). The mean pain control frequency was 1.5 times (0-8 times) in group I, 1.5 times (0-3 times) in group II, and 1 time (0-5 times) in group III, showing the lowest frequency for group III, but without statistical significance (Table 7). The mean duration of antibiotic therapy was 6 days (3-21 days), with no significant difference between group I and group II (Table 7).
Table 7.
Clinical Characteristics Related to Percutaneous Endoscopic Gastrostomy
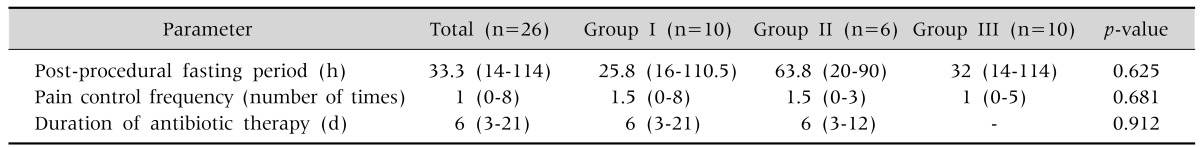
Values are presented as median (range).
Group I: patients underwent the Ponsky-Gauderer pull technique from December 2005 to July 2010, group II: patients underwent large-caliber trocar introducer percutaneous endoscopic gastrostomy from August 2010 to June 2012, group III: patients underwent T-fastener gastropexy from July 2012 to June 2014.
DISCUSSION
In this study, we compared the short-term complications and prognosis between patients undergoing PEG using three different placement techniques: the Ponsky-Gauderer pull technique, the large-caliber trocar introducer PEG technique, and the T-fastener gastropexy technique. The T-fastener gastropexy technique was associated with the lowest incidence of peristomal infection. The incidence of major complications was the highest in the large-caliber trocar introducer PEG group and lowest in the T-fastener gastropexy group. The incidence of all major complications was not significantly different between the Ponsky-Gauderer pull technique and the large-caliber trocar introducer PEG technique, while the T-fastener gastropexy technique was associated with the lowest incidence of complications compared to the other two techniques.
Martins et al. [17] have reported that the gastropexy technique without prophylactic antibiotics was associated with a lower rate of peristomal infection than the pull technique with prophylactic antibiotics. A randomized study by Shigoka et al. [18] that prospectively compared the modified introducer method and the pull methods reported a lower incidence of peristomal infection when using the modified introducer method, but this was not statistically significant. A study by Maetani et al. [19] showed a lower risk of peristomal infection when using the introducer technique compared to the pull technique. It seems that the pull technique is associated with a higher rate of peristomal infection than the introducer technique because the gastrostomy tube passes the oral cavity, which is colonized by normal flora. In our study, we also found a lower incidence of peristomal infection using the T-fastener gastropexy technique compared to the pull technique. However, the large-caliber trocar introducer PEG technique was associated with a higher rate of peristomal infection compared to the pull technique, suggesting no advantage of using large-caliber trocars in pediatric patients. This will need to be confirmed with further studies.
In a meta-analysis, the incidence of peristomal infection was 10.7% for the pull technique and 0.9% for the introducer technique [20]. In our study, the incidence of peristomal infection for the pull technique was 20%, which was higher than that reported by the meta-analysis, and the incidence was 50% when the large-caliber trocar introducer technique was used, higher than that reported in the meta-analysis. However, we did not observe any case of peristomal infection in the T-fastener gastropexy group, which was similar to the results of the meta-analysis.
The overall incidence of complications was lower for the push technique than the pull technique in the study by Tucker et al. [21]. A study by Köhler et al. [12] reported that the push technique was associated with a higher incidence of complications, such as migration of the gastrostomy tube and blockage of the tube, than the pull technique. In our study, we did not identify a significant difference in the overall incidence of all complications between the three different PEG placement techniques. Taylor et al. [22] reported a 70% incidence rate of PEG complications, most of which occurred within the first 3 months, and of which 88% were minor complications. In the present study, we investigated complications that occurred within the first 10 weeks, and the incidence of complications was 84.6%. Of these, 34.6% were major complications, all of which were associated with other minor complications. Half of the patients had only minor complications, most of which were stomal leakage and granulation tissue formation. In a prospective study by Brewster et al. [23], where patients underwent the modified introducer PEG technique, the incidence of major complications associated with PEG was 14%, which was lower than that of our study. However, most of the major complications in our study were peristomal infections requiring systemic antibiotic therapy. On the contrary, peristomal infection and tube dislodgement or migration each accounted for approximately half of all major complications in the study by Brewster et al. [23]. The incidence of major complications associated with PEG in pediatric patients in the literature ranges between 12.6% and 17.5%, and that of procedure-related death is between 0.2% and 0.9% [24,25,26,27]. In our study, there were 5 cases of complications (19.2%) that required surgical intervention, but there were no cases of death or need for blood transfusion.
Cerebral palsy and hypoxemic ischemic brain injury are the most common underlying disease in patients who require PEG, which is usually associated with severe spasticity and stenosis or deformity of the opening of the esophagus due to prolonged poor feeding. In the introducer technique, the gastrostomy tube is inserted into the stomach through the abdominal wall under endoscopic guidance. Hence, unlike the pull technique (where the gastrostomy tube is tied to the guide wire, inserted into the stomach, and then pulled out through the abdominal wall), it can be performed in handicapped children possessing the above problems. It can also be performed using a small-diameter endoscope under sedation and local anesthesia, in patients who develop low oxygen saturation when using endoscopes with larger diameters. In the present study, 46% of patients underwent PEG under sedation in the endoscopy unit.
The most common indication for PEG is neurodevelopmental disorder with oral motor dysfunction [28]. In our study, 69.2% of patients underwent PEG owing to dysphagia associated with cerebral palsy and epileptic disease. Pediatric patients with neurodevelopmental disorders frequently require anti-reflux surgery. Laparoscopic fundoplication was performed in 4 patients in our study.
Although prophylactic antibiotic therapy before the procedure does not significantly reduce local infection in adult and geriatric patients, it can reduce the infection rate in pediatric patients [29]. A few studies have supported the effectiveness of prophylactic antibiotics in reducing infection [30,31,32]. In our study, 84.6% of patients received prophylactic antibiotics, and only 25% of the 15.4% of patients who did not receive prophylactic antibiotics developed local infection. Therefore, the benefit of prophylactic antibiotics is not clear and will need to be confirmed by further studies.
Nutrition can be safely delivered 24 hours after PEG placement. PEG feeding can begin 3 hours after tube placement if there are no problems [33]. However, the delivery of nutrition is delayed if there are complications such as bleeding or swelling at the procedure site, paralytic ileus, or fever. In our study, the fasting period was not significantly different between the three groups, but was found to be the shortest in the Ponsky-Gauderer technique group and the longest in the large-caliber trocar introducer PEG group. This is likely associated with the higher incidence of major complications in the large-caliber trocar introducer PEG group.
The pain control frequency was lowest in the T-fastener gastropexy group, even though the difference was not significant between the three groups. There is a possibility that the other two techniques were associated with larger skin incisions from using relatively larger gastrostomy tubes. In addition, the large-caliber trocar introducer PEG technique involves simultaneous penetration of the abdominal wall and the gastric wall using a trocar larger than the gastrostomy tube. In contrast, in the T-fastener gastropexy technique, the peel-away sheath dilator is systemically expanded from smaller to larger diameters, and associated with a shorter incision and less tissue damage.
This study has several limitations: the small number of cases from a single institution, retrospective study design, and the fact that the three techniques were performed at different periods, allowing for variation in the experience and skills of the practitioner. Further, the caregivers (parents or employee of the institution) and quality of the environment differed after they were discharged, which may have influenced on the occurrence of complications. These could not be controlled in our study.
In conclusion, the incidence of major complications that required medical or surgical intervention was lowest in the T-fastener gastropexy group, while there was no significant difference in the incidence of minor complications between the three PEG techniques. This group also had the lowest incidence of peristomal infection, which supports the use of this method as a first choice in pediatric patients. Future multicenter studies recruiting a larger number of patients that compare the short- and long-term complications after undergoing PEG with the different techniques will be necessary.
References
- 1.Abuksis G, Mor M, Plaut S, Fraser G, Niv Y. Outcome of percutaneous endoscopic gastrostomy (PEG): comparison of two policies in a 4-year experience. Clin Nutr. 2004;23:341–346. doi: 10.1016/j.clnu.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Gauderer MW, Ponsky JL, Izant RJ., Jr Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15:872–875. doi: 10.1016/s0022-3468(80)80296-x. [DOI] [PubMed] [Google Scholar]
- 3.Ponsky JL, Gauderer MW. Percutaneous endoscopic gastrostomy: a nonoperative technique for feeding gastrostomy. Gastrointest Endosc. 1981;27:9–11. doi: 10.1016/s0016-5107(81)73133-x. [DOI] [PubMed] [Google Scholar]
- 4.El-Matary W. Percutaneous endoscopic gastrostomy in children. Can J Gastroenterol. 2008;22:993–998. doi: 10.1155/2008/583470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch CR, Fang JC. Prevention and management of complications of percutaneous endoscopic gastrostomy (PEG) tubes. Pract Gastroenterol. 2004;28:66–76. [Google Scholar]
- 6.Lee SP, Lee KN, Lee OY, Lee HL, Jun DW, Yoon BC, et al. Risk factors for complications of percutaneous endoscopic gastrostomy. Dig Dis Sci. 2014;59:117–125. doi: 10.1007/s10620-013-2891-7. [DOI] [PubMed] [Google Scholar]
- 7.Russell TR, Brotman M, Norris F. Percutaneous gastrostomy. A new simplified and cost-effective technique. Am J Surg. 1984;148:132–137. doi: 10.1016/0002-9610(84)90300-3. [DOI] [PubMed] [Google Scholar]
- 8.Campoli PM, Cardoso DM, Turchi MD, Ejima FH, Mota OM. Assessment of safety and feasibility of a new technical variant of gastropexy for percutaneous endoscopic gastrostomy: an experience with 435 cases. BMC Gastroenterol. 2009;9:48. doi: 10.1186/1471-230X-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terry NE, Boswell WC, Carney DE, Beck A, Lowe L, Rittmeyer C. Percutaneous endoscopic gastrostomy with T-bar fixation in children and infants. Surg Endosc. 2008;22:167–170. doi: 10.1007/s00464-007-9402-x. [DOI] [PubMed] [Google Scholar]
- 10.Fox VL, Abel SD, Malas S, Duggan C, Leichtner AM. Complications following percutaneous endoscopic gastrostomy and subsequent catheter replacement in children and young adults. Gastrointest Endosc. 1997;45:64–71. doi: 10.1016/s0016-5107(97)70304-3. [DOI] [PubMed] [Google Scholar]
- 11.Fortunato JE, Troy AL, Cuffari C, Davis JE, Loza MJ, Oliva-Hemker M, et al. Outcome after percutaneous endoscopic gastrostomy in children and young adults. J Pediatr Gastroenterol Nutr. 2010;50:390–393. doi: 10.1097/MPG.0b013e3181aed6f1. [DOI] [PubMed] [Google Scholar]
- 12.Köhler G, Kalcher V, Koch OO, Luketina RR, Emmanuel K, Spaun G. Comparison of 231 patients receiving either "pull-through" or "push" percutaneous endoscopic gastrostomy. Surg Endosc. 2014 doi: 10.1007/s00464-014-3673-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Liu R, Jiwane A, Varjavandi A, Kennedy A, Henry G, Dilley A, et al. Comparison of percutaneous endoscopic, laparoscopic and open gastrostomy insertion in children. Pediatr Surg Int. 2013;29:613–621. doi: 10.1007/s00383-013-3313-9. [DOI] [PubMed] [Google Scholar]
- 14.Ljungdahl M, Sundbom M. Complication rate lower after percutaneous endoscopic gastrostomy than after surgical gastrostomy: a prospective, randomized trial. Surg Endosc. 2006;20:1248–1251. doi: 10.1007/s00464-005-0757-6. [DOI] [PubMed] [Google Scholar]
- 15.Pennington C. To PEG or not to PEG. Clin Med. 2002;2:250–255. doi: 10.7861/clinmedicine.2-3-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim CH, Park JM, Cho YK, Lee IS, Kim SW, Choi MG, et al. Successful control of peristomal infection by introducer-type percutaneous endoscopic gastrostomy: a retrospective historical control study. Dig Dis Sci. 2011;56:2024–2029. doi: 10.1007/s10620-011-1570-9. [DOI] [PubMed] [Google Scholar]
- 17.Martins FP, Sousa MC, Ferrari AP. New "introducer" PEG-gastropexy with T fasteners: a pilot study. Arq Gastroenterol. 2011;48:231–235. doi: 10.1590/s0004-28032011000400003. [DOI] [PubMed] [Google Scholar]
- 18.Shigoka H, Maetani I, Tominaga K, Gon K, Saitou M, Takenaka Y. Comparison of modified introducer method with pull method for percutaneous endoscopic gastrostomy: prospective randomized study. Dig Endosc. 2012;24:426–431. doi: 10.1111/j.1443-1661.2012.01317.x. [DOI] [PubMed] [Google Scholar]
- 19.Maetani I, Tada T, Ukita T, Inoue H, Sakai Y, Yoshikawa M. PEG with introducer or pull method: a prospective randomized comparison. Gastrointest Endosc. 2003;57:837–841. doi: 10.1016/s0016-5107(03)70017-0. [DOI] [PubMed] [Google Scholar]
- 20.Campoli PM, de Paula AA, Alves LG, Turchi MD. Effect of the introducer technique compared with the pull technique on the peristomal infection rate in PEG: a meta-analysis. Gastrointest Endosc. 2012;75:988–996. doi: 10.1016/j.gie.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Tucker AT, Gourin CG, Ghegan MD, Porubsky ES, Martindale RG, Terris DJ. 'Push' versus 'pull' percutaneous endoscopic gastrostomy tube placement in patients with advanced head and neck cancer. Laryngoscope. 2003;113:1898–1902. doi: 10.1097/00005537-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Taylor CA, Larson DE, Ballard DJ, Bergstrom LR, Silverstein MD, Zinsmeister AR, et al. Predictors of outcome after percutaneous endoscopic gastrostomy: a community-based study. Mayo Clin Proc. 1992;67:1042–1049. doi: 10.1016/s0025-6196(12)61118-5. [DOI] [PubMed] [Google Scholar]
- 23.Brewster BD, Weil BR, Ladd AP. Prospective determination of percutaneous endoscopic gastrostomy complication rates in children: still a safe procedure. Surgery. 2012;152:714–719. doi: 10.1016/j.surg.2012.07.018. discussion 719-21. [DOI] [PubMed] [Google Scholar]
- 24.Gauderer MW. Percutaneous endoscopic gastrostomy: a 10-year experience with 220 children. J Pediatr Surg. 1991;26:288–292. doi: 10.1016/0022-3468(91)90504-m. discussion 292-4. [DOI] [PubMed] [Google Scholar]
- 25.Khattak IU, Kimber C, Kiely EM, Spitz L. Percutaneous endoscopic gastrostomy in paediatric practice: complications and outcome. J Pediatr Surg. 1998;33:67–72. doi: 10.1016/s0022-3468(98)90364-5. [DOI] [PubMed] [Google Scholar]
- 26.Avitsland TL, Kristensen C, Emblem R, Veenstra M, Mala T, Bjørnland K. Percutaneous endoscopic gastrostomy in children: a safe technique with major symptom relief and high parental satisfaction. J Pediatr Gastroenterol Nutr. 2006;43:624–628. doi: 10.1097/01.mpg.0000229550.54455.63. [DOI] [PubMed] [Google Scholar]
- 27.Vervloessem D, van Leersum F, Boer D, Hop WC, Escher JC, Madern GC, et al. Percutaneous endoscopic gastrostomy (PEG) in children is not a minor procedure: risk factors for major complications. Semin Pediatr Surg. 2009;18:93–97. doi: 10.1053/j.sempedsurg.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Van Biervliet S, Van Renterghem K, Vande Putte D, Vande Velde S, De Bruyne R, Van Winckel M. Gastrostomy use in children: a 3-year single centre experience. Acta Gastroenterol Belg. 2014;77:8–12. [PubMed] [Google Scholar]
- 29.Fröhlich T, Richter M, Carbon R, Barth B, Köhler H. Review article: percutaneous endoscopic gastrostomy in infants and children. Aliment Pharmacol Ther. 2010;31:788–801. doi: 10.1111/j.1365-2036.2010.04246.x. [DOI] [PubMed] [Google Scholar]
- 30.Lipp A, Lusardi G. Systemic antimicrobial prophylaxis for percutaneous endoscopic gastrostomy. Cochrane Database Syst Rev. 2006;(4):CD005571. doi: 10.1002/14651858.CD005571.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25:647–656. doi: 10.1111/j.1365-2036.2007.03247.x. [DOI] [PubMed] [Google Scholar]
- 32.Lipp A, Lusardi G. A systematic review of prophylactic antimicrobials in PEG placement. J Clin Nurs. 2009;18:938–948. doi: 10.1111/j.1365-2702.2008.02585.x. [DOI] [PubMed] [Google Scholar]
- 33.Choudhry U, Barde CJ, Markert R, Gopalswamy N. Percutaneous endoscopic gastrostomy: a randomized prospective comparison of early and delayed feeding. Gastrointest Endosc. 1996;44:164–167. doi: 10.1016/s0016-5107(96)70134-7. [DOI] [PubMed] [Google Scholar]


