Abstract
Purpose
Non-alcoholic fatty liver disease (NAFLD) in children has become an important public health issue because of its high prevalence and severity. Several noninvasive methods for estimating NAFLD are under investigation. We aimed to evaluate the usefulness of serum ferritin as a biomarker of severity of pediatric NAFLD patients.
Methods
A total of 64 NAFLD patient were enrolled from Severance Children's Hospital from March 2010 to February 2013. Serum ferritin levels, liver related laboratory tests, liver magnetic resonance imaging (MRI) (2-dimensional [2D] proton density-fat fraction) and NAFLD severity markers were compared between obese group and overweight group. Correlation analyses were performed between serum ferritin and laboratory values including NAFLD severity markers.
Results
In obese group, serum ferritin, alanine aminotransferase (ALT), total bilirubin, international normalized ratio (INR), MRI 2D proton density-fat fraction, aspartate aminotransferase (AST) to platelet ratio index (APRI) and fibrosis-4 (FIB-4) (an index score calculated from platelet count, ALT, AST and age) were significantly higher than those of overweight group. NAFLD severity markers, APRI and FIB-4, and liver specific important laboratory values, AST, ALT, INR, cholesterol, triglyceride and low density lipoprotein show significant correlation with serum ferritin in NAFLD patients.
Conclusion
Serum ferritin concentrations could be a candidate of useful severity marker in the pediatric NAFLD patients.
Keywords: Non-alcoholic fatty liver disease, Child, Ferritins, Biological markers, Obesity
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum of liver diseases ranging from hepatocellular steatosis to irreversible liver cirrhosis and is closely related to obesity and metabolic syndrome [1]. Metabolic syndrome is characterized by obesity, hyperlipidemia, diabetes mellitus (DM), and insulin resistance. In adults, as well as hypertension, obesity, and DM, the frequency of metabolic syndrome is increasing gradually. Metabolic syndrome can be manifested as NAFLD in the liver [2,3,4]. The prevalence of NAFLD is also increasing due to the increase in the rate of metabolic syndrome. NAFLD is raising the morbidity and mortality rates associated with the liver [5]. In western countries, the morbidity of NAFLD is 20-30% of total adults and 90% of obese adults. Moreover, non-alcoholic steatohepatitis (NASH) is affecting an estimated 2-3% of the general population and up to 37% of obese individuals [1]. Approximately 10% of patients with NAFLD progress to NASH, and 8-26% of NASH patients progress to cirrhosis [2].
The prevalence of NAFLD in children and young adults is also increasing rapidly. Studies have reported about 3% prevalence of NAFLD in the general pediatric population and 53% in obese children [1]. Due to the Westernized lifestyle and diet, pediatric NAFLD is expected to increase significantly in the future globally. Furthermore, NAFLD can have more severe influence to children due to longer duration of the disease. Therefore, as with adults, the importance of the early diagnosis and proper treatment of NAFLD and NASH in children is increasing in order to prevent liver cirrhosis and hepatocellular carcinoma.
NAFLD can be diagnosed after the exclusion of other liver diseases and identifying image studies of the fatty liver [6]. To diagnose NASH, various methods-including magnetic resonance imaging (MRI) proton density-fat fraction (PDFF), which has been actively studied recently-are being used. MRI PDFF is a non-invasive and quantitative means of quantifying hepatic steatosis in patients with NAFLD [7]. However, the exact cut-off value has yet not been established, and the MRI equipment itself is not easily obtained due to its high price. Confirmation of the disease can be made only by pathology, which is characterized as steatosis, lobular inflammation, and hepatocellular ballooning [2,8]. However, liver biopsy is invasive in children. Furthermore, there is the possibility of sampling error of liver biopsy specimens [9]. Therefore, to follow up on the progression of NAFLD, repeated liver biopsies are nearly impossible, especially for children. Thus, researchers are seeking non-invasive and cost-effective tools for the diagnosis of NAFLD and NASH. They are making efforts to develop biological markers to predict the progression from simple fatty liver to NASH, liver fibrosis, cirrhosis, and hepatocellular carcinoma [10]. In recent adult studies, serum ferritin levels were reported to be an independent predictor of advanced hepatic fibrosis in patients with NAFLD based on its correlation with hepatic inflammation and hepatic iron storage [10]. Serum ferritin levels are known to be elevated in patients with NAFLD and seem to be related to insulin resistance and hepatocyte damage [11]. So researchers are interested in serum ferritin as a biomarker that can reflect the inflammatory change of the liver for children as well. In fact, pediatric gastroenterologists are more earnestly seeking simple and noninvasive biologic markers for NAFLD. If simple and non-invasive biologic markers for NAFLD are found, it will be very helpful in disease management due to improved compliance of follow-up. Also early detection or screening could be possible for the pediatric NAFLD patients who may progress to liver cirrhosis. Proper medical treatment to the patients could be provided at an appropriate time accordingly. We also can expect an additional effect that might reduce patient's medical expenses from other expensive and invasive diagnostic methods and its possible complications. For these reasons, we were to investigate the clinical significance of serum ferritin in pediatric NAFLD patients as a candidate of effective and less-invasive biological marker.
MATERIALS AND METHODS
Study design
A total of 64 patients diagnosed as NAFLD at Severance Children's Hospital, Seoul, Korea, from March 2010 to February 2013 were included in this study. We selected patients whose repetitive alanine aminotransferase (ALT) was abnormal (30 U/L or more for men and 19 U/L or more for women) [12] for unknown reasons in the regular check-up. Patients with ALT elevation for certain reasons (e.g., hepatitis B, hepatitis C, hepatitis A, Wilson's disease, hemochromatosis, etc.) were excluded. No patients in the population reported active substance abuse or significant systemic illnesses or were taking drugs known to cause hepatic steatosis. All patients had a diagnosis of NAFLD based on cryptogenic etiology and MRI-PDFF imaging with steatosis >5% [7]. Laboratory tests (e.g., iron profiles, liver function tests, and lipid profiles), MRI PDFF values were obtained from patients' medical records and analyzed. NAFLD severity markers-such as the aspartate aminotransferase (AST) to platelet (PLT) ratio index (APRI), AST/ALT ratio (AAR), and fibrosis-4 (FIB-4)-that are known as predictors of liver fibrosis were also calculated and analyzed [11,13].
1. Differences between obese and overweight group
We aimed to evaluate whether the body mass index (BMI) influences the variables associated liver and serum ferritin. Therefore, we examined the differences in the laboratory values, MRI PDFF values and NAFLD severity makers between overweight and obese groups, which were divided by BMI percentile. Obesity was defined as a BMI in the 95th percentile or higher, while overweight was defined as a BMI in the 85th-95th percentile considering age and sex.
2. Correlation of laboratory values and NAFLD severity markers with serum ferritin level
To evaluate the significance of serum ferritin level, correlation between several liver specific laboratory values, MRI 2-dimensional (2D) PDFF and NAFLD severity markers with serum ferritin were analyzed.
Statistical analysis
Baseline characteristics were presented as the median and range in parentheses. The obese group and overweight group were compared using Mann-Whitney U test. Correlations between laboratory and serum ferritin were assessed by Spearman analysis. For better understanding, the scatter plot of NAFLD severity markers was displayed with Spearman correlation coefficient. All tests were two-sided and a p-value of 0.05 was considered to be statistically significant. Also, statistical analyses were conducted with IBM SPSS Statistics version 20.0 (IBM Co., Armonk, NY, USA). The study was approved by the institutional review board of our institution, and it was conducted in compliance with the Declaration of Helsinki.
RESULTS
Characteristics of pediatric NAFLD patients
According to the patients' BMIs, all included patients were labeled as more than overweight. The laboratory test results and patient characteristics of the 64 NAFLD patients are presented in Table 1. In the table, the patients were divided into two groups, obese and overweight, according to their BMI. All the variables were described with the median and range in parentheses. Other variables shows no significant differences except the median value of MRI 2D PDFF between the two groups (p=0.0194). APRI and FIB showed significantly high values in the obese group (p=0.0463 and p=0.001, respectively).
Table 1.
Characteristics of Patients of Non-Alcoholic Fatty Liver Disease (NAFLD) Children
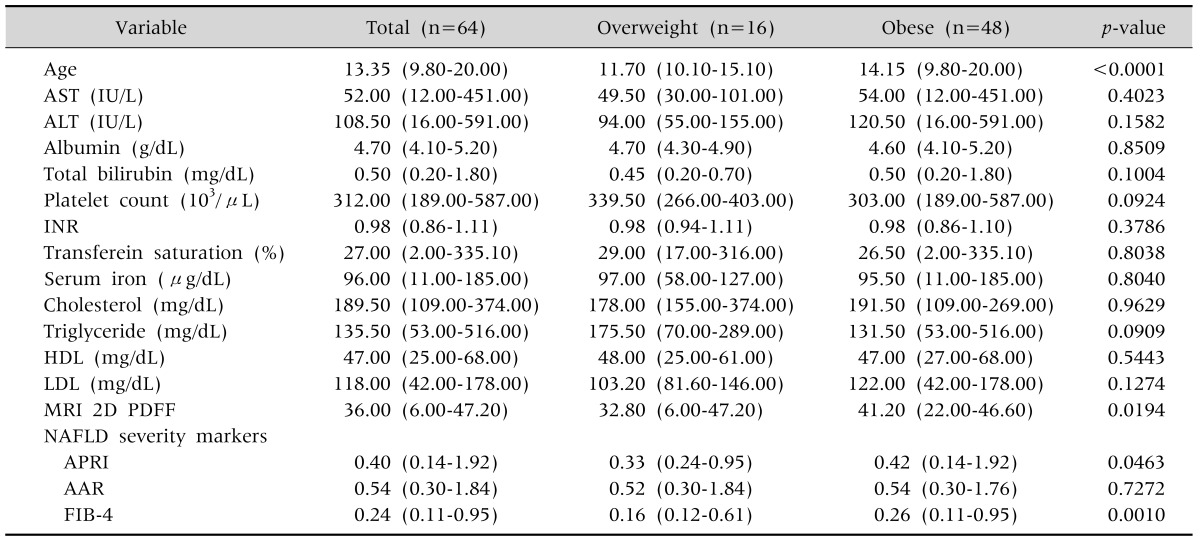
Values are presented as median (minimum-maximum).
AST: aspartate aminotransferase, ALT: alanine aminotransferase, INR: international normalized ratio, HDL: high density lipoprotein, LDL: low density lipoprotein, MRI 2D PDFF: magnetic resonance imaging 2-dimensional proton density-fat fraction, APRI: AST to platelet ratio index, AAR: AST/ALT ratio, FIB-4: fibrosis-4.
1. Differences between obese and overweight group
There were 48 patients in the obese group and 16 in the overweight group. As shown in Table 2, the values of ALT, total bilirubin, ferritin, and MRI 2D PDFF were significantly higher in the obese group than the overweight group (p<0.05). The values of the NAFLD severity markers, APRI and FIB-4, were 0.61±0.42 and 0.30±0.16, respectively, in the obese group, which are both significantly higher than the corresponding values for the overweight group.
Table 2.
Laboratory Value Differences between Obese and Overweight Group
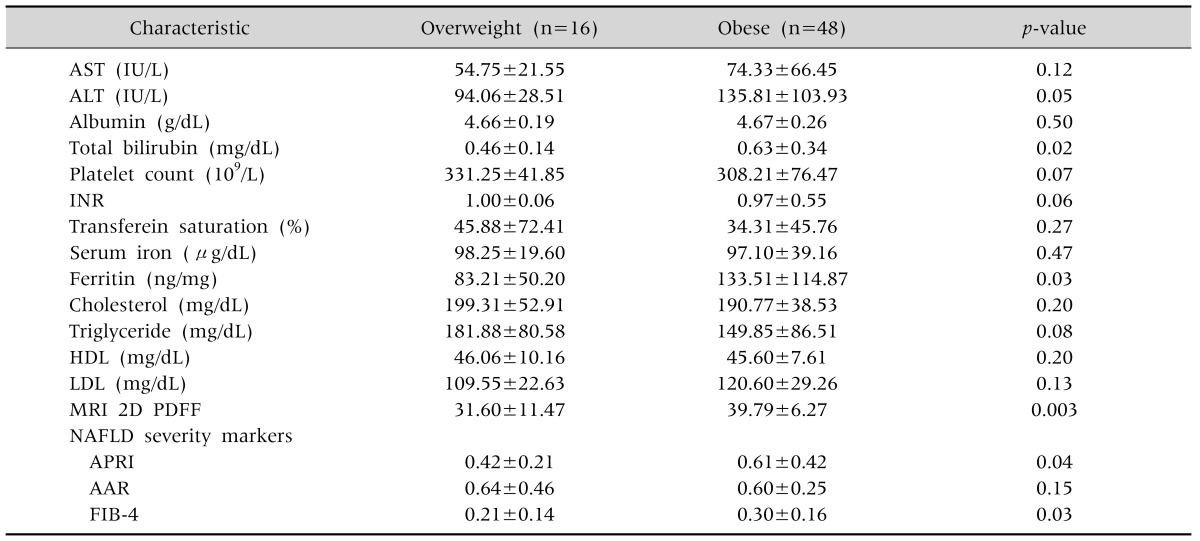
Values are presented as mean±standard deviation.
AST: aspartate aminotransferase, ALT: alanine aminotransferase, INR: international normalized ratio, HDL: high density lipoprotein, LDL: low density lipoprotein, MRI 2D PDFF: magnetic resonance imaging 2-dimensional proton density-fat fraction, NAFLD: non-alcoholic fatty liver disease, APRI: AST to platelet ratio index, AAR: AST/ALT ratio, FIB-4: fibrosis-4.
2. Correlation between laboratory values and serum ferritin
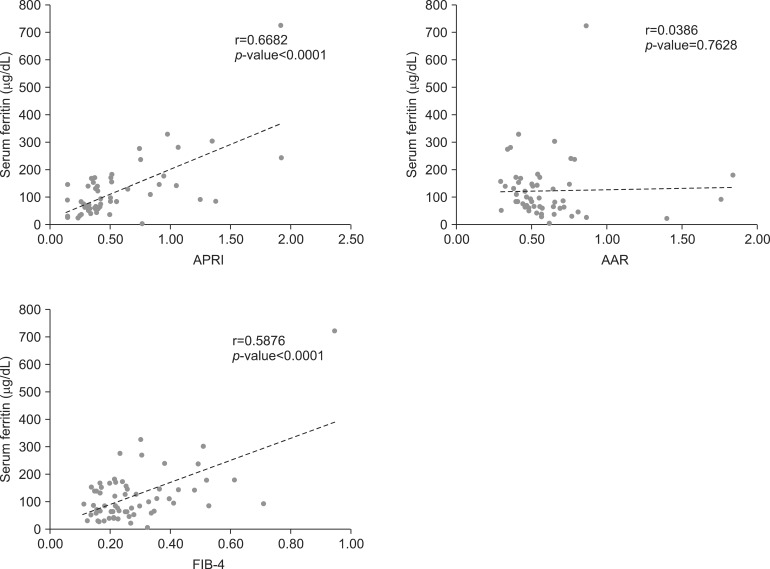
As shown in Table 3, AST, ALT, international normalized ratio, cholesterol, triglyceride and low density lipoprotein showed significant correlations with serum ferritin. The correlation coefficients of the NAFLD severity markers, APRI and FIB-4, were 0.6682 (0.5014-0.7830) and 0.5876 (0.3956-0.7261) respectively, which were statistically significant. AAR did not show any significant correlation with serum ferritin. Likewise, the scatter plot of APRI and FIB-4 tended to increase as the serum ferritin increased significantly (p<0.0001 and p<0.0001, respectively) (Fig. 1). In addition, there was a significant correlation between BMI and serum ferritin (Table 3).
Table 3.
Correlation between Laboratory Values and Serum Ferritin
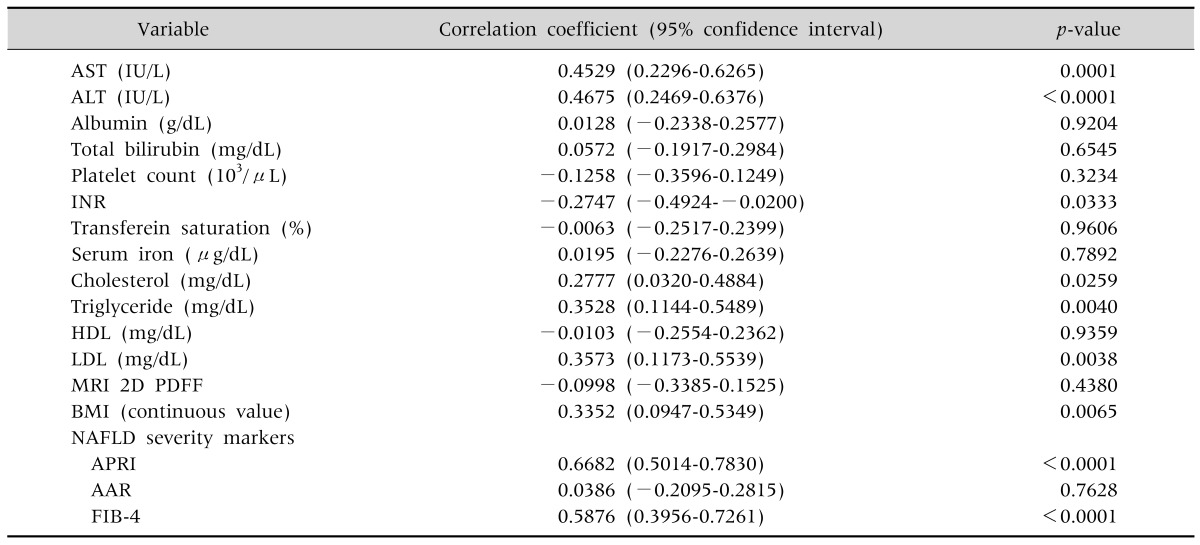
AST: aspartate aminotransferase, ALT: alanine aminotransferase, INR: international normalized ratio, HDL: high density lipoprotein, LDL: low density lipoprotein, MRI 2D PDFF: magnetic resonance imaging 2-dimensional proton density-fat fraction, NAFLD: non-alcoholic fatty liver disease, BMI: body mass index, NAFLD: non-alcoholic fatty liver disease, APRI: AST to platelet ratio index, AAR: AST/ALT ratio, FIB-4: fibrosis-4.
Fig. 1.
(A) Scatter plot between APRI and serum ferritin. (B) Scatter plot between AAR and serum ferritin. (C) Scatter plot between FIB-4 and serum ferritin. The scatter plot of APRI and FIB-4 tended to increase as the serum ferritin increased significantly (p<0.0001 and p<0.0001, respectively). AST: aspartate aminotransferase, APRI: AST to platelet ratio index, ALT: alanine aminotransferase, AAR: AST/ALT ratio, FIB-4: fibrosis-4.
DISCUSSION
Recently, NAFLD has become a very important disease in the pediatric population. As liver biopsies are invasive, reliable and noninvasive biomarkers are essential for examining pediatric NAFLD. In this study, we examined whether serum ferritin could be an effective and less-invasive biological marker that could be applied to pediatric patients to predict their disease severity.
The three-hit hypothesis is the widely known NAFLD pathogenesis that a progression to NASH and fibrosis depends on additional factors such as free fatty acids, inflammatory cytokines and adipokines, oxidative stress, and mitochondrial dysfunction in the base of obesity and insulin resistance [1]. Serum ferritin is the primary tissue for iron-storage protein in the liver, where most extra body iron is stored. Ferritin levels can be elevated secondary to obesity, chronic alcohol consumption, chronic inflammation including viral hepatitis, histiocytic neoplasm, and steatohepatitis [10]. Hyperferritinemia has been previously observed in obesity-related chronic inflammatory conditions such as DM, metabolic syndrome, liver cirrhosis, and NAFLD [10]. As the ferritin concentration increases, the risk of significant liver disease also increases. In NAFLD, increased ferritin levels are considered an expression of metabolic syndrome and of hepatic damage, because of inflammatory cytokine activation [12]. In addition, considering the pathophysiology from a different standpoint, the histological evidence of hepatic iron accumulation has been reported to be strongly associated with a hepatic fibrosis in NAFLD patients in large multicenter studies [14]. Hepatic iron accumulation produces inflammatory cytokines, and they induce hepatic fibrosis [8]. According to another adult study by Kowdley et al. [10], the histological features of NAFLD, which include steatosis, hepatocellular ballooning, and fibrosis, were more severe in patients with increased serum ferritin. They concluded that serum ferritin is associated with hepatic iron deposition and worsened histological activity in patients with NAFLD. However, they also said that hyperferritinemia was associated with the histologic findings of NAFLD patients whose histological findings did not include detectable iron deposition.
In this study, the MRI 2D PDFF values and liver-related laboratory marker values, including serum ferritin, were higher in the obese group than the overweight group, which is concordant with the previous results of adult studies that indicated that NAFLD and NASH are positively correlated with obesity [2,8,15,16]. NAFLD can be divided into five grades according to the results of liver biopsy, from grade-0 to grade-4. Grade-4 indicates liver cirrhosis. According to Permutt et al. [7], the mean MRI 2D PDFF value increased significantly with histology-determined steatosis grade: <5.0% at grade-0, 5.0-8.9% at grade-1, 8.9-16.3% at grade-2, and 16.3-25.0% at grade-3; with p≤0.0001. However, no significant correlation in the MRI 2D PDFF values at grade-4 was found. They thought that this is because the results of the MRI 2D PDFF were well correlated with low-grade hepatic steatosis, but steatosis was not linearly correlated with NAFLD progression. Hepatic steatosis may be replaced by collagen in severe NAFLD or cirrhosis. As a result, in severe NAFLD or cirrhosis, low hepatic steatosis could be found, and there might be no differences between MRI 2D PDFF in cirrhosis and MRI 2D PDFF in grade-1 steatosis. Therefore, in patients with NAFLD, a low amount of hepatic steatosis on imaging may not indicate mild NAFLD, so other parameters should be considered to distinguish low-grade steatosis from liver cirrhosis [7]. In this study, we tried to separate the whole population into five grades (grade 0-4). However, our population was divided into two of grade-0, two of grade-1, 11 of grade-2, and 51 of grade-3, so there were some limitations to our ability to show the relationship between MRI 2D PDFF and serum ferritin, because of the largely deviated populations of the groups. The reason that the correlation between serum ferritin and MRI 2D PDFF was not good could be explained as the severity of our patients was higher (the median of the MRI 2D PDFF is 36, the average is 33.68).
Although liver biopsy remains the gold standard for the diagnosis and staging of NASH, we could not perform liver biopsies on all patients. Therefore, we applied NAFLD severity markers. Using APRI scores, McPherson et al. [17] demonstrated an area under the receiver operating characteristic curve (AUROC) of 0.67 for advanced fibrosis in a study of 145 adult NAFLD subjects. The AUROC values for AAR and FIB-4 were found to be 0.742 and 0.802, respectively, for advanced fibrosis in another study of 541 NAFLD patients [18]. In our study, the correlation coefficients of APRI and FIB-4 showed 0.6682 (0.5014-0.7830) and 0.5876 (0.3956-0.7261), respectively. They both showed good statistical correlation with serum ferritin (p<0.0001). These facts suggest the clinical usefulness of serum ferritin in pediatric NAFLD patients. We think that these NAFLD markers can make up for some of the limitations of our study. By using BMI as a continuous value, we found a good correlation with serum ferritin. These results show that serum ferritin is correlated with obesity and hepatocyte inflammation.
The pathophysiology of NAFLD is closely correlated with metabolic syndrome. Obese patients with Type 2 DM and NAFLD have been reported to have an 80% morbidity rate [19]. Ultrasound examinations are widely implemented as an initial non-invasive diagnostic imaging tool, and some reports indicate that ultrasonography results correlate with liver biopsy and liver-related blood test results [20]. However, it is an inadequate screening tool, because of the large deviation of results according to examiners and the risk of failure in diagnosing early fatty liver [11,16,19]. An MRI can provide a significantly higher sensitivity and specificity of diagnosis of NAFLD: 98% and 99%, respectively. However, patient compliance is low due to its high cost [5]. This is why serum ferritin arouses interest in many adult studies as an independent predictor of liver fibrosis. According to a study by Manousou et al. [12], serum ferritin has relatively high accuracy: 78-85% sensitivity and 60-67% specificity. Although serum ferritin alone is insufficient for diagnosing NAFLD, it could be used to rule out NAFLD in those with underlying obesity or DM and high serum AST and ALT levels. In addition, serum ferritin could be used as a marker for NAFLD patients to determine the appropriate time to undergo a liver biopsy for the screening of NASH or cirrhosis [12,21].
In recent studies, Plasma caspase-generated cytokeratin-18 fragments (CK-18) as a biological indicator other than serum ferritin were introduced, which is indicative of the index of the apoptosis of hepatocytes in NASH. It seems to show a high specificity for the diagnosis of NASH in several studies [22]. CK-18 is a major intermediate filament protein in the liver. Because of obesity-related liver damage, injured hepatocytes precede to apoptosis. Then, CK-18 fragments are released from hepatocytes and can be detected in serum [23,24]. However, according to a recent study of the multicenter, Cusi et al. [19], Plasma CK-18 was raised significantly with any increase in steatosis, inflammation, and fibrosis, but because of low sensitivity, there was significant vagueness in the prediction of disease severity. Therefore, more research is warranted for larger populations, to develop simple and non-invasive biological markers that can provide an alternative for the histologic results of liver biopsy.
In this study, we applied serum ferritin to pediatric NAFLD patients based on previous adult studies. The results of the comparison of various liver-related tests, MRI findings, and NAFLD severity markers showed that serum ferritin was related with the severity of NAFLD. However, due to the limitations of the pediatric population, we could not compare the liver biopsy tissue of each patient with their serum ferritin level. If we could obtain the liver biopsy results of the patients divide the patients into several grades according to their results, we could describe the diagnostic value of the serum ferritin in NAFLD patients more precisely; that is the limitation of this study. In summary, serum ferritin, as a biological indicator, could have a close correlation with the severity of NAFLD in children. However, when using serum ferritin as a noninvasive marker, other factors that can affect serum ferritin levels should be controlled.
References
- 1.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651–5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fon Tacer K, Rozman D. Nonalcoholic fatty liver disease: focus on lipoprotein and lipid deregulation. J Lipids. 2011;2011:783976. doi: 10.1155/2011/783976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Utzschneider KM, Largajolli A, Bertoldo A, Marcovina S, Nelson JE, Yeh MM, et al. Serum ferritin is associated with non-alcoholic fatty liver disease and decreased B-cell function in non-diabetic men and women. J Diabetes Complications. 2014;28:177–184. doi: 10.1016/j.jdiacomp.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowman JK, Tomlinson JW, Newsome PN. Systematic review: the diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2011;33:525–540. doi: 10.1111/j.1365-2036.2010.04556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam B, Younossi ZM. Treatment options for nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2010;3:121–137. doi: 10.1177/1756283X09359964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valenti L, Dongiovanni P, Fargion S. Diagnostic and therapeutic implications of the association between ferritin level and severity of nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:3782–3786. doi: 10.3748/wjg.v18.i29.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 10.Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, et al. NASH Clinical Research Network. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77–85. doi: 10.1002/hep.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:666–675. doi: 10.1038/nrgastro.2013.175. [DOI] [PubMed] [Google Scholar]
- 12.Manousou P, Kalambokis G, Grillo F, Watkins J, Xirouchakis E, Pleguezuelo M, et al. Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non-alcoholic fatty liver disease patients. Liver Int. 2011;31:730–739. doi: 10.1111/j.1478-3231.2011.02488.x. [DOI] [PubMed] [Google Scholar]
- 13.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 14.Nelson JE, Wilson L, Brunt EM, Yeh MM, Kleiner DE, Unalp-Arida A, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology. 2011;53:448–457. doi: 10.1002/hep.24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giorgio V, Prono F, Graziano F, Nobili V. Pediatric non alcoholic fatty liver disease: old and new concepts on development, progression, metabolic insight and potential treatment targets. BMC Pediatr. 2013;13:40. doi: 10.1186/1471-2431-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto E, Taniai M, Tokushige K. Characteristics and diagnosis of NAFLD/NASH. J Gastroenterol Hepatol. 2013;28(Suppl 4):64–70. doi: 10.1111/jgh.12271. [DOI] [PubMed] [Google Scholar]
- 17.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–1269. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 18.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cusi K, Chang Z, Harrison S, Lomonaco R, Bril F, Orsak B, et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60:167–174. doi: 10.1016/j.jhep.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 20.Ballestri S, Lonardo A, Romagnoli D, Carulli L, Losi L, Day CP, et al. Ultrasonographic fatty liver indicator, a novel score which rules out NASH and is correlated with metabolic parameters in NAFLD. Liver Int. 2012;32:1242–1252. doi: 10.1111/j.1478-3231.2012.02804.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim YS, Jung ES, Hur W, Bae SH, Choi JY, Song MJ, et al. Noninvasive predictors of nonalcoholic steatohepatitis in Korean patients with histologically proven nonalcoholic fatty liver disease. Clin Mol Hepatol. 2013;19:120–130. doi: 10.3350/cmh.2013.19.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzpatrick E, Mitry RR, Quaglia A, Hussain MJ, DeBruyne R, Dhawan A. Serum levels of CK18 M30 and leptin are useful predictors of steatohepatitis and fibrosis in paediatric NAFLD. J Pediatr Gastroenterol Nutr. 2010;51:500–506. doi: 10.1097/MPG.0b013e3181e376be. [DOI] [PubMed] [Google Scholar]
- 24.Feldstein AE, Alkhouri N, De Vito R, Alisi A, Lopez R, Nobili V. Serum cytokeratin-18 fragment levels are useful biomarkers for nonalcoholic steatohepatitis in children. Am J Gastroenterol. 2013;108:1526–1531. doi: 10.1038/ajg.2013.168. [DOI] [PubMed] [Google Scholar]