Abstract
Background
Determination of the benefits and limitations of specific physiologic tests has not been well studied in long-term clinical pediatric trials.
Objective
To determine the utility of impulse oscillometry in a long-term comparison of three controller regimens in children with persistent asthma.
Methods
Children 6 to 14 years of age with mild to moderate persistent asthma were characterized with oscillometry and spirometry before entry into a clinical trial and then serially during 48 weeks of therapy with either an inhaled corticosteroid, a combination inhaled corticosteroid with a long-acting beta-agonist, or a leukotriene receptor antagonist.
Results
The FEV1/ FVC ratio as well as the FEF25–75 in terms of spirometric parameters and the reactance area (XA) from impulse oscillometry appeared to complement information provided by FEV1 when comparing the tests and factors that appeared to predict a response to treatment. Reactance area was unique in that it, as distinct from spirometric variables, reflected ongoing improvement during the latter part of the trial. In general, improvements in XA during the latter part of the study occurred independent of indices of atopy and the level of airway responsiveness.
Conclusion
Assessment of respiratory mechanics over time with oscillometry may offer additional insights into response of asthmatic patients to therapy. In particular, the pattern of improvement seen in XA over the course of therapy suggests this test may detect alterations in airway mechanics not reflected by spirometry. The possibility that changes in reactance area reflect ongoing improvement in small airway function deserves additional study.
Keywords: pediatric asthma, impulse oscillometry, spirometry, therapy of asthma
INTRODUCTION
The ability to easily evaluate respiratory function in children with asthma is of central importance in terms of following the course of disease. This is especially important when tracking the effects of therapy.1 With respect to asthma clinical research, physiologic tests are commonly used to define the effects of intervention. While a variety of tests may be utilized, values obtained from spirometry are most commonly reported due in part to the large volume of information on the use of tests such as FEV1. However, this approach may have limitations. For example, spirometric tests may be less helpful than other measures of respiratory function when assessing airway lability in preschool children at risk for persistent asthma.2 Thus, the issue of what physiologic tests will be most informative in long-terms studies requires evaluation.
Forced oscillation is one of several techniques that have been used to obtain measures of respiratory function in subjects with lung disease.3,4 This method involves the application of pressure waves to the airway opening via a mouthpiece, and measuring respiratory system impedance from which resistance and reactance can be derived.5 Results from studies to date suggest a reasonable agreement with more traditional measures of lung function in children with asthma.6 In this respect, there are reports of use of this technique in young children with acute asthma.7,8 This technique has also been used to quantify the response to methacholine6,7,9,10 and histamine9 challenges in young asthmatics. The results of these and other studies as reviewed by Oostveen et al3 and Goldman5 suggest forced oscillation may have advantages when evaluating acute responses to therapy and may also prove useful in following the course of the disease.
The focus of this work is on a comparison of spirometric measures with values obtained by impulse oscillometry (IOS) as methods to follow the course of children with asthma that have been treated in differing ways. The latter is a forced oscillation technique that involves application of a rectangular pulse signal to the airways.11 Subjects assessed were participants in the clinical trial entitled the “Pediatric Asthma Controller Trial” (PACT).12 This study utilized a cohort of children with mild to moderate persistent asthma enrolled to compare the effectiveness of 3 treatment regimens in achieving asthma control. Performance of spirometry and IOS before, during, and at the end of the trial allowed comparison of differing ways of monitoring respiratory function over a prolonged period of therapy.
METHODS
Study Population
The study population for the PACT trial has been described.12 Briefly, children with asthma ages 6 to less than 14 years of age were screened, characterized, and randomized at 5 clinical centers of the Childhood Asthma Research and Education (CARE) Network funded by the National Heart, Lung, and Blood Institute (NHLBI). PACT was designed to compare the effectiveness of 3 treatment regimens in achieving asthma control in school-aged children with mild-moderate persistent asthma. Inclusion criteria included physician-diagnosed asthma, the ability to perform reproducible spirometry, and an FEV1 (measured more than 4 hours since use of a bronchodilator) of at least 80% predicted normal at screening and at least 70% predicted at randomization. Each child had airway lability as defined by methacholine responsiveness with a PC20 ≤ 12.5 m g/ ml (see below). Exclusion criteria included systemic corticosteroid use within 4 weeks prior to enrollment. Two or more hospitalizations or 4 or more courses of systemic corticosteroids in the past year also precluded participation. A listing of inclusion and exclusion criteria has been presented.12 Informed consent consisted of signing a copy of the consent form approved by the Institutional Review Board of the subject’s respective study institution . Assent from the child was also obtained.
Protocol
All participants had mild-moderate persistent asthma, as defined by diary-reported symptoms, beta-agonist use or peak flows obtained during the final week of a 2 to 4 week run-in period. After run-in, participants were assigned to one of three treatment arms: fluticasone 100 mcg morning and 100 mcg evening (Flovent Diskus®, GlaxoSmithKline) plus placebo oral drug in the evening (fluticasone monotherapy); fluticasone 100 mcg/ salmeterol 50 mcg (Advair Diskus®, GlaxoSmithKline) in the morning and salmeterol 50 mcg (Serevent Diskus®, GlaxoSmithKline) in the evening plus placebo oral drug in the evening (PACT combination); or placebo Diskus® in the morning and placebo Diskus® in the evening plus montelukast 5 mg (Singulair®, Merck) in the evening (montelukast monotherapy). Treatment assignment followed a double-blind, randomized parallel group design, stratified by center.
Pulmonary Function Testing: Spirometry and IOS
Spirometry and IOS were performed by CARE-certified technicians with over-reading performed to insure quality control. The tests were performed using a pneumotachograph-type spirometer interfaced with a personal computer system (Jaeger-Toennies GmbH, Hoechberg, Germany). Equipment and testing procedures for the maximal expiratory flow volume (MEFV) maneuvers met American Thoracic Society (ATS) 1994 Spirometry standards13,14 with techniques modified for children less than 8 years of age as described by Eigen et al 15 and Arets et al.16 Age, gender, and ethnicity appropriate prediction equations were used to calculate percent of predicted values for spirometric parameters.17
IOS was performed by applying a rectangular pulse signal to airways with a pressure step wave every 250 milliseconds through a loudspeaker to the airway opening via a mouthpiece through which the subjects breathed normally (tidal breathing) for 30 seconds at a time (see below). The subject wore a nose clip during sampling with the subject or parent/ guardian gently holding the sides of the face to decrease the shunt compliance of the cheeks. Repeated measurements took a few minutes to perform, with at least 3 IOS procedures performed both before and after bronchodilator. Measurements were made using commercially available equipment (Master Screen, E. Jaeger, Würzburg, Germany) and were processed on a Dell computer. Measurements were carried out during stable tidal breathing over a 30 second interval of time with the indices obtained being the total respiratory resistance (R) and reactance (X) as a function of frequency. The former was calculated from pressure and flow signals where pressure was in phase with flow while the latter computation involved pressure out of phase with flow. Reactance at low frequencies reflects the pressures needed to overcome the viscoelastic properties of the lung. In addition to resistance and reactance, the resonant frequency (Fres) was recorded. This is the frequency at which reactance is zero. Also recorded was the reactance area (XA), an integrated response index for reactance developed by Goldman 5 reflecting the integral of the negative values of X from 5 Hz to Fres. This value may in part reflect small airway function. The duration of each measurement was 30 seconds, yielding a total of 120 impulses from which the mean values of R and X were calculated at discrete frequencies from 5 to 35 Hz. During data acquisition, pressure and flow traces were graphically displayed in real time. Measurements were accepted when the tracings showed uninterrupted breathing during data acquisition. Measurements were rejected if disturbed by coughing, breathholding, swallowing, or vocalization. The values from 3 acceptable 30-second periods of data collection were averaged in terms of IOS parameters. The value of R10 was monitored to assess repeatability of the 30 seconds of data collection. The coherence values at 10 Hz had to be ≥ 0.80 while values of R10 had to be within a 20% range (calculated from the largest value for R10). Predicted values for R5, R10, X5 and Fres are from Lechtenbörger et al (unpublished communication) based on 614 healthy children and adolescents from 5 to 17 years of age. These values represent the default normal reference values for the equipment used in this study. For XA, normal values for children of the age range employed in this study have not been established. Thus, the latter is reported as an absolute value with changes tracked over the course of the study. For XA, improvement is reflected by a decrease in this value.
Methacholine Challenge
A CARE-certified technician measured airway responsiveness by the decrease in FEV1 after administering increasing concentrations of methacholine (Provocholine®, Methapharm, Coral Springs, Fl). This drug was delivered by the small volume nebulizer-tidal breathing technique (Wright nebulizer, Medi-tech Ltd, Montreal, Quebec) according to a standardized procedure18 and ATS 2000 methacholine challenge testing guidelines.19 The test was performed at least 4 hours after the last use of a short-acting bronchodilator and/ or caffeine consumption, and at least 4 weeks after the last oral corticosteroid use or respiratory tract infection. Baseline FEV1 values equaled or exceeded 70% of predicted prior to the test. A diluent step was performed and utilized as the reference value for calculation of PC20.
Measurement of Biomarkers
Exhaled nitric oxide (eNO) was measured employing the online technique recommended by the American Thoracic Society20 using the NIOX® system (Aerocrine AB, Stockholm, Sweden). This technique is described by Strunk et al.21 Measurement of eNO was obtained prior to spirometry. Peripheral blood eosinophil counts were obtained by automated assay at each center. Immediate hypersensitivity skin tests were performed with 8 common aeroallergens as outlined previously.21 Testing was performed in accordance with a study specific protocol.22,23
Statistical Analysis
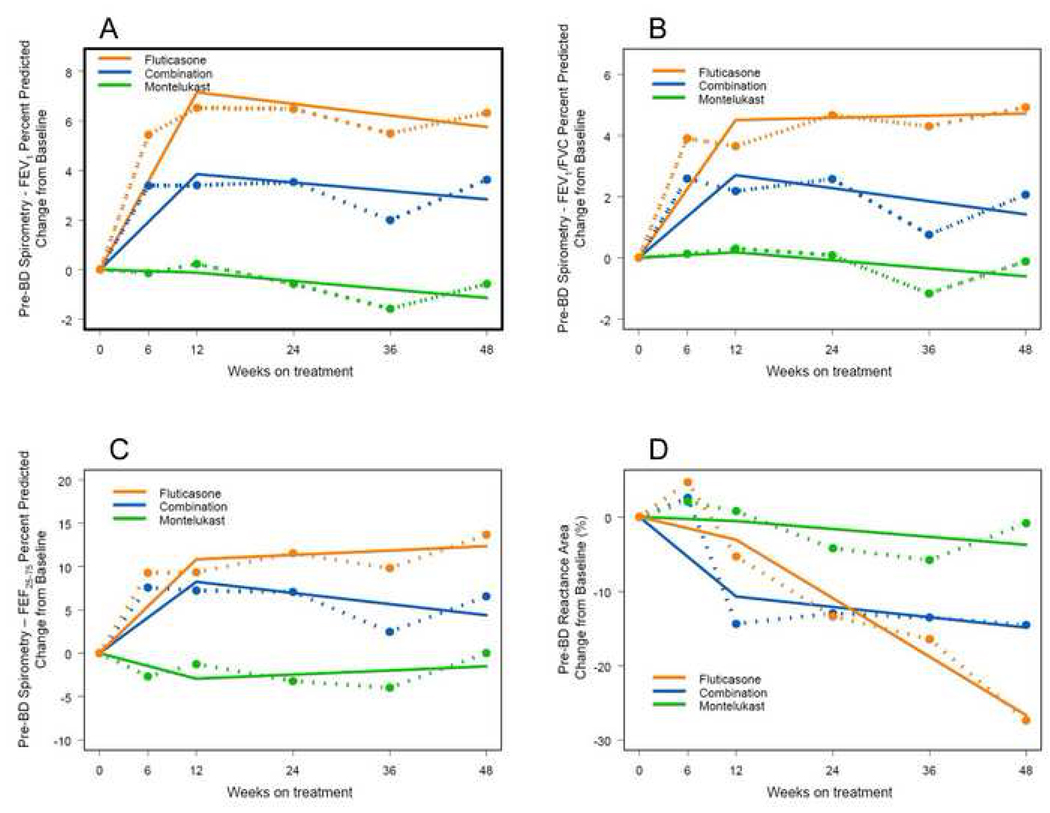
Given the time course and pattern of the responses to therapy in terms of FEV1 and FEV1/ FVC as reported in the PACT trial12 and reproduced in part in Figures 1 and 2 (dashed lines), a regression model with a change point at 12 weeks was used to describe changes in lung function over time. The term “change point” refers to the time at which the temporal profile of the outcome changes. In this case, the form of the regression model is a straight line from time zero to 12 weeks, connected to another straight line extending from 12 to 48 weeks (Figure 1; regression model shown by straight lines with change point at 12 weeks). All outcomes were defined in terms of change from baseline and thus, the regression line begins at zero on the y-axis. The slope of the line from baseline to 12 weeks represents the initial response to treatment while the slope of the line from 12 to 48 weeks represents later changes following the initial response.
FIG 1.
Changes in FEV1 (A), FEV1/ FVC (B), FEF25–75 (C), and XA (D) over time are displayed for three treatment groups as both mean data at each measurement point (dashed lines) and as a regression model with a change point at 12 weeks (solid lines). The statistical analyses using the regression model are summarized in Tables I and II. Over the first 12 weeks of therapy, the slopes for FEV1, FEV1/ FVC, and FEF25–75 were significant in a positive direction for combination and fluticasone therapy. However, for these spirometric parameters, the pattern over the last period of therapy (12–48 weeks) was different with all slopes close to zero. Conversely, XA significantly improved in the fluticasone group during the latter period as reflected by the negative slope for change in XA.
The regression models were fit within the framework of repeated measures analysis for longitudinal data.24 The results of the regression models are presented as population averages, unadjusted for other covariates. Residual analysis was performed to verify that the model was appropriate for the data. All analyses were performed using SAS Version 9.2 statistical software. Significance was established at p < 0.05, 2-tailed.
Protocol Review
An Institutional Review Board at each center plus an NHLBI Protocol Review Committee and an NHLBI Data Safety Monitoring Board approved the PACT protocol. The latter committees were formed specifically for the CARE Network.
RESULTS
Demographics /Clinical Characteristics of Study Participants
Demographic characteristics were presented by Sorkness et al.12 Of the 285 randomized subjects, 252 completed the study. The three treatment groups were well matched, with characteristics at baseline of mild-to-moderate asthma. Of the scheduled visits, 97% were completed. Adherence to study medications was high as presented in the main outcome paper.12 In terms of the primary outcome (asthma control days), fluticasone monotherapy and PACT combination were comparable and superior to montelukast.
Spirometry and IOS Results
As presented in the original manuscript, prebronchodilator FEV1 (% predicted) and FEV1/ FVC (expressed as a percent) increased significantly more with fluticasone monotherapy than with PACT combination and montelukast monotherapy. The mean change in FEV1 % predicted from baseline was 6.32% with fluticasone monotherapy and 3.62% with PACT combination while the mean change for FEV1/ FVC was 3.95% and 1.76% respectively. Montelukast did not improve theses measure of lung function.
The results of fitting the FEV1 and FEV1/ FVC data to the change-point model are shown in Figure 1. The associated parameter estimates are summarized in Tables I and II. The parameter estimates represent the changes in FEV1 and FEV1/ FVC per week. The p-values reported in Table I are those associated with testing whether the slope parameter for each treatment is different from zero. The p-values in Table II are those associated with testing whether the treatments have equal slopes. Over the first 12 weeks of therapy, the slopes for these measures of airways obstruction were significant in a positive direction for combination and fluticasone therapy (Table I) with all groups differing from the two others (Table II). However, the pattern over the last period of therapy (12–48 weeks) was different. Slopes for both tests of lung function were close to zero with the probabilities not significant. Thus, these measures of lung function improved initially in two of the three treatment arms and then were fairly static for the remainder of the study.
TABLE I.
Slopes for tests of lung function in first 12 weeks and from 12 to 48 weeks of treatment
| Lung Function |
Time | Treatment | Estimate (95% CI) | Probability |
|---|---|---|---|---|
| FEV1 |
0–12 weeks |
Combination | 0.320 (0.201, 0.439) | <0.0001 |
| Fluticasone | 0.596 (0.477, 0.715) | <0.0001 | ||
| Montelukast | −0.011 (−0.128, 0.107) | 0.8589 | ||
|
12–48 weeks |
Combination | −0.028 (−0.096, 0.039) | 0.4138 | |
| Fluticasone | −0.039 (−0.105, 0.027) | 0.2483 | ||
| Montelukast | −0.028 (−0.094, 0.038) | 0.4010 | ||
| FEV1/FVC |
0–12 weeks |
Combination | 0.225 (0.135, 0.315) | <0.0001 |
| Fluticasone | 0.375 (0.287, 0.463) | <0.0001 | ||
| Montelukast | 0.015 (−0.071, 0.101) | 0.7323 | ||
|
12–48 weeks |
Combination | −0.036 (−0.086, 0.015) | 0.1693 | |
| Fluticasone | 0.006 (−0.043, 0.055) | 0.8140 | ||
| Montelukast | −0.022 (−0.070, 0.026) | 0.3753 | ||
| FEF25–75 |
0–12 weeks |
Combination | 0.686 (0.461, 0.910) | <0.0001 |
| Fluticasone | 0.902 (0.671, 1.134) | <0.0001 | ||
| Montelukast | −0.245 (−0.477, −0.012) | 0.0393 | ||
|
12–48 weeks |
Combination | −0.107 (−0.235, 0.022) | 0.1044 | |
| Fluticasone | 0.042 (−0.087, 0.171) | 0.5238 | ||
| Montelukast | 0.040 (−0.090, 0.169) | 0.5483 | ||
| XA |
0–12 weeks |
Combination | −0.893 (−1.544, −0.242) | 0.0073 |
| Fluticasone | −0.256 (−0.889, 0.378) | 0.4293 | ||
| Montelukast | −0.047 (−0.697, 0.603) | 0.8876 | ||
|
12–48 weeks |
Combination | −0.116 (−0.484, 0.253) | 0.5394 | |
| Fluticasone | −0.656 (−1.008, −0.303) | 0.0003 | ||
| Montelukast | −0.088 (−0.451, 0.274) | 0.6325 | ||
TABLE II.
Differences in slopes for tests of lung function in the first 12 weeks and from 12 to 48 weeks of treatment
| Lung Function |
Time | Treatment | Estimate (95% CI) | Probability |
|---|---|---|---|---|
| FEV1 |
0–12 weeks |
C vs. F | −0.275 (−0.443, −0.107) | 0.0014 |
| C vs. M | 0.331 (0.164, 0.499) | 0.0001 | ||
| F vs. M | 0.606 (0.439, 0.774) | <.0001 | ||
|
12–48 weeks |
C vs. F | 0.011 (−0.084, 0.105) | 0.8247 | |
| C vs. M | 0.000 (−0.094, 0.094) | 0.9988 | ||
| F vs. M | −0.011 (−0.104, 0.083) | 0.8237 | ||
| FEV1/FVC |
0–12 weeks |
C vs. F | −0.150 (−0.276, −0.024) | 0.0199 |
| C vs. M | 0.210 (0.086, 0.335) | 0.0010 | ||
| F vs. M | 0.360 (0.237, 0.483) | <.0001 | ||
|
12–48 weeks |
C vs. F | −0.041 (−0.112, 0.029) | 0.2489 | |
| C vs. M | −0.014 (−0.084, 0.056) | 0.7008 | ||
| F vs. M | 0.028 (−0.041, 0.096) | 0.4292 | ||
| FEF25–75 |
0–12 weeks |
C vs. F | −0.217 (−0.539, 0.106) | 0.1887 |
| C vs. M | 0.930 (0.607, 1.254) | <.0001 | ||
| F vs. M | 1.147 (0.819, 1.475) | <.0001 | ||
|
12–48 weeks |
C vs. F | −0.149 (−0.331, 0.033) | 0.1099 | |
| C vs. M | −0.146 (−0.329, 0.036) | 0.1162 | ||
| F vs. M | 0.002 (−0.180, 0.185) | 0.9803 | ||
| XA |
0–12 weeks |
C vs. F | −0.637 (−1.546, 0.271) | 0.1694 |
| C vs. M | −0.846 (−1.766, 0.074) | 0.0717 | ||
| F vs. M | −0.209 (−1.116, 0.699) | 0.6522 | ||
|
12–48 weeks |
C vs. F | 0.540 (0.030, 1.050) | 0.0382 | |
| C vs. M | −0.027 (−0.544, 0.490) | 0.9184 | ||
| F vs. M | −0.567 (−1.073, −0.062) | 0.0281 | ||
C = Combination; F = Fluticasone; M = Montelukast
Changes over time for FEF25–75 (expressed as a percent of predicted) for all 3 treatment groups are shown in Figure 1C. The statistical analysis in terms of the slopes and comparisons between groups is presented in Tables I and II respectively. Over the first 12 weeks of therapy, the slopes for this measure of airways obstruction were significant in a positive direction for combination and fluticasone therapy (Table I) and significant in a negative direction for the montelukast group. During this initial period of treatment, the groups receiving combination and fluticasone therapy were significantly different from the group receiving montelukast (Table II). Once again, the pattern over the last period of therapy (12–48 weeks) was different. Slopes were close to zero with the probabilities not significant. Thus, FEF25–75 improved initially in two of the three treatment arms and then was fairly static for the duration of the study for all treatment arms.
For XA, the patterns of change over time were different than those seen with spirometric parameters. The changes in absolute value of XA for the three treatment groups are shown graphically in Figure 1D while the slopes and their estimates for the first and second phases of treatment are listed in Table I. During the initial 12 weeks of therapy, the only group in which the slope was significantly different than zero was the one receiving combination therapy. During the subsequent 36 weeks of treatment, the group receiving fluticasone showed significant improvement as reflected by the negative slope for change in XA over time (Table I), with the differences between the fluticasone group and the other two groups being significant (Table II).
Relationship of Biomarkers and Level of Reactivity to Changes in XA
The possibility of correlations existing between potential biomarkers of disease and the variable (XA) that improved with fluticasone therapy during the latter part of the study was also addressed. Listed in E Table I of the Online Repository are the slopes of XA during the latter part of therapy for subjects treated with fluticasone. The subjects were divided into those with eNO values of less than 25 versus those with values of 25 or higher, subjects with negative versus those with positive skin tests, and participants with eosinophil percentages on their blood counts of less than 4% versus those with values of 4% and higher. As listed in E Table I, the slope estimates and the probabilities were comparable in all groups with the exception of the grouping taking into consideration eosinophil percentages in blood. In this analysis, those that would be considered to have a biomarker compatible with more active disease had a slope estimate that was significant. When eNO or skin test reactivity were used to define the sub-groupings, statistical significance was found regardless of eNO levels or whether the subjects had reactions to aeroallergens.
In addition to the three biomarkers, the subjects receiving fluticasone were also assessed in terms of slope estimates and probabilities depending on their methacholine responsiveness. For this analysis, subjects were divided into those with a PC20 less than 2.0 mg/ ml and those with a value equal to or greater than 2.0 mg/ ml. As seen in E Table I, the p values were both significant. The slope estimates were greater and the probabilities greater in the group that was less reactive in terms of methacholine responsiveness.
DISCUSSION
Assessment of lung function with IOS was easily accomplished in children with persistent asthma who were 6 to 14 years of age. This study found one IOS parameter (XA) demonstrated continued improvement over a prolonged period of time compared to spirometric variables. As such, IOS may offer a valuable adjunct to spirometry in following the course of subjects enrolled in clinical studies. In terms of biomarkers, a higher level of circulating eosinophils identified subjects more likely to respond in terms of improvements in XA with inhaled corticosteroid therapy. Elevated eNO, skin test reactivity to aeroallergens, and the level of airway responsiveness did not distinguish those with improvements in XA.
The value of assessing objective measures of lung function that include spirometric parameters in children with asthma is well established. For example, Bye et al 25 argued that failure to perform spirometry in children with asthma results in underdiagnosis of airflow obstruction. More recently, Fuhlbrigge and colleagues26 demonstrated a strong association between FEV1 expressed as a percent of predicted and the risk of an asthma attack over the subsequent year. In terms of clinical research in asthma, spirometric variables are commonly used to define entry criteria and to gauge the effects of intervention. However, use of FEV1 may have limitations in terms of both clinical care and research. In this respect, the question has been raised about whether FEV1 is the best spirometric measure of severity in childhood asthma.27,28 Thus, the issue of what physiologic tests other than FEV1 will be of use in following the course of subjects with persistent asthma over a prolonged period of treatment was a focus of this work.
The design of the PACT study provided a unique opportunity to compare methods of physiologically characterizing school-age children with persistent asthma over time when they were treated with three distinct medical regimens. These children were characterized by respiratory physiology, symptom burden, and methacholine responsiveness. The study design also allowed assessment of biomarkers in subjects so that changes in physiologic measurements could be compared to markers of disease activity when therapy began.
In terms of spirometry, we focused on FEV1 as well as the ratio of FEV1 to FVC and FEF25–75 for reasons given previously. While the initial report of the results of this study focused on both FEV1 as well as FEV1/ FVC,12 we felt it important to address the changes over time in more detail with a random effects model that addresses both acute and more chronic changes over time. Our focus in this analysis on FEF25–75 was based on the argument that this test as well as FEV1/ FVC may more accurately measure lung function impairment than FEV1 in children. The former parameter (FEF25–75) has been felt to possibly reflect small airway function29,30 and this also had appeal in terms of our analysis. Reactance area, which reflects the integrated sum of the negative reactance values below the resonant frequency, is also felt to reflect at least in part peripheral airway function.5,31 Thus, our analysis yielded information on spirometric variables that may be useful in gauging response, but also on a spirometric parameter as well as an IOS parameter that may reflect events within more peripheral airways.
Our analysis of these tests of lung function demonstrated important trends in terms of therapeutic responses. For FEV1 as well as FEV1/ FVC and FEF25–75, significant changes in slopes were limited to the first 12 weeks of therapy with the improvements maintained but not significantly increasing over the latter part of treatment. The improvements were seen in the groups receiving fluticasone monotherapy and combination therapy. For XA, the pattern was different. For this test, only the group receiving combination therapy exhibited a change in slope that was significantly different from zero in the initial 12 weeks of treatment. In addition, during the subsequent 36 weeks of treatment, the group receiving fluticasone showed significant improvement as reflected by the negative slope of XA over time (Table I) with the differences between the fluticasone and other two groups being significant (Table II).
Comment is necessary regarding the changes that occur in XA over time when the results are expressed in absolute units rather than as a percent of predicted. This IOS parameter is a reflection of both the resonant frequency and X5. Both parameters normally decrease with growth, and thus XA is also expected to normally decrease in value over time with linear growth. However, as noted previously, predicted values of XA for children over the range of ages and heights used in this study are not available. Thus, we elected to use absolute values instead of extrapolated normal values from other data sets. With this approach, the question must be asked if the changes seen with fluticasone therapy only reflect changes that occur with growth over the period of study. This is unlikely for several reasons. First, as reported by Sorkness et al,12 differences among the three therapies in this study in terms of growth effects were not significant, including when stratified for age. Comparable growth was seen in all three treatment groups over the course of treatment, ranging from a mean of 5.3 cm in the fluticasone monotherapy and combination therapy groups to 5.7 cm in the montelukast monotherapy group. Second, during the last 36 weeks of treatment, the group receiving fluticasone showed not only a significant improvement as reflected by the negative slope for change in XA over time (Table I), but a significant difference in slope when compared to the other two groups (Table II). Third, if stature had been significantly impaired in the groups receiving inhaled corticosteroids, changes in XA values over time would be expected to be less and not greater in magnitude. Therefore, for these reasons, we believe that the changes in XA over time in the corticosteroid monotherapy group reflect more than just alterations that normally occur with growth.
Both FEF25–75 and XA may reflect events within more peripheral airways. Yet, these two measures differed in terms of the patterns of improvement seen during the study. This was especially evident in terms of the improvement seen with corticosteroid monotherapy for XA but not FEF25–75 during the latter part of therapy. Thus, XA may be more sensitive to alterations in peripheral airway function than the former. Another possibility is that the improvement in XA over time reflects not only small airway function, but other mechanical events within the lung. Unfortunately, other tests of airway function that might shed light on this issue were not part of this study. More information on what is occurring over time in terms of air trapping as reflected by the residual volume and ratio of residual volume to total lung capacity might be helpful. Use of quantitative computed tomography might also help assess events within peripheral airways.30
In older children, the CARE Network has previously reported that treatment of mild - moderate asthma in subjects 6 to 17 years of age with an inhaled corticosteroid led to not only improvement in spirometry but also to significant improvement in R5 and XA.32 While resistance measures at various frequencies including R5 and R10 were obtained by IOS during PACT and analyzed as part of this work, the changes were not of similar magnitude or as consistent as those seen with XA (CARE Network, unpublished observations). Thus, we focused on XA alone for this report.
Biomarkers that reflect an atopic state have been used to determine if they help predict the response to therapy. In another CARE network study, a favorable response (in terms of FEV1) to 8 weeks of fluticasone therapy was associated with higher baseline (pretreatment) levels of eNO, total eosinophils counts, and levels of serum IgE.33 In terms of biomarkers used in PACT, only peripheral eosinophil percentages identified subjects more likely to respond in terms of improvements in XA with inhaled corticosteroid therapy. Improvements in XA were seen regardless of eNO levels and skin test responses. Improvements in XA also occurred independent of the level of airway responsiveness. This observation also differed from the previous study where improvement in FEV1 was associated with an initially lower PC20 (greater airway reactivity) to methacholine.33 These observations suggest improvements seen in XA with corticosteroid therapy over time may reflect effects that occur independent of atopy.
These data suggest that IOS may be informative in longer-term studies and may reflect improvements in lung function not reflected by spirometric parameters. This latter concept is supported by other studies. In adults with asthma, Evans and colleagues 34 suggested that IOS is a more sensitive measure of change in airway function than spirometry when assessing the response to room temperature and cold temperature exercise challenges. In older children with asthma, Goldman et al 35 found significant differences in some IOS parameters (including both R5 measured during inspiration and XA) but not spirometric indices when assessing adolescents daily over a period of three days. In preschool children, Marotta et al2 reported that IOS responses to bronchodilator demonstrated significantly greater change than observed with FEV1 in those 4-year-old children most likely to have persistent asthma. Conventional spirometry did not establish similar statistically significant findings. Thus, IOS might provide a u seful d iagnostic tool when monitoring for the development of early asthma. In addition, IOS might also be helpful in obtaining objective outcome measures in studies of early intervention when spirometric measures are not as easily obtained.36 Both issues require additional study in preschool children with wheezing that may progress to chronic asthma.37
In summary, analysis of the response of PACT participants to an ICS, combination therapy, and a LTRA using IOS was feasible, and revealed differences in the time course of improvement compared with spirometric variables. Additional investigations to define benefits and limitations of this method of physiologic assessment over prolonged periods of time appear justified. In addition, additional study to address the possibility that the changes seen in XA in this study reflected continuing improvement in small airway function deserves attention.
Supplementary Material
Acknowledgments
This work was supported by Grants U10HL064287, 5U10HL064288, 5U10HL064295, 5U10HL064305, and 5U10HL064307 from the National Heart, Lung and Blood Institute. This study was carried out in part in the General Clinical Research Centers at National Jewish Medical and Research Center (M01 RR00051), Washington University School of Medicine (M01 RR00036), and the University of Wisconsin (MO1 RR03186).
Abbreviations
- ATS
American Thoracic Society
- CARE
Childhood Asthma Research and Education Network
- eNO
exhaled nitric oxide
- FEF25–75
forced expiratory flow from 25 to 75% of forced vital capacity
- FEV1
volume (liters) of air expired in the first second of forced expiration
- Fres
resonant frequency
- FVC
forced vital capacity
- Hz
Hertz (cycles per second)
- ICS
inhaled corticosteroids
- IOS
impulse oscillometry
- LABA
long-acting beta-agonist
- LTRA
leukotriene receptor antagonist
- MDI
metered dose inhaler
- NHLBI
National Heart, Lung and Blood Institute
- NIH
National Institutes of Health
- PACT
Pediatric Asthma Controller Trial
- PC20
methacholine dose that decreases FEV1 by 20%
- PEF
peak expiratory flow
- R5, R10
resistance of the respiratory system at 5 and 10 Hz
- X5
reactance of the respiratory system at 5 Hz
- XA
reactance area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Implications: Assessment of respiratory mechanics over time with impulse oscillometry may offer important information about the pulmonary response of asthmatic patients to therapy that is not reflected by spirometric parameters.
REFERENCES
- 1.Larsen GL, Kerby GS, Guilbert TW, Morgan WJ. Functional assessment of asthma. In: Leung DYM, Sampson HA, Geha RS, Szefler SJ, editors. Pediatric Allergy: Principles and Practice. St. Louis: Mosby: 2003. pp. 357–365. [Google Scholar]
- 2.Marotta A, Klinnert MD, Price MR, Larsen GL, Liu AH. Impulse oscillometry provides an effective measure of lung dysfunction in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol. 2003;112:317–322. doi: 10.1067/mai.2003.1627. [DOI] [PubMed] [Google Scholar]
- 3.Oostveen E, MacLeod D, Lorino H, Farré R, Hantos Z, Desager L, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22:1026–1041. doi: 10.1183/09031936.03.00089403. [DOI] [PubMed] [Google Scholar]
- 4.Desager KN, Marchal F, van de Woestijne KP. Forced oscillation technique. In: Stocks J, Sly PD, Tepper RS, Morgan WJ, editors. Infant Respiratory Function Testing. New York, New York: Wiley-Liss; 1996. pp. 355–378. [Google Scholar]
- 5.Goldman MD. Clinical application of forced oscillation. Pulm Pharm Ther. 2001;14:341–350. doi: 10.1006/pupt.2001.0310. [DOI] [PubMed] [Google Scholar]
- 6.Duiverman EJ, Neijens HJ, van der Snee-van Smaalen M, Kerribijn KF. Comparison of forced oscillometry and forced expirations for measuring dose-related responses to inhaled methacholine in asthmatic children. Bull Eur Physiopathol Respir. 1986;22:433–436. [PubMed] [Google Scholar]
- 7.Klug B, Bisgaard H. Measurement of lung function in awake 2–4-year-old asthmatic children during methacholine challenge and acute asthma: a comparison of the impulse oscillation technique, the interrupter technique, and transcutaneous measurement of oxygen versus whole-body plethysmography. Pediatr Pulmonol. 1996;21:290–300. doi: 10.1002/(SICI)1099-0496(199605)21:5<290::AID-PPUL4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Ducharme FM, Davis GM. Measurement of respiratory resistance in the emergency department. Feasibility in young children with acute asthma. Chest. 1997;111:1519–1525. doi: 10.1378/chest.111.6.1519. [DOI] [PubMed] [Google Scholar]
- 9.Duiverman EJ, Neijens HJ, van Strik R, van der Snee-van Smaalen M, Kerribijn KF. Bronchial responsiveness in asthmatic children aged 3 to 8 years measured by forced pseudo-random noise oscillometry. Bull Eur Physiopathol Respir. 1986;22:27–34. [PubMed] [Google Scholar]
- 10.Vink GR, Arets HGM, van der Laag J, van der Ent CK. Impulse oscillometry: a measure for airway obstruction. Pediatr Pulmonol. 2003;35:214–219. doi: 10.1002/ppul.10235. [DOI] [PubMed] [Google Scholar]
- 11.Hellinckx J, De Boeck K, Bande-Knops J, van der Poel M, Demedts M. Bronchodilator response in 3–6.5 years old healthy and stable asthmatic children. Eur Respir J. 1998;12:438–443. doi: 10.1183/09031936.98.12020438. [DOI] [PubMed] [Google Scholar]
- 12.Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild -moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society Committee of Proficiency Standards for Clinical Pulmonary Function Laboratories. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 14.Childhood Asthma Management Program Spirometry Manual, Version 3.0. Springfield, VA: National Technical Information Service; 1994. Childhood Asthma Management Program. [Google Scholar]
- 15.Eigen H, Bieler H, Grant D, Christoph K, Terrill D, Heilman DK, et al. Spirometric pulmonary function in healthy preschool children. Am J Respir Crit Care Med. 2001;163:619–623. doi: 10.1164/ajrccm.163.3.2002054. [DOI] [PubMed] [Google Scholar]
- 16.Arets HGM, Brackel HJL, van der Ent CK. Forced expiratory manoeuvres in children: do they meet ATS and ERS criteria for spirometry? Eur Respir J. 2001;18:655–660. doi: 10.1183/09031936.01.00204301. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 18.Childhood Asthma Management Program for Methacholine Challenge Testing, Version 3.0. Springfield, VA: National Technical Information Service; 1994. Childhood Asthma Management Program. [Google Scholar]
- 19.American Thoracic Society Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. Guidelines for methacholine and exercise challenge testing – 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society. Recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children - 1999. Am J Respir Crit Care Med. 1999;160:2104–2117. doi: 10.1164/ajrccm.160.6.ats8-99. [DOI] [PubMed] [Google Scholar]
- 21.Strunk RC, Szefler SJ, Phillips BR, Zeiger RS, Chinchilli VM, Larsen G, et al. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol. 2003;112:883–892. doi: 10.1016/j.jaci.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Childhood Asthma Management Program Allergy and Skin Test Manual, Version 2.0. Springfield, VA: National Technical Information Service; 1994. Childhood Asthma Management Program. [Google Scholar]
- 23.Mitchell H, Senturia Y, Gergen P, Baker D, Joseph C, McNiff-Mortimer K. Design and methods of the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24:237–252. doi: 10.1002/(sici)1099-0496(199710)24:4<237::aid-ppul3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 24.Omar RZ, Wright EM, Turner RM, Thompson SG. Analyzing repeated measurements data: A practical comparison of methods. Stat Med. 1999;18:1587–1603. doi: 10.1002/(sici)1097-0258(19990715)18:13<1587::aid-sim141>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 25.Bye MR, Kerstein D, Barsh E. The importance of spirometry in the assessment of childhood asthma. Am J Dis Child. 1992;146:977–978. doi: 10.1001/archpedi.1992.02160200099037. [DOI] [PubMed] [Google Scholar]
- 26.Fuhlbrigge AL, Kitch BT, Paltiel AD, Kuntz KM, Neumann PJ, Dockery DW, et al. FEV1 is associated with risk of asthma attacks in a pediatric population. J Allergy Clin Immunol. 2001;107:61–67. doi: 10.1067/mai.2001.111590. [DOI] [PubMed] [Google Scholar]
- 27.Spahn JD, Cherniack R, Paull K, Gelfand EW. Is forced expiratory volume in one second the best measure of severity in childhood asthma? Am J Respir Crit Care Med. 2004;169:784–786. doi: 10.1164/rccm.200309-1234OE. [DOI] [PubMed] [Google Scholar]
- 28.Paull K, Covar R, Jain N, Gelfand EW, Spahn JD. Do NHLBI lung function criteria apply to children? A cross-sectional evaluation of childhood asthma at National Jewish Medical and Research Center, 1999–2002. Pediatr Pulmonol. 2005;39:311–317. doi: 10.1002/ppul.20161. [DOI] [PubMed] [Google Scholar]
- 29.Wagner EM, Liu MC, Weinmann GG, Permutt S, Bleecker ER. Peripheral lung resistance in normal and asthmatic subjects. Am Rev Respir Dis. 1990;141:584–588. doi: 10.1164/ajrccm/141.3.584. [DOI] [PubMed] [Google Scholar]
- 30.Jain N, Covar RA, Gleason MC, Newell JD, Jr, Gelfand EW, Spahn JD. Quantitative computed tomography detects peripheral airway disease in asthmatic children. Pediatr Pulmonol. 2005;40:211–218. doi: 10.1002/ppul.20215. [DOI] [PubMed] [Google Scholar]
- 31.Frei J, Jutla J, Kramer G, Hatzakis GE, Ducharme FM, Davis GM. Impulse oscillometry. Reference values in children 100 to 150 cm in height and 3 to 10 years of age. Chest. 2005;128:1266–1273. doi: 10.1378/chest.128.3.1266. [DOI] [PubMed] [Google Scholar]
- 32.Zeiger RS, Szefler SJ, Phillips BR, Schatz M, Martinez FD, Chinchilli VM, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Szefler SJ, Phillips B, Martinez FD, Chinchilli VM, Lemanske RF, Jr, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Evans TM, Rundell KW, Beck KC, Levine AM, Baumann JM. Airway narrowing measured by spirometry and impulse oscillometry following room temperature and cold temperature exercise. Chest. 2005;128:2412–2419. doi: 10.1378/chest.128.4.2412. [DOI] [PubMed] [Google Scholar]
- 35.Goldman MD, Carter R, Klein R, Fritz G, Carter B, Pachucki P. Within - and between-day variability of respiratory impedance, using impulse oscillometry in adolescent asthmatics. Pediatr Pulmonol. 2002;34:312–319. doi: 10.1002/ppul.10168. [DOI] [PubMed] [Google Scholar]
- 36.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Eng J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 37.Larsen GL, Kang J-KB, Guilbert T, Morgan W. Assessing respiratory function in young children: Developmental considerations. J Allergy Clin Immunol. 2005;115:657–666. doi: 10.1016/j.jaci.2004.12.1112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.