Abstract
Gene translation modeling and prediction is a fundamental problem that has numerous biomedical implementations. In this work we present a novel, user-friendly tool/index for calculating the mean of the typical decoding rates that enables predicting translation elongation efficiency of protein coding genes for different tissue types, developmental stages, and experimental conditions. The suggested translation efficiency index is based on the analysis of the organism’s ribosome profiling data. This index could be used for example to predict changes in translation elongation efficiency of lowly expressed genes that usually have relatively low and/or biased ribosomal densities and protein levels measurements, or can be used for example for predicting translation efficiency of new genetically engineered genes. We demonstrate the usability of this index via the analysis of six organisms in different tissues and developmental stages. Distributable cross platform application and guideline are available for download at: http://www.cs.tau.ac.il/~tamirtul/MTDR/MTDR_Install.html
Keywords: codon decoding rate, codon usage bias, gene translation, ribosome profiling, transcript evolution
Gene translation is a fundamental intracellular process. Thus, the ability to predict gene translation elongation efficiency (i.e., a gene’s translation rate) is a central challenge related to all biomedical disciplines.
Currently, there are no direct measures of genes translation efficiency that do not include “components” of other gene expression stages, such as transcription and/or posttranslational regulatory steps (e.g., mRNA degradation, protein degradation, and protein synthesis rate). For example, conventional nondirect proxies of translation efficiency include messenger RNA (mRNA) levels, protein abundance, or the normalization of the two aforementioned factors. One drawback of these proxies is the fact that they are not available for the majority of organisms in various experimental conditions and tissues. In addition, these proxies are not highly reliable for lowly expressed genes, nor they can predict the translation efficiency of new engineered genes expressed in the same cell conditions.
Recently, ribosome profiling was suggested for measuring some aspects of the translation process at nucleotide resolution (Ribo-seq) (Ingolia et al. 2009). This method can potentially estimate the relative time ribosomes spend on the organismal mRNA molecules, at nucleotide resolution. Thus, ribosome profiles reflect in vivo the translation process of specific tissues and developmental stages or conditions. As a result, it was suggested to estimate the general translation efficiency of genes by calculating their mean average footprint read counts (Ingolia et al. 2009).
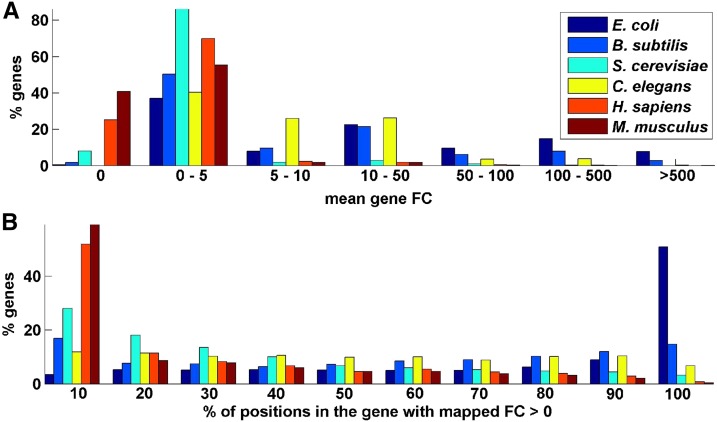
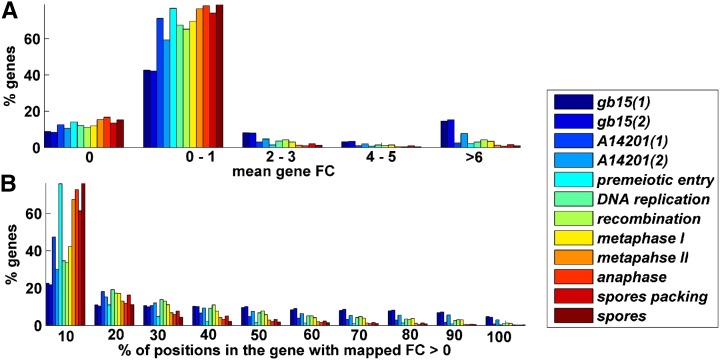
However, resulting ribosome profiles are reliable only for highly expressed genes, thus restricting the ability of the method to accurately measure translation efficiency of the remaining of the genes or to predict translation efficiency of newly engineered genes in similar cellular conditions. For example, as can be seen in Figure 2, for S. cerevisiae, H. sapiens, and M. musculus only 13.9–23.7% of the genes include more than 50% positions with nonzero mapped read counts; similarly, only 8.5–11.8% of their genes include mean footprint count (FC; per nucleotide) larger than 2.
Figure 2.
Footprint count (FC) statistics over all genes of six organisms. (A) Histogram of average read counts. (B) Histogram of percentage of positions with a positive number of mapped read counts. As can be seen in all analyzed organisms, most of the genes have very low read counts.
Additional conventional approach/indexes for estimating translation efficiency are based on various measures of codon distribution/bias within the opening reading frame (ORF) (Sharp and Li 1987; Wright 1990; dos Reis et al. 2004; Fox and Erill 2010; Sabi and Tuller 2014). These indexes were found to be correlative with the protein abundance in the cell for S. cerevisiae, E. coli, and C. elegans (dos Reis et al. 2004; Tuller et al. 2010b; Sabi and Tuller 2014). However, these indexes are not condition nor tissue specific and may not be directly related to translation but to other steps of gene expression and gene evolution (Sharp and Li 1987; Plotkin and Kudla 2011).
In contrast to the previous suggested indexes, the mean of the typical decoding rates (MTDR) index (Dana and Tuller 2014) is based on the estimation of the typical codon decoding times from Ribo-seq data, thus potentially capturing aspects of translation elongation in specific tissues, developmental stages, and/or conditions. Specifically, the MTDR index calculates the geometrical mean of the estimated typical nominal translation rates of a gene’s codons after filtering biases and phenomena such as ribosomal traffic jams and translational pauses (Dana and Tuller 2014) (see also Figure 1 and the section Materials and Methods), reflecting the mean typical translation elongation rate of a gene. Thus, this index could be used to predict all genes’ translation efficiency, including newly engineered genes.
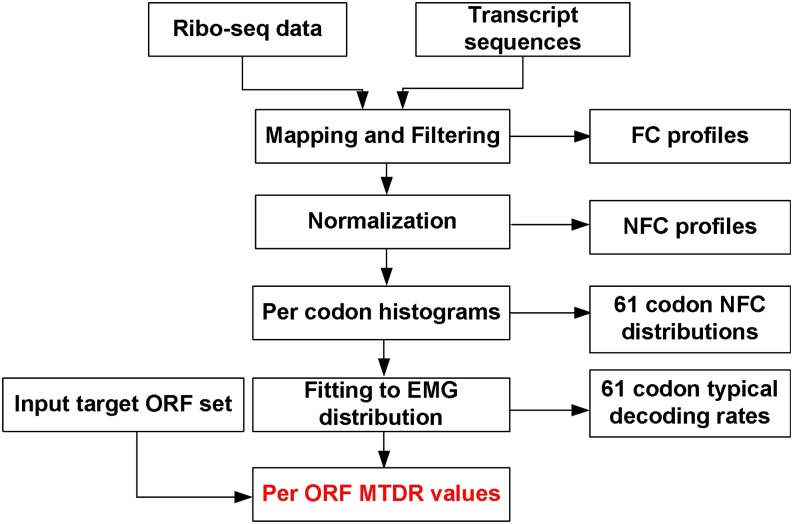
Figure 1.
Diagram showing the mean of the typical decoding rates (MTDR) calculation process. Ribo-seq data are created per selected organism (condition and tissue specific). Resulting mRNA fragments are then mapped to transcript sequences resulting for each gene its ribosomal profile. Genes with mean footprint counts (FC) lower than one are filtered. To enable comparison between footprint counts of different genes with different mRNA levels and initiation rates, FC of each gene are normalized, resulting in normalized footprint count (NFC) profiles. Then for each codon type a NFC distribution is created. By fitting the NFC distribution to an exponentially modified Gaussian distribution type, the typical decoding rate of each codon is estimated. Finally, for each one of the input target open reading frames (ORFs), the mean typical decoding rates of its codons (MTDR index) is calculated.
In this study we estimate the typical codons translation time of additional organisms in different conditions and tissues (see Table 1) from ribosomal profiling data (see the section Materials and Methods). We also demonstrate the advantages of the MTDR index in predicting various gene expression measurements and compare it with previously suggested translation efficiency indexes (which are based on codon distributions). Finally, we provide a cross-platform tool for calculating the MTDR index of all ORFs in these organisms and conditions.
Table 1. Analyzed organisms and tissues/conditions.
| Organism | Condition/Tissue |
|---|---|
| E. coli (Li et al. 2012) | − |
| B. subtilis (Li et al. 2012) | − |
| S. cerevisiae | Exponential (Ingolia et al. 2009), (Brar et al. 2012) |
| DNA replication (Brar et al. 2012) | |
| Recombination (Brar et al. 2012) | |
| Metaphase II (Brar et al. 2012) | |
| Anaphase (Brar et al. 2012) | |
| Spore packing (Brar et al. 2012) | |
| Spores (Brar et al. 2012) | |
| C. elegans (Stadler et al. 2012) | L4 (Stadler et al. 2012) |
| L2 | |
| L1 | |
| M. musculus | Embryonic stem cells (Ingolia et al. 2011) |
| Neutrophils (Guo et al. 2010) | |
| Embryonic fibroblast (Lee et al. 2012) | |
| H. sapiens | HEK293 (Lee et al. 2012) |
Materials and Methods
Calculating the normalized footprint count (NFC) distribution
As seen in Figure 2, the majority of genes’ ribosome profiles have less than 50% of codons mapped with read counts. Therefore, to avoid analyzing unreliable ribosome profiles that could biases estimations, only genes with a median FC greater than one were included in the analysis (Dana and Tuller 2014). In addition, previous studies indicated an increase of FC at the beginning of the ORF (Ingolia 2010; Ingolia et al. 2011) and for some organisms at the end of ORF (Li et al. 2012); therefore, the first and last 20 codons were excluded from the analysis. Moreover, to prevent analysis of unreliable reads, codons with FC values less than one were excluded from the analysis (Li et al. 2012).
To enable comparison of footprint counts of a codon type from genes with different mRNA levels and initiation rates, FC of each codon were first normalized by the average FC of each gene (Li et al. 2012; Qian et al. 2012; Dana and Tuller 2014), resulting in NFC. This normalization enables measuring the relative time a ribosome spends translating each codon in a specific gene relative to other codons in it, while considering the total number of codons in the gene. Then, for each codon type a vector consisting of NFC values originating from all analyzed genes was generated, creating the “NFC distribution” of a codon.
Estimating the codons’ typical decoding time
Based on the characteristics of the NFC distributions, we suggest that their topology could result from a superposition of two distributions/components (Dana and Tuller 2014): the first one describes the “typical” decoding time of the ribosomes, which was modeled by a normal distribution characterized by its mean μ and variance .
The second component describes relatively rare translational pauses and ribosomal interactions such as traffic jams due to the codons’ different translation efficiency and was modeled by a random variable with an exponentially distribution, characterized by one parameter λ.
The summation of two independent normal and exponential random variables corresponding to the distributions mentioned above results in an exponentially modified Gaussian distribution. The parameters were estimated by fitting the measured NFC distributions to the exponentially modified Gaussian distribution, under the log-likelihood criterion. The μ parameter is referred as the typical decoding time of a codon. For more details, see Dana and Tuller (2014). Then, the MTDR index of a gene was defined as the geometrical mean of its codons translation rates.
Protein abundance and mRNA levels measurements
E. coli protein abundance measurements were downloaded from the PRIDE database (Vizcaino et al. 2013), and mRNA level measurements were taken from another source (Taniguchi et al. 2010). C. elegans mRNA levels were downloaded from Kirienko and Fay (2007). B. subtilis protein abundance measures were used as published by Chi et al. (2011), and mRNA levels were downloaded from another source (Nicolas et al. 2012). S. cerevisiae protein abundance measures were averaged from four quantitative large-scale measurements: two large scale measurements in two conditions (Newman et al. 2006), and a large-scale protein abundance measurement from two sources (Ghaemmaghami et al. 2003; Lee et al. 2011). mRNA levels were determined by averaging large scale measurements of mRNA levels (Wang et al. 2002; Ingolia et al. 2009).
Estimating the contribution of the MTDR index and other translation efficiency indexes to predicting protein abundance
To estimate whether the MTDR index improves the prediction protein levels relative to other translation efficiency indexes, we have modeled the relationship between protein abundance and the different translation efficiency indexes MTDR, transfer RNA (tRNA) adaptation index [tAI (dos Reis and Wernisch 2009)], and codon adaptation index [CAI (Sharp and Li 1987)], using a linear regressor (Seber and Lee 2012).
where the coefficients were estimated to minimize the mean square error difference between the prediction vector and the real protein abundance measurements .
For each one of the coefficients confidence intervals (Kendall and Stuart 1979) were calculated to determine the reliability of the estimates (at 95% confidence interval). A coefficient’s confidence interval that does not contain zeroes implies that it significantly contributes to the regression, i.e., the coefficient is not zero (Kendall and Stuart 1979).
Results
The estimated codons decoding times correlate with measures of codon usage bias
We started our analysis by comparing the typical decoding times estimated from the ribosome profiling data (µ) to other estimations of codon translation efficiency estimated using additional methods which are based on codon distributions. These include: 1) decoding time based on the codon adaptation index (), which calculates the codon bias in highly expressed genes (Sharp and Li 1987) and 2) decoding time based on the tRNA adaptation index () (dos Reis et al. 2004), which takes into consideration the number of tRNA copies in the genome recognizing each codon and additional codon/anticodon interactions. As seen in Table 2, the correlation between µ values and the other codon decoding time estimators is significant in all analyzed organisms (0.42 < P < 0.83; P < 0.00065).
Table 2. Spearman correlation between µ and various methods for estimating the typical codon decoding time.
| Estimation method | E. coli | B. subtilis | S. cerevisiae | C. elegans |
|---|---|---|---|---|
| r = 0.55 | r = 0.66 | r = 0.56 | r = 0.67 | |
| p = 4*10−6 | p = 8.8*10−9 | p = 3.2*10−6 | p = 4.3*10−9 | |
| r = 0.42 | r = 0.51 | r = 0.47 | r = 0.83 | |
| p = 0.00065 | p = 3.1*10−115 | p = 0.00013 | p = 1.6*10−16 |
In addition, we also compared the values to decoding times measured by using various experimental methods. For example, the amino acid insertion time per codon in E. coli was estimated using known biochemistry factors (Fluitt et al. 2007). Spearman correlation between insertion time and µ was 0.43 (P = 0.00051), whereas the correlation between insertion time and was lower: 0.35/0.31 (P = 0.0062/0.017), supporting the conjecture that µ values are better direct estimators of the amino acid insertion time. In another study (Chu et al. 2014) the authors found that for S. cerevisiae the GAG codon is more slowly translated than the GAA codon. This result is also supported by the µ estimations (GAG: 0.24 vs. GAA: 0.21). The same trend was also observed for the values (GAG: 0.016 vs. GAA: 0.006) and values (GAG: 2.44 vs. GAA: 1.1). In another study (Kemp et al. 2013) it was shown that in S. cerevisiae, replacing codons the codons “CAA” by the codons “CAG,” which are decoded by a rare tRNA, near the 5′ end of an ORF reduces luciferase expression by 60%. Indeed the estimated µ decoding time of the CAG codon was found to be greater than of the CAA codon (0.25 vs. 0.15). An additional work (Letzring et al. 2010) studied the effect of synonymous codon repeats of luciferase expression in S. cerevisiae; to validate our µ estimations, we calculated Spearman correlation between translation efficiency changes with respect to the wild type and the estimated µ values and found them to be significantly correlative (r = −0.33, P = 0.013).
The MTDR index predicts protein levels in standard conditions with comparable quality as other codon bias measures
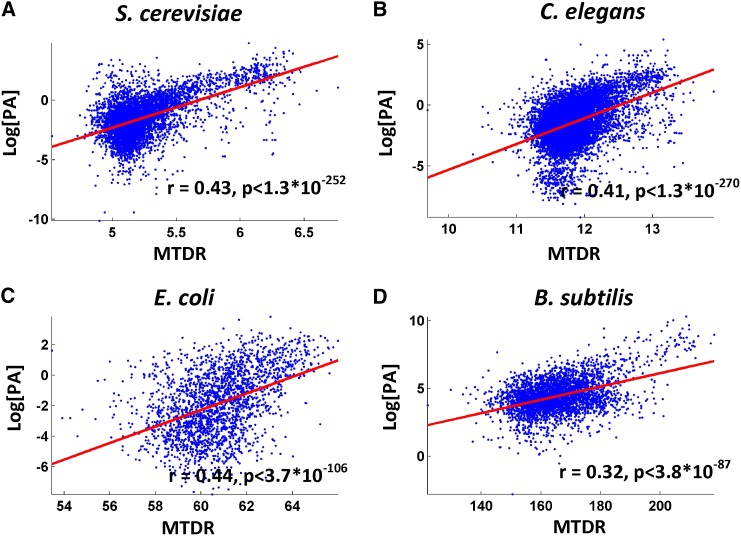
The usability of the suggested MTDR index was validated for four different organisms that have abundant large scale protein and mRNA levels measurements (E. coli, B. subtilis, C. elegans, and S. cerevisiae). Spearman correlation between the MTDR index to various proxies of translation efficiency such as protein abundance resulted in significant correlations (0.32< r < 0.44; P < 3.8*10−87; see also Figure 3 and Table 3). Similarly, a significant partial Spearman correlation between protein abundance and MTDR index given mRNA levels (partial correlation) was observed (0.23 < r < 0.5; P < 4.2*10−86; see Table 3).
Figure 3.
Protein abundance (PA) vs. mean of the typical decoding rates (MTDR) and Spearman correlation between MTDR index and protein abundance for various organisms.
Table 3. Spearman correlation between protein abundance (also when controlling for mRNA levels) and various translation efficiency indexes.
| Organism | Correlation Between Index and PA |
Correlation Between Index and PA Given mRNA Levels |
||||
|---|---|---|---|---|---|---|
| MTDR | CAI | tAI | MTDR | CAI | tAI | |
| E. coli | r = 0.44 | r = 0.56 | r = 0.55 | r = 0.5 | r = 0.61 | r = 0.48 |
| p < 3.7*10−106 | p = 3.8*10−184 | p =1.2*10−178 | p < 1.1*10−38 | p = 3.4*10−62 | p = 3.4*10−35 | |
| B. subtilis | r = 0.32 | 0.37 | r = 0.41 | r = 0.26 | r = 0.280 | r = 0.32 |
| p < 3.8*10−87 | p = 1.5*10−117 | p = 4.7*10−146 | p < 3.7*10−43 | p = 7.1*10−67 | p = 8.6*10−108 | |
| C. elegans | r = 0.41 | r = 0.52 | r = 0.56 | r = 0.23 | r = 0.35 | r = 0.41 |
| p < 1*10−270 | p = 0 | p = 0 | p < 4.2*10−86 | p = 3.7*10−206 | p = 6.1*10−293 | |
| S. cerevisiae | r = 0.43 | r = 0.44 | r = 0.47 | r = 0.3 | r = 0.33 | r = 0.37 |
| p < 1.3*10−252 | p = 1.1*10−275 | p = 2.6**10−314 | p < 1.2*10−102 | p = 5.3*10−125 | p = 1.1*10−66 | |
PA, protein abundance; MDTR, mean of the typical decoding rates; CAI, codon adaptation index; tAI, tRNA adaptation index.
Measures based on codon usage bias resulted in similar correlations with protein levels in standard conditions (Table 3); these results can be explained among others, by the fact that codon bias usage measures various (direct and indirect) aspects of gene expression (Chamary et al. 2006; Hershberg and Petrov 2008; Plotkin and Kudla 2011; Sauna and Kimchi-Sarfaty 2011) (Tuller and Zur, unpublished data), by the fact that ribosome profiling data are probably “noisier” and more biased than genomic sequence data (Dana and Tuller 2012; Gerashchenko and Gladyshev 2014), and by the fact that in this study we consider protein levels in “standard”/exponential conditions which are probably reflected more properly by codon bias usage.
For some of the analyzed organisms, a decrease in correlation between the different translation efficiency indexes and protein abundance was observed when controlling for mRNA levels. As genes with greater mRNA levels potentially consume a greater percentage of the ribosomes in the cell, they are expected to undergo stronger selection forgreater translation elongation speed (and/or other aspects of translation efficiency) to reduce ribosome utilization (Tuller et al. 2010a; Plotkin and Kudla 2011). Therefore we expect to see a positive correlation between measures of translation efficiency and mRNA levels (and not only with protein abundance); as a result, the partial correlation between protein levels and translation efficiency indexes decreases when controlling for mRNA levels.
Because the correlation between the MTDR index and protein abundance was found to be similar to the correlation between protein abundance and the other two translation efficiency indexes, we wanted to assess whether the MTDR index provides additional information with respect to the other two. To this end, we calculated a linear regressor based on tAI, CAI, and MTDR for predicting protein abundance levels (see the section Materials and Methods). Then, we checked the 95% confidence intervals of each one of the translation efficiency indexes and found that they do not include the value 0 for the variable MTDR (see the section Materials and Methods), demonstrating that the MTDR index contributes additional information to protein abundance prediction given the other two indexes.
Finally, it should be noted that in endogenous genes it is impossible to prove the direction of causality between the various translation efficiency indexes and protein abundance based on correlation; a correlation may suggest that 1) codons with greater µ values or codons that are recognized by more abundant tRNA molecule tend to improve translation rate and thus increase protein levels (e.g., Letzring et al. 2010; Tuller et al. 2010b); 2) genes with greater expression levels are selected to have codons with a greater MTDR/tAI index due to reasons not directly related, increasing the number of proteins per mRNA [for example, global ribosomal allocation (Kudla et al. 2009)].
Using the MTDR index for predicting ribosomal load
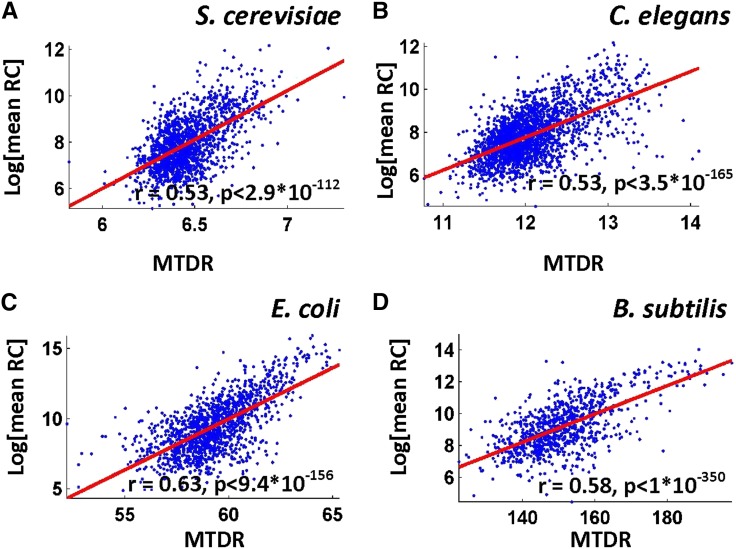
To demonstrate that the MTDR index can predict ribosomal densities of each one of the analyzed organisms we used 50% of the highly expressed genes to compute the typical codon decoding times. Next, we computed the MTDR index of the genes in the rest 50% of the highly expressed genes (that were not used for computing the typical decoding times). Finally, we computed the correlation between the MTDR index and the actual mean read count of these genes. As can be seen in Figure 4 and in Table 4, in almost all analyzed organisms/conditions the correlations are positive and significant (the top correlation is 0.8, P = 1.4*10−131). This result demonstrates that the MTDR index could be used as a good predictor of the ribosome load (number of ribosomes per mRNA * number of mRNA molecules), probably since highly translated genes tend to undergo selection for greater codon elongation rate, for example, to improve ribosomal allocation and translation cost (Kudla et al. 2009; Tuller et al. 2010a). For comparison, we also calculated the correlation between the tAI and CAI indexes and ribosomal load (see Table 4). These indexes resulted in similar correlations for the different “typical”/exponential stage conditions, however, produced a lower correlation in atypical conditions such as the different S. cerevisiae meiosis stages and for some M. musculus tissue types.
Figure 4.
Ribosomal load, as estimated by the mean footprint counts per gene vs. mean of the typical decoding rates (MTDR) index for various organisms and Spearman correlation between these factors.
Table 4. Correlation between MTDR index and ribosomal load for the different tissues/conditions of the analyzed organisms.
| Organism | Correlation Between MTDR Index and Ribosomal Load | Correlation Between tAI Index and Ribosomal Load | Correlation between CAI Index and Ribosomal Load |
|---|---|---|---|
| E. coli (Li et al. 2012) | r = 0.63 p = 9.4*10−156 | r = 0.56 p = 1.3*10−117 | r = 0.60 p = 5.7*10−142 |
| B. subtilis (Li et al. 2012) | r = 0.58 p = 0 | r = 0.33 p = 0 | r = 0.56 p = 0 |
| S. cerevisiae – Exponential (Ingolia et al. 2009), | r = 0.53 p = 2.9*10−112 | r = 0.62 p = 5.5*10−163 | r = 0.65 p = 3*10−184 |
| S. cerevisiae – Exponential (gb15) (Brar et al. 2012) | r = 0.32 p = 1*10−22 | r = 0.29 p = 2.3*10−18 | r = 0.32 p = 1.3*10−22 |
| S. cerevisiae – Exponential (A14201) (Brar et al. 2012) | r = 0.80 p = 1.4**10−131 | r = 0.77 p = 7.9*10−116 | r = 0.80 p = 1.1*10−134 |
| S. cerevisiae – DNA replication (Brar et al. 2012) | r = 0.26 p = 1.9*10−8 | r = 0.11 p = 0.023 | r = 0.09 p = 0.048 |
| S. cerevisiae – Recombination (Brar et al. 2012) | r = 0.31 p = 2.4*10−12 | r = 0.20 p = 7.6*10−6 | r = 0.20 p = 1.4*10−5 |
| S. cerevisiae – Metaphase II (Brar et al. 2012) | r = 0.46 p = 4.3*10−8 | r = -0.12 p = 0.15 | r = -0.12 p = 0.18 |
| S. cerevisiae – Anaphase (Brar et al. 2012) | r = 0.23 p = 0.027 | r = 0.10 p = 0.31 | r = 0.11 p = 0.26 |
| S. cerevisiae – Spore packing (Brar et al. 2012) | r = 0.50 p = 0 | r = -0.15 p = 0.045 | r = -0.16 p = 0.029 |
| S. cerevisiae – Spores (Brar et al. 2012) | r = 0.35 p = 0.00015 | r = -0.11 p = 0.25 | r = -0.08 p = 0.39 |
| C. elegans – L4 (Stadler et al. 2012) | r = 0.53 p = 3.5*10−165 | r = 0.45 p = 1.8*10−112 | r = 0.49 p = 1.9*10−132 |
| C. elegans – L2 (Stadler et al. 2012) | r = 0.48 p = 1.6*10−197 | r = 0.47 p = 1.4*10−185 | r = 0.51 p = 1.4*10−219 |
| C. elegans – L1 (Stadler et al. 2012) | r = 0.51 p = 5.9*10−10 | r = 0.42 p = 2.8*10−72 | r = 0.46 p = 9.3*10−86 |
| M. musculus – Embryonic stem cells(Ingolia et al. 2011) | r = 0.02 p = 0.35 | r = 0.07 p = 0.0095 | r = 0.07 p = 0.0046 |
| M. musculus – Neutrophils (Guo et al. 2010) | r = 0.22 p = 1.2*10−13 | r = 0.07 p = 0.012 | r = 0.12 p = 5.4*10−5 |
| M. musculus – Embryonic fibroblast (Lee et al. 2012) | r = 0.41 p = 4.1*10−15 | r = 0.22 p = 3.6*10−5 | r = 0.22 p = 3.4*10−5 |
| H. sapiens – HEK293 (Lee et al. 2012) | r = 0.17 p = 1.9*10−9 | r = 0.15 p = 9.2*10−8 | r = 0.15 p = 3.4*10−8 |
MDTR, mean of the typical decoding rates; tAI, tRNA adaptation index; CAI, codon adaptation index.
It should be mentioned that a decrease in this correlation was observed for non-exponential stages in S. cerevisiae and in greater eukaryotes such as H. sapiens and M musculus. This could result from: 1) a greater level of noise (see Figure 5) and biases caused by additional and/or more complicated biological mechanisms (e.g., due substantial alternative splicing in mammals the mapping of reads to exons is less trivial) (Engstrom et al. 2013); 2) the fact that mammals have smaller effective population size and thus lower selection pressure related to some translation aspects (Charlesworth 2009; dos Reis and Wernisch 2009); and 3) since growth rate is strongly related to fitness in unicellular organisms but not in mammals, there is lower effect on ribosomal allocation on the organisms fitness in mammals than in the rest of the analyzed organism (Rocha 2004; dos Reis and Wernisch 2009).
Figure 5.
Footprint count (FC) statistics for S. cerevisiae different meiosis stages. (A) Histogram of average read counts. (B) Histogram of percentage of positions with a positive number of mapped read counts. As can be seen in all of the organisms, most of the genes have very low read counts.
Assessing the ability of the MTDR index to predict translation efficiency in different experimental conditions
Codon bias indexes (e.g., CAI, tAI) are based solely on static information encoded in the genome or general chemical properties; thus, they cannot differentiate among different experimental conditions. One of the major advantages of the MTDR index is that it is condition specific. To demonstrate this advantage, we estimated the typical decoding times of S. cerevisiae in starvation conditions (Ingolia et al. 2009). Translation efficiency (defined as protein abundance normalized by mRNA levels) was calculated for rich (yeast extract peptone dextrose) and minimal (synthetic defined) media [protein abundance and mRNA levels measured per cell were taken from a previous study (Newman et al. 2006)].
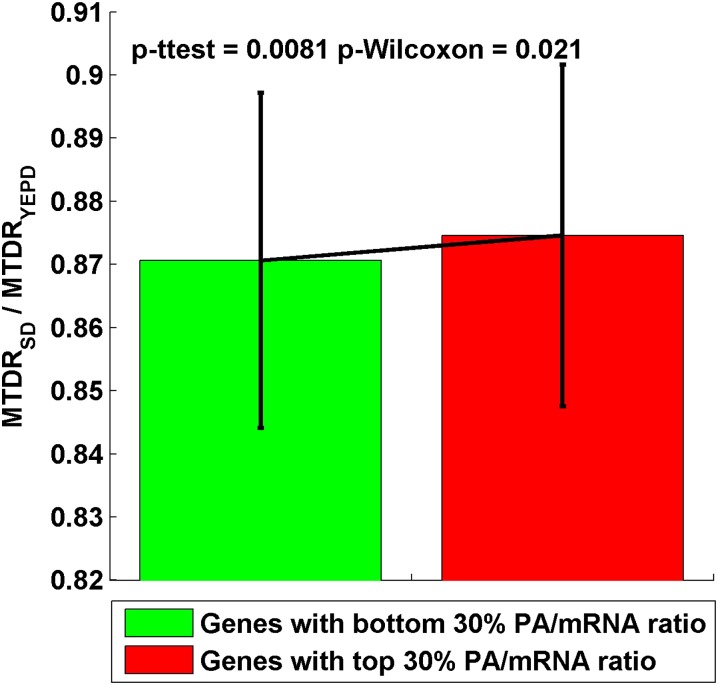
We found that the MTDR values of the genes with top/bottom 30% translation efficiency ratios are significantly different (t-test: P = 0.0081; Wilcoxon test: P = 0.021): genes with a greater change in their translation efficiency have a greater change in their estimated MTDR index (in the same direction). This result demonstrates the ability of the suggested index to estimate gene expression in different conditions (Figure 6).
Figure 6.
The ration between mean of the typical decoding rates (MTDR) index calculated in starvation (synthetic defined [SD]) and rich medium (yeast extract peptone dextrose [YEPD]) for genes with bottom/up 30% PA/mRNA ratio in SD/YEPD conditions (green/red).
In addition, we also found that Spearman correlation between the MTDR ratio and ribosomal load ratio for different tissue/developmental conditions in C. elegans, S. cerevisiae, and M. musculus (Table 5) was significantly positive, (0.17 < r < 0.72; P < 0.0003), indicating that the MTDR index could predict changes in ribosomal load, contrary to the static tAI and CAI indexes.
Table 5. Spearman correlation between MTDR index ratios and ribosomal load ratio for different tissues/developmental stages in C. elegans, S. cerevisiae (partial list) and M. musculus.
| Compared Conditions | Corr(MTDR1/MTDR2, meanRC1/meanRC2) | Compared Conditions | Corr(MTDR1/MTDR2, meanRC1/meanRC2) |
|---|---|---|---|
| C. elegans L4 vs. L2 | r = 0.53 P = 0 | S. cerevisiae premeiotic entry vs. metaphase I | r = 0.40 P = 0.00045 |
| C. elegans L4 vs. L1 | r = 0.51 P = 4.5*10−52 | S. cerevisiae premeiotic entry vs. spore packing | r = 0.33 P = 0.044 |
| C. elegans L2 vs. L1 | r = 0.51 P = 0 | S. cerevisiae premeiotic entry vs. spores | r = 0.56 P = 0.00042 |
| S. cerevisiae gb15 vs. A14201 | r = 0.59 P = 5.8*10−8 | S. cerevisiae DNA replication vs. metaphase II | r = 0.44 P = 0.014 |
| S. cerevisiae gb15 vs. premeiotic entry | r = 0.42 P = 0.00011 | S. cerevisiae recombination vs. metaphase II | r = 0.54 P = 3.7*10−5 |
| S. cerevisiae gb15 vs. recombination | r = 0.32 P = 2*10−6 | S. cerevisiae metaphase I vs. metaphase II | r = 0.45 P = 6.7*10−5 |
| S. cerevisiae gb15 vs. metaphase II | r = 0.55 P = 5.2*10−6 | S. cerevisiae metaphase II vs. anaphase | r = 0.45 P = 0.0031 |
| S. cerevisiae gb15 vs. spore packing | r = 0.34 P = 0.00074 | S. cerevisiae metaphase II vs. spore packing | r = 0.38 P = 0.055 |
| S. cerevisiae gb15 vs. spores | r = 0.45 P = 0.0013 | S. cerevisiae spore packing vs. spores | r = 0.52 P = 0.00079 |
| S. cerevisiae A14201 vs. premeiotic entry | r = 0.72 P = 0.0031 | M. musculus embryonic stem cells vs. neutrophils | r = 0.17 P = 0.0003 |
| S. cerevisiae A14201 vs. recombination | r = 0.56 P = 6.5*10−5 | M. musculus embryonic stem cells vs. fibroblast | r = 0.25 P = 0.00074 |
| S. cerevisiae A14201 vs. spore packing | r = 0.56 P = 0.0096 | M. musculus neutrophils vs. fibroblast | r = 0.31 P = 0.00023 |
Application
The MTDR application enables calculating the translation efficiency of various genes according to their ORF sequence. The input of the application includes the selection of one of the organisms and its tissue/condition and a file containing the ORFs of the requested genes (in FASTA format or text format where each ORF is defined in a separate line). ORFs could also be straightforwardly inserted in a textbox. The application returns an output file which includes for each of the ORFs their MTDR index. The available organisms and conditions/tissues are depicted in Table 1. The distributable cross platform application and user manual are available for download at:
Acknowledgments
This research is partially supported by research grants from the German-Israeli Foundation (GIF) and Israel Cancer Research Fund (ICRF).
Footnotes
Communicating editor: A. M. Dudley
Literature Cited
- Brar G. A., Yassour M., Friedman N., Regev A., Ingolia N. T., et al. , 2012. High-resolution view of the yeast meiotic program revealed by ribosome profiling, Science 335, 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamary J. V., Parmley J. L., Hurst L. D., 2006. Hearing silence: non-neutral evolution at synonymous sites in mammals. Nat. Rev. Genet. 7: 98–108. [DOI] [PubMed] [Google Scholar]
- Charlesworth B., 2009. Fundamental concepts in genetics: effective population size and patterns of molecular evolution and variation. Nat. Rev. Genet. 10: 195–205. [DOI] [PubMed] [Google Scholar]
- Chi B. K., Gronau K., Mader U., Hessling B., Becher D., et al. , 2011. S-bacillithiolation protects against hypochlorite stress in Bacillus subtilis as revealed by transcriptomics and redox proteomics. Mol. Cell Proteomics 10: M111 009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D., Kazana E., Bellanger N., Singh T., Tuite M. F., et al. , 2014. Translation elongation can control translation initiation on eukaryotic mRNAs. EMBO J. 33: 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana A., Tuller T., 2012. Determinants of translation elongation speed and ribosomal profiling biases in mouse embryonic stem cells. PLOS Comput. Biol. 8: e1002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana A., Tuller T., 2014. The effect of tRNA levels on decoding times of mRNA codons. Nucleic Acids Res. 42: 9171–9181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Reis M., Savva R., Wernisch L., 2004. Solving the riddle of codon usage preferences: a test for translational selection. Nucleic Acids Res. 32: 5036–5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Reis M., Wernisch L., 2009. Estimating translational selection in eukaryotic genomes. Mol. Biol. Evol. 26: 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom P. G., Steijger T., Sipos B., Grant G. R., Kahles A., et al. , 2013. Systematic evaluation of spliced alignment programs for RNA-seq data. Nat. Methods 10: 1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluitt A., Pienaar E., Viljoen H., 2007. Ribosome kinetics and aa-tRNA competition determine rate and fidelity of peptide synthesis. Comput. Biol. Chem. 31: 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. M., Erill I., 2010. Relative codon adaptation: a generic codon bias index for prediction of gene expression. DNA Res. 17: 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko M. V., Gladyshev V. N., 2015. Translation inhibitors cause abnormalities in ribosome profiling experiments. Nucleic Acids Res. 42: e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami S., Huh W. K., Bower K., Howson R. W., Belle A., et al. , 2003. Global analysis of protein expression in yeast. Nature 425: 737–741. [DOI] [PubMed] [Google Scholar]
- Guo H., Ingolia N. T., Weissman J. S., Bartel D. P., 2010. Mammalian microRNAs predominantly act to decrease target mRNA levels, Nature 466, 835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberg R., Petrov D. A., 2008. Selection on codon bias. Annu. Rev. Genet. 42: 287–299. [DOI] [PubMed] [Google Scholar]
- Ingolia N. T., 2010. Genome-wide translational profiling by ribosome footprinting. Methods Enzymol. 470: 119–142. [DOI] [PubMed] [Google Scholar]
- Ingolia N. T., Ghaemmaghami S., Newman J. R., Weissman J. S., 2009. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia N. T., Lareau L. F., Weissman J. S., 2011. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell 147: 789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A. J., Betney R., Ciandrini L., Schwenger A. C., Romano M. C., et al. , 2013. A yeast tRNA mutant that causes pseudohyphal growth exhibits reduced rates of CAG codon translation. Mol. Microbiol. 87: 284–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall M., Stuart A., 1979. The Advanced Theory of Statistics. Vol. 2, Ed. 1. Inference and Relationship. Griffin, London. [Google Scholar]
- Kirienko N. V., Fay D. S., 2007. Transcriptome profiling of the C. elegans Rb ortholog reveals diverse developmental roles. Dev. Biol. 305: 674–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla G., Murray A. W., Tollervey D., Plotkin J. B., 2009. Coding-sequence determinants of gene expression in Escherichia coli. Science 324: 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. V., Topper S. E., Hubler S. L., Hose J., Wenger C. D., et al. , 2011. A dynamic model of proteome changes reveals new roles for transcript alteration in yeast. Mol. Syst. Biol. 7: 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Liu B., Huang S. X., Shen B., Qian S. B., et al. , 2012. Global mapping of translation initiation sites in mammalian cells at single-nucleotide resolution, Proceedings of the National Academy of Sciences 109, E2424–E2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzring D. P., Dean K. M., Grayhack E. J., 2010. Control of translation efficiency in yeast by codon-anticodon interactions. RNA 16: 2516–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. W., Oh E., Weissman J. S., 2012. The anti-Shine-Dalgarno sequence drives translational pausing and codon choice in bacteria. Nature 484: 538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J. R., Ghaemmaghami S., Ihmels J., Breslow D. K., Noble M., et al. , 2006. Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature 441: 840–846. [DOI] [PubMed] [Google Scholar]
- Nicolas P., Mader U., Dervyn E., Rochat T., Leduc A., et al. , 2012. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science 335: 1103–1106. [DOI] [PubMed] [Google Scholar]
- Plotkin J. B., Kudla G., 2011. Synonymous but not the same: the causes and consequences of codon bias. Nat. Rev. Genet. 12: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W., Yang J. R., Pearson N. M., Maclean C., Zhang J., 2012. Balanced codon usage optimizes eukaryotic translational efficiency. PLoS Genet. 8: e1002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha E. P., 2004. Codon usage bias from tRNA’s point of view: redundancy, specialization, and efficient decoding for translation optimization. Genome Res. 14: 2279–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabi R., Tuller T., 2014. Modelling the efficiency of codon-tRNA interactions based on codon usage bias. DNA Res. 21: 511–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauna Z. E., Kimchi-Sarfaty C., 2011. Understanding the contribution of synonymous mutations to human disease. Nat. Rev. Genet. 12: 683–691. [DOI] [PubMed] [Google Scholar]
- Seber G. A., Lee A. J., 2012. Linear Regression Analysis. John Wiley & Sons, New York. [Google Scholar]
- Sharp P. M., Li W. H., 1987. The codon Adaptation Index—a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 15: 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler M., Artiles K., Pak J., Fire A., 2012. Contributions of mRNA abundance, ribosome loading, and post- or peri-translational effects to temporal repression of C. elegans heterochronic miRNA targets, Genome Res. 22, 2418–2426. 2410.1101/gr.136515.136111. Epub 132012 Aug 136511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Y., Choi P. J., Li G. W., Chen H., Babu M., et al. , 2010. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science 329: 533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuller T., Carmi A., Vestsigian K., Navon S., Dorfan Y., et al. , 2010a An evolutionarily conserved mechanism for controlling the efficiency of protein translation. Cell 141: 344–354. [DOI] [PubMed] [Google Scholar]
- Tuller T., Waldman Y. Y., Kupiec M., Ruppin E., 2010b Translation efficiency is determined by both codon bias and folding energy. Proc. Natl. Acad. Sci. USA 107: 3645–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuller T., Zur H., 2014. Multiple Roles of the Coding Sequence 5' End in Gene Expression Regulation. To appear in Nucleic Acids Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaino J. A., Cote R. G., Csordas A., Dianes J. A., Fabregat A., et al. , 2013. The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 41: D1063–D1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu C. L., Storey J. D., Tibshirani R. J., Herschlag D., et al. , 2002. Precision and functional specificity in mRNA decay. Proc. Natl. Acad. Sci. USA 99: 5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F., 1990. The ’effective number of codons’ used in a gene. Gene 87: 23–29. [DOI] [PubMed] [Google Scholar]