Weber et al. report that neutrophils are required for both the sensitization and elicitation phase of contact hypersensitivity. Their results identify a novel role for neutrophils in shaping the adaptive immune response.
Abstract
Allergic contact dermatitis and its animal model, contact hypersensitivity (CHS), are T cell–mediated inflammatory skin diseases induced by contact allergens. Though numerous cellular and molecular players are known, the mechanism of chemical-induced sensitization remains poorly understood. Here, we identify neutrophils as crucial players in the sensitization phase of CHS. Genetic deficiency of neutrophils caused by myeloid-specific deletion of Mcl-1 or antibody-mediated depletion of neutrophils before sensitization abrogated the CHS response. Neutrophil deficiency reduced contact allergen-induced cytokine production, gelatinase release, and reactive oxygen species production in naive mice. Mast cell deficiency inhibited neutrophil accumulation at the site of sensitization. In turn, neutrophils were required for contact allergen-induced release of further neutrophil-attracting chemokines, migration of DCs to the draining lymph nodes, and priming of allergen-specific T cells. Lymph node cells from mice sensitized in the absence of neutrophils failed to transfer sensitization to naive recipients. Furthermore, no CHS response could be induced when neutrophils were depleted before elicitation or when normally sensitized lymph node cells were transferred to neutrophil-deficient recipients, indicating an additional role for neutrophils in the elicitation phase. Collectively, our data identify neutrophils to be critically involved in both the sensitization and elicitation phase of CHS.
Contact hypersensitivity (CHS), the animal model of human allergic contact dermatitis (ACD), is an inflammatory skin disease triggered by repeated exposure to contact allergens. CHS is a delayed-type hypersensitivity reaction mediated by T cells recognizing hapten-modified self-peptides in the context of MHC molecules (Vocanson et al., 2009). The first sensitization phase of the CHS response is characterized by activation of DCs, their migration to the skin-draining lymph nodes, and the priming of allergen-specific T cells. The second elicitation phase is dominated by recruitment and activation of effector T cells to the site of allergen challenge and T cell–mediated tissue damage.
Contact allergens activate the innate immune system by complex mechanisms involving Toll-like receptors, the NLRP3 inflammasome, and endogenous danger signals such as extracellular ATP, fragments of the extracellular matrix component hyaluronic acid and ROS (Martin et al., 2008; Schmidt et al., 2010; Weber et al., 2010; Esser et al., 2012). Innate immune cells such as DCs and mast cells have been shown to be crucial for the sensitization phase of CHS (Martin et al., 2008; Weber et al., 2010; Dudeck et al., 2011; Martin, 2012). However, the contribution of other innate immune cells to the sensitization phase of CHS is poorly understood.
Neutrophils provide the first line of defense against invading bacterial and fungal pathogens (Mócsai, 2013), but their improper activation may also contribute to tissue damage during various diseases (Mantovani et al., 2011; Németh and Mócsai, 2012). Neutrophils can exert a robust antimicrobial and proinflammatory reaction through ROS production, exocytosis of granule proteins (including proteases such as gelatinase), and the release of various cytokines (Mantovani et al., 2011). Interestingly, neutrophils are found in the inflammatory skin lesions of ACD patients (Goebeler et al., 2001). Studies using anti–Gr-1 antibodies before allergen reexposure suggested a role for neutrophils in the elicitation phase of CHS (Engeman et al., 2004), though interpretation of those experiments is complicated by the depletion of various other lineages such as inflammatory monocytes, macrophages, DCs and activated T cells by anti–Gr-1 antibodies (Dunay et al., 2008; Wojtasiak et al., 2010). The role of neutrophils in the sensitization phase of CHS has not yet been investigated.
The aforementioned issues prompted us to test the role of neutrophils in both phases of the CHS response using genetic deletion and antibody-mediated depletion approaches combined with trans-sensitization by adoptive transfer of lymph node cells to naive recipients. Our results provide the first evidence for a critical role for neutrophils in the sensitization phase of CHS.
RESULTS AND DISCUSSION
Genetic deficiency of neutrophils abrogates the CHS response
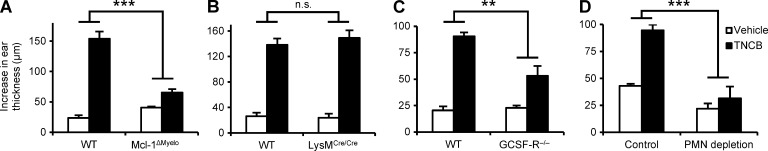
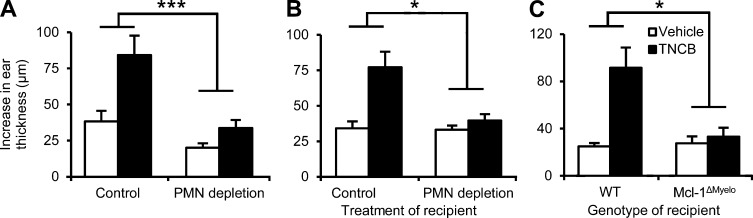
To investigate the role of neutrophils in CHS, we used mice with a myeloid-specific conditional deletion of the antiapoptotic Mcl-1 protein (LysMCre/CreMcl-1flox/flox mutants referred to as Mcl-1ΔMyelo mice). Those mice have a selective neutrophil deficiency caused by the requirement of Mcl-1 for the survival of neutrophils, whereas other myeloid-lineage cells (even those that express the LysMCre knock-in allele) are not affected because they do not rely on Mcl-1 for their survival (Dzhagalov et al., 2007). As shown in Fig. 1 A, the Mcl-1ΔMyelo mutation abrogated the ear thickness increase upon reexposure of 2,4,6-trinitrochlorobenzene (TNCB)-sensitized mice to TNCB challenge (P = 2.9 × 10−9), indicating that neutrophil-deficient mice are resistant to CHS.
Figure 1.
Neutrophils are essential for the CHS response. Mice were sensitized with TNCB or acetone and were challenged with TNCB 5 d after sensitization. The increase in ear thickness 24 h after challenge is depicted. (A and B) CHS response in WT, Mcl-1ΔMyelo, and LysMCre/Cre mice. (C) CHS response in bone marrow chimeras with WT or GCSF-R−/− hematopoietic compartment. (D) CHS response in WT mice treated with a neutrophil-depleting anti-Ly6G antibody (PMN depletion) or a control rat IgG 24 h before sensitization. The graphs show mean and SEM from 8–13 (A), 4–6 (B), 5–8 (C), or 5–24 (D) individual mice per group from 3 (A–C) or 5 (D) independent experiments. **, P < 0.01; ***, P < 0.002; n.s., statistically not significant.
We also assessed the effect of the Mcl-1ΔMyelo mutation on various leukocyte lineages, including known crucial players in CHS. We observed a nearly complete absence of neutrophils in the peripheral blood (98% reduction; P = 2.41 × 10−14) and spleen (91% reduction; P = 0.015) of Mcl-1ΔMyelo mice, whereas the number of circulating monocytes and T cells (P = 0.11 and 0.84, respectively), and splenic dendritic cells (P = 0.35) were not affected, and the number of splenic macrophages was even slightly, but not significantly (P = 0.081), increased (Fig. S1, A and B). Interestingly, Mcl-1ΔMyelo mice did not show any gross phenotypes and survived normally up to 6 mo of age, suggesting that the low but clearly present number of neutrophils was sufficient to cope with the commensal flora under our animal housing conditions.
Because the LysMCre component of the Mcl-1ΔMyelo mutation is a loss-of-function knock-in mutation of the lysozyme M-encoding gene (Clausen et al., 1999), we also tested whether LysMCre/Cre mice are resistant to CHS. As shown in Fig. 1 B, the LysMCre/Cre mutation did not affect CHS development (P = 0.51), indicating that the defective response in Mcl-1ΔMyelo mice is not caused by the lack of lysozyme M.
As an additional model of neutrophil deficiency, we also tested the effect of genetic deletion of the G-CSF receptor. For those experiments, we used bone marrow chimeras generated by transplanting bone marrow cells from G-CSF receptor-deficient mice (Hermans et al., 2003) on the FVB/N genetic background into WT FVB/N recipients. Such chimeras had a 92% reduction of circulating neutrophil counts compared with control WT chimeras (P = 1.67 × 10−12). As shown in Fig. 1 C, G-CSF receptor deficiency caused a substantial reduction in the CHS response (P = 0.0068).
Collectively, the aforementioned observations provide the first genetic evidence for a functional role of neutrophils in contact hypersensitivity, both on the C57BL/6 and the FVB/N genetic backgrounds. The less dramatic effect of G-CSF receptor deficiency was likely due to the less severe reduction of circulating neutrophil numbers in those animals.
Neutrophil depletion abrogates the CHS response
As another approach to test the functional importance of neutrophils during the sensitization phase of CHS, we depleted neutrophils using an antibody against the Ly6G antigen (Charmoy et al., 2011). As shown in Fig. S1 (C and D), whereas neutrophil depletion dramatically reduced circulating (P = 0.00037) and splenic (P = 9.6 × 10−5) neutrophil numbers, it did not affect circulating monocytes or T cells (P = 0.38 and 0.43, respectively), or splenic macrophages or dendritic cells (P = 0.88 and 0.46, respectively). Importantly, as shown in Fig. 1 D, depletion of neutrophils 24 h before sensitization abrogated the overall CHS response (P = 1.1 × 10−10), providing an independent confirmation of the role of neutrophils in contact hypersensitivity.
Kinetic analysis of neutrophil depletion
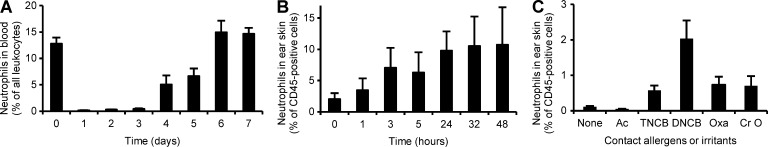
We next tested the time-course of circulating neutrophil numbers after antibody-mediated neutrophil depletion. As shown in Fig. 2 A, circulating neutrophils were almost completely absent 1 d after the depletion (which corresponds to the time of sensitization in CHS experiments) and remained at very low levels for two additional days. Neutrophil numbers were normalized (P = 0.40) 6 d after depletion, which corresponds to the time of elicitation. Those results suggest that the effect of neutrophil depletion on the CHS response is caused by the absence of neutrophils during the sensitization phase, rather than a prolonged effect causing neutrophil depletion also during the elicitation phase.
Figure 2.
Neutrophils in the CHS sensitization phase. (A) Analysis of the number of circulating neutrophils before or at the indicated times after treatment with the neutrophil-depleting anti-Ly6G antibody. (B and C) Infiltration of neutrophils was tested at the indicated times after sensitization with TNCB (B) or 24 h after sensitization with TNCB, DNCB, oxazolone (Oxa), or croton oil (Cr O) on the ears. Neutrophil infiltration was assessed by digestion of the ear skin followed by flow cytometry. The graphs show mean and SEM from 7–26 (A), 8–11 (B), or 9 (C) mice per group from 6 (A) or 4 (B and C) independent experiments.
Neutrophils infiltrate the sensitization site
Next, we tested whether neutrophils infiltrate the sensitization site. To this end, we digested TNCB-treated skin samples of naive WT mice and quantified the number of neutrophils by flow cytometry. We observed a profound infiltration of neutrophils to the skin beginning at a few hours after sensitization and plateauing at around 24 h (Fig. 2 B). Other contact allergens such as 2,4-Dinitrochlorobenzene (DNCB) or oxazolone and the irritant croton oil also induced recruitment of neutrophils to the skin of naive WT animals (Fig. 2 C).
Neutrophils are required for the contact allergen-induced inflammatory response
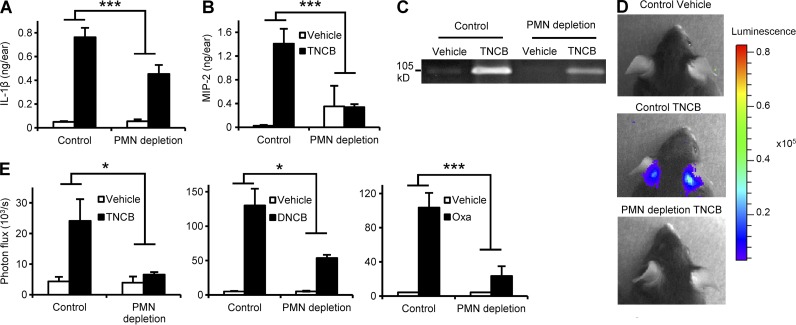
To analyze the function of neutrophils during the sensitization phase of CHS, we tested various signs of contact allergen-induced skin inflammation in naive WT mice. First, we measured the level of IL-1β, a master regulator of the inflammatory reaction and a known central component of the CHS response (Shornick et al., 1996; Weber et al., 2010), at the sensitization site. As shown in Fig. 3 A, neutrophil depletion strongly inhibited the TNCB-induced up-regulation of IL-1β in the skin of naive WT mice (P = 0.0019).
Figure 3.
Neutrophil depletion suppresses the contact allergen-induced inflammatory response. Naive mice were treated with a neutrophil-depleting anti-Ly6G antibody (PMN depletion) or control rat IgG, sensitized with TNCB, DNCB, oxazolone (Oxa), or vehicle control on both ears 24 h later, and the development of an inflammatory environment was tested after an additional 24 h. (A and B) Analysis of IL-1β and MIP-2 levels in the ear tissue by ELISA. (C) Analysis of gelatinase levels by in-gel zymography. (D and E) Analysis of ROS production by in vivo bioluminescence. C and D show representative images from 3 independent experiments. Bar graphs show mean and SEM from 4–5 (A and B) or 5–16 (E) individual mice from 3 (A and B) or 2–3 (E) independent experiments. *, P < 0.05; ***, P < 0.002.
Recent studies on arthritis development indicated that neutrophils trigger a positive-feedback loop by releasing mediators attracting additional neutrophils to the site of inflammation (Kim et al., 2006; Kovács et al., 2014). This coordinated action, termed neutrophil swarming (Chtanova et al., 2008), is partially mediated by neutrophil-derived CXC chemokines (KC and MIP-2, also known as CXCL1 and CXCL2, respectively) acting as potent neutrophil chemoattractants. As shown in Fig. 3 B, sensitization of naive control mice with TNCB triggered robust up-regulation of MIP-2, which was strongly reduced in neutrophil-depleted mice (P = 0.0013). Similar results were obtained with KC (unpublished data).
Another function of neutrophils is the release of proteolytic enzymes such as gelatinase, which leads to extracellular matrix degradation, possibly contributing to the CHS response (Wang et al., 1999). In-gel zymography of tissue extracts revealed up-regulation of gelatinase activity in the affected skin of TNCB-sensitized naive control animals, which was strongly decreased upon prior depletion of neutrophils (Fig. 3 C). Recent studies also demonstrated a crucial role for ROS in the skin in the sensitization and elicitation phases of CHS (Esser et al., 2012). Because neutrophils are able to release large amounts of ROS, we tested in vivo ROS production using a bioluminescence approach. Sensitization with TNCB caused significant ROS production in the skin of naive mice, which was strongly reduced (P = 0.029) by prior depletion of neutrophils (Fig. 3, D and E). In addition, the contact allergens DNCB and oxazolone also potently induced in vivo ROS production, which was also inhibited by neutrophil depletion (P = 0.018 and 0.0015, respectively; Fig. 3 E).
Collectively, neutrophils are required for a broad spectrum of contact sensitizer-induced local inflammatory skin reactions, including up-regulation of the master regulator of contact hypersensitivity, coordination of neutrophil swarming behavior, and release of proteolytic enzymes and ROS.
Mast cells mediate neutrophil recruitment during the sensitization phase of CHS
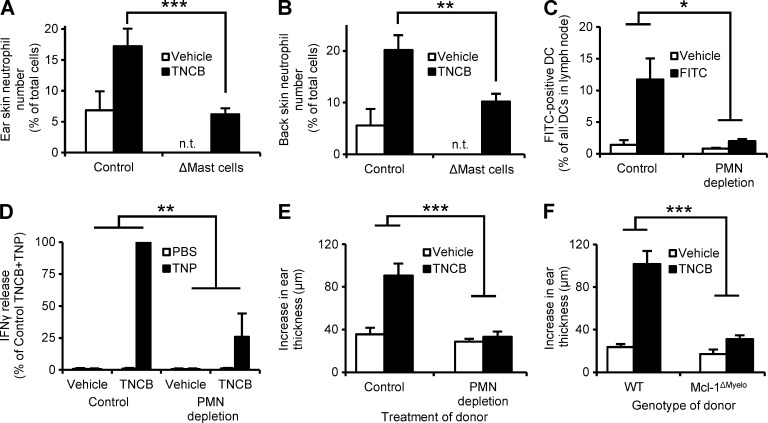
As mast cells are important innate immune mediators of the CHS response (Dudeck et al., 2011), we next tested the relationship between neutrophils and mast cells during contact sensitization. To this end, we tested the effect of diphtheria toxin (DT)–induced mast cell deficiency, made possible by Mcpt5-Cre–induced expression of the DT receptor in skin-resident connective tissue type mast cells (Dudeck et al., 2011). As expected, TNCB treatment of the ears or the back skin of naive DT-treated Mcpt5-Cre–negative control mice triggered a robust neutrophil infiltration (Fig. 4, A and B). However, this response was substantially reduced in similarly treated Mcpt5-Cre+ iDTR (i.e., mast cell–depleted) mice (P = 0.0008 and 0.0069 for the ears and the back skin, respectively; Fig. 4, A and B). This indicates that mast cells trigger neutrophil recruitment upon allergen exposure of the skin during the sensitization phase of the CHS response.
Figure 4.
Neutrophils are recruited by mast cells and trigger DC migration and T cell priming. (A and B) Mice with a DT-induced mast cell deficiency (ΔMast cells) or control animals were sensitized with TNCB on the ears (A) or the back skin (B) and the number of infiltrating neutrophils was determined 24 h later. (C) Mice were treated with the neutrophil-depleting anti-Ly6G antibody (PMN depletion) or control antibody, and FITC or vehicle was applied to the skin 24 h later, and the number of FITC-positive DCs in the draining lymph nodes was determined after an additional 24 h. (D) Neutrophil-depleted and control mice were sensitized with TNCB or acetone. Their draining lymph node cells were isolated 5 d after sensitization, restimulated by TNP-modification, and their IFN-γ production was measured by ELISA. (E and F) Neutrophil-depleted (E), Mcl-1ΔMyelo (F), or the appropriate control mice were sensitized with TNCB or acetone, their draining lymph node cells were isolated 5 d after sensitization, and transferred to naive mice, which were then challenged with TNCB and the increase in their ear thickness was measured. Graphs show mean and SEM from 4–15 (A-B), 6–8 (C), 8–9 (E), and 7–9 (F) individual mice or 3 independent samples (D) per group from 2 (A–C) or 3 (D–F) independent experiments. In (D), data are expressed in percentage of the IFN-γ release in nondepleted TNCB+TNP samples, which corresponded to 1.5 ± 0.6 ng IFN-γ per 106 cells. n.t., not tested. *, P < 0.05; **, P < 0.01; ***, P < 0.002.
We have also tested whether TNCB can directly activate neutrophils in vitro. We have not been able to detect any respiratory burst response when neutrophils were stimulated using a wide concentration range of TNCB and under varying experimental conditions (unpublished data). Those results are in agreement with an indirect mechanism of neutrophil activation during the CHS sensitization phase.
The role of neutrophils in DC migration and priming of allergen-specific T cells
A critical step in the CHS sensitization phase is the priming of allergen-specific T cells by DCs that migrate from the affected skin to the draining lymph nodes in an IL-1β–dependent manner (Cumberbatch et al., 2002). Because we observed reduced IL-1β production in neutrophil-depleted mice, we next addressed whether neutrophils are required for contact allergen-induced migration of DCs to the draining lymph nodes. As shown in Fig. 4 C, the contact allergen FITC triggered DC migration to the local draining lymph nodes and this response was severely impaired in neutrophil-depleted mice (P = 0.044).
Because DC migration is a crucial step in the sensitization of naive mice, we tested whether priming of allergen-specific T cells was altered in neutrophil-depleted mice. We assessed IFN-γ production by allergen-specific T cells from neutrophil-depleted or control-treated mice in response to allergen reexposure during an in vitro restimulation assay (Fig. 4 D). Lymph node cells from TNCB-sensitized mice were restimulated 5 d after sensitization by modification with TNBS, the water soluble analogue of TNCB that modifies proteins to generate TNP epitopes for T cells. Although lymph node cells from sensitized nondepleted mice responded with strong production of IFN-γ, a strongly decreased IFN-γ release was observed from the lymph node cells of neutrophil-depleted mice (P = 0.0036; Fig. 4 D), indicating a critical role for neutrophils in contact allergen-induced T cell priming.
We performed adoptive CHS (trans-sensitization or passive CHS) experiments to further investigate the role of neutrophils in the priming of allergen-specific T cells. The skin-draining lymph node cells were isolated 5 d after TNCB sensitization, and were transferred to naive recipients, which were then challenged by TNCB application. As shown in Fig. 4 (E and F), mice receiving lymph node cells from TNCB-sensitized undepleted WT donors displayed a strong ear swelling response. No such response could be observed in recipients of lymph node cells from TNCB-sensitized neutrophil-depleted (P = 0.00068; Fig. 4 E) or genetically neutrophil-deficient (P = 0.00025; Fig. 4 F) donors.
Collectively, neutrophil deficiency at the time of sensitization leads to defective allergen-induced migration of DCs to the draining lymph nodes and defective priming of allergen-specific T cells. These results indicate that neutrophils are indispensable for the sensitization phase of CHS.
Neutrophils play a crucial role in the elicitation phase of CHS
Prior studies showed that treatment of mice with an anti-Gr-1 antibody before elicitation inhibited the CHS response, indicating an important role for Gr-1–positive cells (possibly neutrophils) in the elicitation phase of CHS (Engeman et al., 2004). We also aimed to assess the role of neutrophils in the elicitation phase using the more neutrophil-specific anti-Ly6G antibody and the neutrophil-deficient Mcl-1ΔMyelo mouse strain. As shown in Fig. 5 A, anti–Ly6G-mediated depletion of neutrophils 24 h before elicitation in sensitized mice abrogated the ear swelling response (P = 1.6 × 10−7). Additional adoptive transfer experiments revealed that no TNCB-induced ear swelling response could be observed when lymph node cells isolated from TNCB-sensitized WT donor mice were adoptively transferred to neutrophil-depleted or Mcl-1ΔMyelo recipient mice (P = 0.011 and 0.029, respectively; Fig. 5, B and C). Those results provide direct evidence for the essential role of neutrophils in the elicitation phase of CHS.
Figure 5.
Neutrophils are required for the elicitation phase of CHS. (A) Mice were sensitized by TNCB or acetone, treated with the neutrophil-depleting anti-Ly6G antibody (PMN depletion) 4 d later, and challenged with TNCB after an additional 24 h. The increase of ear thickness during an additional 24 h was measured. (B and C) Nondepleted WT naive mice were sensitized by TNCB or acetone, and their lymph node cells were isolated 5 d later and injected into neutrophil-depleted (B), Mcl-1ΔMyelo (C), or appropriate control recipients which were then challenged with TNCB and the increase of their ear thickness after 24 h was measured. Graphs show mean and SEM from 3–12 (A) 11–12 (B), or 4–9 (C) individual mice per group from 3 (A and C) or 4 (B) experiments. *, P < 0.05; ***, P < 0.002.
In summary, we demonstrate that neutrophils are crucially involved in both the sensitization and elicitation phases of CHS. In case of sensitization, mast cells trigger the recruitment of neutrophils, which further promote their own recruitment in a swarming manner, and are then required for contact allergen-induced local inflammation, activation, and migration of DCs and the subsequent priming of allergen-specific T cells. These results indicate that neutrophils play critical roles in various phases and diverse models of allergic skin inflammation, making them attractive targets for the development of future therapeutic strategies.
MATERIALS AND METHODS
Animals.
Mice carrying the Mcl1tm1Ywh (Mcl-1flox) floxed allele of the Mcl-1–encoding gene (Dzhagalov et al., 2007) were obtained from Y. He (Duke University, Durham, NC) and were crossed to mice carrying the Lyz2tm1(cre)Ifo (LysMCre) knock-in strain expressing the Cre recombinase in the myeloid compartment (Clausen et al., 1999). LysMCre/CreMcl-1flox/+ mice were used to maintain the strain and to obtain LysMCre/CreMcl-1flox/flox homozygous animals (referred to as Mcl-1ΔMyelo mice). The genotype of the mice was confirmed by allele-specific PCR reactions. Due to the limited availability of Mcl-1ΔMyelo mice, bone marrow chimeras with an Mcl-1ΔMyelo hematopoietic system were occasionally generated as previously described (Jakus et al., 2009) and used for CHS experiments. Identical results were obtained with intact Mcl-1ΔMyelo mice and Mcl-1ΔMyelo bone marrow chimeras. All mice were on the C57BL/6 genetic background. Control C57BL/6 animals were obtained from our colony or purchased from the Hungarian National Institute of Oncology (Budapest, Hungary). G-CSF receptor-deficient (Csf3rtm1Eur/tm1Eur, referred to as GCSF-R−/−) mice on the FVB/N genetic background (Hermans et al., 2003) were generously provided by I.P. Touw (Erasmus University, Rotterdam, Netherlands). Mice were kept in individually sterile ventilated cages (Tecniplast) in a conventional facility.
Mcpt5-Cre iDTR mice (C57BL/6J background) were generated as previously described (Dudeck et al., 2011) and housed at the Experimental Centre at the University of Technology Dresden, Medical Faculty Carl-Gustav Carus, under specific pathogen–free conditions.
All procedures were in accordance with institutional guidelines on animal welfare and were approved by the Semmelweis University Animal Experimentation Review Board or the Landesdirektion Dresden.
Chemicals and antibodies.
TNCB, DNCB, oxazolone, FITC, luminol, and croton oil were obtained from Sigma-Aldrich. Isoflurane was purchased from Baxter, and Liberase II kit was obtained from Roche. Antibodies specific for the following surface markers were used: CD4 (L3T4), CD8 (53–6.7), CD45.2 (104), CD11c (HL3), I-Ab (AF6-120.1), CD11b (M1/70), Gr-1 (RB6-8C5), CD3 (145-2C11), Ly6G (1A8), and CCR7 (4B12) were obtained from BD; F4/80 (BM8) were purchased from eBioscience; F4/80 (Cl:A3-1) was purchased from AbD Serotec; and anti-Ly6A (7/4) was obtained from Abcam. The IL-1β and the MIP-2 ELISA kits were purchased from R&D Systems, and the OptEIA murine IFN-γ ELISA kit was obtained from BD. The neutrophil-depleting anti-Ly6G antibody NIMP-R14 (Lopez et al., 1984; Charmoy et al., 2011) was purified from Hybridoma Supernatant and used for neutrophil depletion as previously described (Sesarman et al., 2008).
Digestion of skin samples.
Ear or back skin from mice was collected and cut into small pieces, and then digested with the Liberase II kit (Roche) on an Eppendorf Thermomixer at 1,400 rpm for 1 h at 37°C according to the manufacturer’s protocol. Single-cell suspensions were obtained by passing the digest through a 40-µm cell strainer (BD), after which they were analyzed by flow cytometry.
Depletion of neutrophils and mast cells.
Neutrophils were depleted by an i.p. injection of 62.5 µg NIMP-R14 anti-Ly6G antibody in PBS. Control mice received 62.5 µg rat IgG2b, κ (BioLegend) or PBS.
To induce efficient depletion of mast cells, Mcpt5-Cre+ iDTR+ mice received 2 successive i.v. injections of 25 ng/g body weight DT in weekly intervals, and 2 successive intradermal injections of 5 ng/g DT into the ear 6 d and 2 d before allergen application onto the ear. Experiments were performed at least 1 wk after the second systemic DT treatment. DT-treated Cre– littermates served as mast cell–competent controls.
Flow cytometry.
The number of various leukocyte types was tested by flow cytometry from blood and spleen samples. Neutrophils were identified as Ly6G-positive or, in the case of Ly6G-mediated depletion, as Ly6A-positive cells in the characteristic forward and side scatter gates. Blood T cells were identified by CD3 staining; blood monocytes as Ly6G-negative/CD11b-positive leukocytes; splenic macrophages as F4/80 and CD11b double-positive cells; and splenic DCs as CD11c-positive and I-Ab-high double-positive cells.
Contact hypersensitivity.
For sensitization, the mice were treated with epicutaneous application of 100 µl 3% TNCB in acetone or acetone alone as a vehicle control to the shaved abdominal skin. 5 d after sensitization, the initial ear thickness of the mice was measured, using a pocket thickness gauge (Mitutoyo). After the measurement, all mice (even vehicle-sensitized ones) were challenged by epicutaneous application of 20 µl 1% TNCB on both ears. The ear thickness was measured 24 h after the challenge. The increase in ear thickness as difference between the values before and 24 h after the challenge are displayed. For sensitization of mice on the ear skin, 20 µl of the contact allergen or irritant were applied to the ear. The following concentrations were used: 1% (vol/vol) croton oil in 4:1 acetone/olive oil, 3% (wt/vol) DNCB in acetone, and 3% (wt/vol) oxazolone in EtOH. Acetone, EtOH, or 4:1 acetone/olive oil were used as vehicle controls.
Passive CHS model.
For the passive (adoptive transfer) CHS model, mice were sensitized by epicutaneous application of 100 µl 3% TNCB to the shaved abdominal skin and 20 µl 1% TNCB to the dorsum of both ears. 5 d after sensitization, the mice were sacrificed, the superficial inguinal and auricular lymph nodes were collected, and a single-cell suspension was prepared. 2 × 107 lymph node cells were transferred by i.v. injection to each naive recipient mouse. Directly after the injection of the lymph node cells, the mice were challenged and the increase in ear thickness was measured as described above.
Ex vivo analysis of inflammatory cytokines.
Ear skin was ground up in liquid nitrogen and extracted using a Triton X–based lysis buffer containing protease and phosphatase inhibitors (Jakus et al., 2009). Cytokine levels were measured by the IL-1β and the MIP-2 Quantikine ELISA kits (R&D Systems) according to the manufacturer’s description.
Gelatinase assay.
To assess the gelatinase activity from ex vivo ear skin samples, mouse ears from two mice were ground up in liquid nitrogen and extracted in 1 ml PBS immediately after collection. The extract was then used to perform in-gel zymography assays as previously described (Futosi et al., 2012).
In vivo ROS measurement.
In vivo measurement of ROS in mice was performed 24 h after allergen application to the skin using a luminol-based assay. In brief, mice were injected with 200 mg/kg luminol 10 min before measurement. The mice were anaesthetized and ROS production was measured in a Perkin Elmer IVIS 100 system with an exposure time of 5 min.
DC migration assay.
To assess the migration of DCs to the lymph nodes, we applied 20 µl of a 0.4% solution in 1:1 acetone/dibutyl phthalate of the contact allergen FITC to the ear skin of neutrophil-depleted or control-treated naive mice. 24 h after allergen application, we sacrificed the mice, collected the auricular lymph nodes and analyzed FITC fluorescence in the DC compartment.
Restimulation of LN cells.
Lymph node cells from sensitized mice were collected 5 d after sensitization. A single-cell suspension of the lymph node cells was modified by incubation for 7 min at 37°C in the dark in a 3-mM solution of 2,4,6-Trinitrobenzene sulfonic acid in PBS (TNBS), the water soluble equivalent of TNCB, or PBS as control treatment. After washing thoroughly, 106 cells were incubated in 100 µl RP-10 medium (Martin, 2004) in a 96-well plate for 48 h. After the incubation, the contact allergen-induced IFN-γ production was measured by ELISA according to the manufacturer’s instructions.
Presentation of data and statistical analysis.
Experiments were performed the indicated number of times. Bar graphs show mean and SEM of all mice or samples from the indicated number of independent experiments. Statistical analysis was performed by the StatSoft STATISTICA software on all individual data points by determining the significance of the interaction between the two independent variables using two-way factorial ANOVA, except for the mast cell depletion experiments where a one-way ANOVA followed by Bonferroni’s post-hoc test was used and the analysis of blood and spleen leukocyte populations which was tested by Student’s t test. P values <0.05 were considered statistically significant.
Online supplemental material.
Fig. S1 shows leukocyte populations of neutrophil-deficient and neutrophil-depleted mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20130062/DC1.
Supplementary Material
Acknowledgments
We thank Krisztina Futosi for help with methods and experiments and Edina Simon, Anna Tóth, Eva Bachtanian and Verena Weber for excellent technical assistance; Youwen He for the mice carrying the Mcl-1flox allele; Ivo P. Touw for GCSF-R–/– bone marrow cells; and Gábor Bánhegyi for access to equipment.
This work was supported by the European Research Council (Starting Independent Investigator Award No. 206283 to A. M.), the European Union’s FP7 Cooperation Program (TARKINAID project No 282095 to A. M.), the Lendület program of the Hungarian Academy of Sciences (LP2013-66 to A. M.) and the German Research Foundation (DFG grants DU1172/2 (priority program 1468) and DU1172/3 (priority program 1394) to A.D.). A. M. was a recipient of a Wellcome Trust International Senior Research Fellowship (Grant No. 087782). F.C.W. was supported by an EMBO short term fellowship (ASTF 56.00-2011) and an EAACI Exchange Research Fellowship.
The authors declare no competing financial interest.
Footnotes
Abbreviations used:
- ACD
- allergic contact dermatitis
- CHS
- contact hypersensitivity
- DNCB
- 2,4-Dinitrochlorobenzene
- DT
- diphteria toxin
- PMN
- polymorphonuclear cells
- ROS
- reactive oxygen species
- TNCB
- 2,4,6-Trinitrochlorobenzene
References
- Charmoy M., Milon G., and Tacchini-Cottier F.. 2011. Role of Neutrophils in the Early Shaping of the Leishmania major Specific Immune Response in Experimental Murine Cutaneous Leishmaniasis. Neutrophils in Infectious Diseases. Tacchini-Cottier F., and van Zandbergen G., Bentham Science Publishers Ltd. pp. 49–58. [Google Scholar]
- Chtanova T., Schaeffer M., Han S.J., van Dooren G.G., Nollmann M., Herzmark P., Chan S.W., Satija H., Camfield K., Aaron H., et al. 2008. Dynamics of neutrophil migration in lymph nodes during infection. Immunity. 29:487–496 10.1016/j.immuni.2008.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen B.E., Burkhardt C., Reith W., Renkawitz R., and Förster I.. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8:265–277 10.1023/A:1008942828960 [DOI] [PubMed] [Google Scholar]
- Cumberbatch M., Dearman R.J., Groves R.W., Antonopoulos C., and Kimber I.. 2002. Differential regulation of epidermal langerhans cell migration by interleukins (IL)-1alpha and IL-1beta during irritant- and allergen-induced cutaneous immune responses. Toxicol. Appl. Pharmacol. 182:126–135 10.1006/taap.2002.9442 [DOI] [PubMed] [Google Scholar]
- Dudeck A., Dudeck J., Scholten J., Petzold A., Surianarayanan S., Köhler A., Peschke K., Vöhringer D., Waskow C., Krieg T., et al. 2011. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 34:973–984 10.1016/j.immuni.2011.03.028 [DOI] [PubMed] [Google Scholar]
- Dunay I.R., Damatta R.A., Fux B., Presti R., Greco S., Colonna M., and Sibley L.D.. 2008. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 29:306–317 10.1016/j.immuni.2008.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhagalov I., St John A., and He Y.W.. 2007. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 109:1620–1626 10.1182/blood-2006-03-013771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeman T., Gorbachev A.V., Kish D.D., and Fairchild R.L.. 2004. The intensity of neutrophil infiltration controls the number of antigen-primed CD8 T cells recruited into cutaneous antigen challenge sites. J. Leukoc. Biol. 76:941–949 10.1189/jlb.0304193 [DOI] [PubMed] [Google Scholar]
- Esser P.R., Wölfle U., Dürr C., von Loewenich F.D., Schempp C.M., Freudenberg M.A., Jakob T., and Martin S.F.. 2012. Contact sensitizers induce skin inflammation via ROS production and hyaluronic acid degradation. PLoS ONE. 7:e41340 10.1371/journal.pone.0041340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futosi K., Németh T., Pick R., Vántus T., Walzog B., and Mócsai A.. 2012. Dasatinib inhibits proinflammatory functions of mature human neutrophils. Blood. 119:4981–4991 10.1182/blood-2011-07-369041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebeler M., Trautmann A., Voss A., Bröcker E.V., Toksoy A., and Gillitzer R.. 2001. Differential and sequential expression of multiple chemokines during elicitation of allergic contact hypersensitivity. Am. J. Pathol. 158:431–440 10.1016/S0002-9440(10)63986-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans M.H., van de Geijn G.J., Antonissen C., Gits J., van Leeuwen D., Ward A.C., and Touw I.P.. 2003. Signaling mechanisms coupled to tyrosines in the granulocyte colony-stimulating factor receptor orchestrate G-CSF-induced expansion of myeloid progenitor cells. Blood. 101:2584–2590 10.1182/blood-2002-07-2062 [DOI] [PubMed] [Google Scholar]
- Jakus Z., Simon E., Frommhold D., Sperandio M., and Mócsai A.. 2009. Critical role of phospholipase Cγ2 in integrin and Fc receptor-mediated neutrophil functions and the effector phase of autoimmune arthritis. J. Exp. Med. 206:577–593 10.1084/jem.20081859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N.D., Chou R.C., Seung E., Tager A.M., and Luster A.D.. 2006. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J. Exp. Med. 203:829–835 10.1084/jem.20052349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács M., Németh T., Jakus Z., Sitaru C., Simon E., Futosi K., Botz B., Helyes Z., Lowell C.A., and Mócsai A.. 2014. The Src family kinases Hck, Fgr, and Lyn are critical for the generation of the in vivo inflammatory environment without a direct role in leukocyte recruitment. J. Exp. Med. 211:1993–2011 10.1084/jem.20132496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A.F., Strath M., and Sanderson C.J.. 1984. Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. Br. J. Haematol. 57:489–494 10.1111/j.1365-2141.1984.tb02923.x [DOI] [PubMed] [Google Scholar]
- Mantovani A., Cassatella M.A., Costantini C., and Jaillon S.. 2011. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11:519–531 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- Martin S.F.2004. T lymphocyte-mediated immune responses to chemical haptens and metal ions: implications for allergic and autoimmune disease. Int. Arch. Allergy Immunol. 134:186–198 10.1159/000078765 [DOI] [PubMed] [Google Scholar]
- Martin S.F.2012. Contact dermatitis: from pathomechanisms to immunotoxicology. Exp. Dermatol. 21:382–389 10.1111/j.1600-0625.2012.01471.x [DOI] [PubMed] [Google Scholar]
- Martin S.F., Dudda J.C., Bachtanian E., Lembo A., Liller S., Dürr C., Heimesaat M.M., Bereswill S., Fejer G., Vassileva R., et al. 2008. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J. Exp. Med. 205:2151–2162 10.1084/jem.20070509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mócsai A.2013. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 210:1283–1299 10.1084/jem.20122220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh T., and Mócsai A.. 2012. The role of neutrophils in autoimmune diseases. Immunol. Lett. 143:9–19 10.1016/j.imlet.2012.01.013 [DOI] [PubMed] [Google Scholar]
- Schmidt M., Raghavan B., Müller V., Vogl T., Fejer G., Tchaptchet S., Keck S., Kalis C., Nielsen P.J., Galanos C., et al. 2010. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat. Immunol. 11:814–819 10.1038/ni.1919 [DOI] [PubMed] [Google Scholar]
- Sesarman A., Mihai S., Chiriac M.T., Olaru F., Sitaru A.G., Thurman J.M., Zillikens D., and Sitaru C.. 2008. Binding of avian IgY to type VII collagen does not activate complement and leucocytes and fails to induce subepidermal blistering in mice. Br. J. Dermatol. 158:463–471 10.1111/j.1365-2133.2007.08388.x [DOI] [PubMed] [Google Scholar]
- Shornick L.P., De Togni P., Mariathasan S., Goellner J., Strauss-Schoenberger J., Karr R.W., Ferguson T.A., and Chaplin D.D.. 1996. Mice deficient in IL-1beta manifest impaired contact hypersensitivity to trinitrochlorobenzone. J. Exp. Med. 183:1427–1436 10.1084/jem.183.4.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocanson M., Hennino A., Rozières A., Poyet G., and Nicolas J.F.. 2009. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 64:1699–1714 10.1111/j.1398-9995.2009.02082.x [DOI] [PubMed] [Google Scholar]
- Wang M., Qin X., Mudgett J.S., Ferguson T.A., Senior R.M., and Welgus H.G.. 1999. Matrix metalloproteinase deficiencies affect contact hypersensitivity: stromelysin-1 deficiency prevents the response and gelatinase B deficiency prolongs the response. Proc. Natl. Acad. Sci. USA. 96:6885–6889 10.1073/pnas.96.12.6885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F.C., Esser P.R., Müller T., Ganesan J., Pellegatti P., Simon M.M., Zeiser R., Idzko M., Jakob T., and Martin S.F.. 2010. Lack of the purinergic receptor P2X(7) results in resistance to contact hypersensitivity. J. Exp. Med. 207:2609–2619 10.1084/jem.20092489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtasiak M., Pickett D.L., Tate M.D., Londrigan S.L., Bedoui S., Brooks A.G., and Reading P.C.. 2010. Depletion of Gr-1+, but not Ly6G+, immune cells exacerbates virus replication and disease in an intranasal model of herpes simplex virus type 1 infection. J. Gen. Virol. 91:2158–2166 10.1099/vir.0.021915-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.