Abstract
The US health care system provides a patchwork of services, known as the safety-net, for the uninsured, under-insured and indigent populations who would otherwise have little access to health care services. Individuals who rely on safety-net facilities are from racial/ethnic minority groups, have low socioeconomic status and often have low health literacy and/or and limited English proficiency. They shoulder a disproportionate burden of chronic kidney disease (CKD) in the United States and experience excess CKD-associated morbidity and mortality. Suboptimal delivery of CKD care may be contributing and is an area of active translational research. Several initiatives that show promise in improving safety-net CKD care delivery include those that enhance diagnostic and management skills of primary care providers, rely on comprehensive care management programs led by non-physicians, and leverage technology to enhance patient access to virtual nephrology expertise. Uncovering better ways to translate scientific evidence into practice for vulnerable patients with CKD is a formidable challenge that will require national surveillance of CKD quality measures across diverse ambulatory health systems, including safety-nets. Only then will the nephrology community be to identify and share best practices to enhance health and mitigate disparities of care among patients with CKD.
Keywords: safety-net, CKD, kidney disease, quality, vulnerable populations
What is the United States safety-net?
The US health care system provides a patchwork of services for the uninsured, under-insured and indigent populations who would otherwise have little access to health care services. This patchwork, known as the health care safety-net, includes federally and community funded community health centers, county health departments, local access-to-care programs such as homeless health centers and church-based health clinics, and services provided by public hospitals to vulnerable populations.1 Safety-net facilities provide medical services to all individuals regardless of their insurance or migrant status at no cost or on a sliding scale based on income. As such, they generally operate as nonprofit organizations and rely heavily on public and private funding to subsidize care for the poor. The federal government has traditionally been a strong partner in this regard. In 1991, the Federally Qualified Health Center benefit was added to the Medicare program to enhance the provision of ambulatory care to underserved urban and rural communities. Subsequently, between 1994 and 2001, the federal Consolidated Health Center Program, which pays for primary care and preventive services for underserved populations, grew from covering 7.3 million to 10.3 million individuals. In 2001, capacity to care for the underserved was further expanded by the Health Center Growth Initiative. By 2007, approximately 16.1 million individuals received care from the safety-net. Now, with implementation of the Affordable Care Act and current/expected Medicaid expansion in 25 states and the District of Columbia, the United States is poised to further expand its ability to care for vulnerable populations that depend on safety-net institutions for health care.
Sociodemographic characteristics of safety-net patients with CKD
Individuals who rely on safety-net facilities for medical care often have limited socioeconomic means, are from racial/ethnic minority groups, and have low health literacy and/or and limited English proficiency.2, 3 As has been extensively documented throughout this issue, these groups shoulder a disproportionate burden of chronic kidney disease (CKD) in the United States as well as co-morbid conditions that serve as risk factors for CKD development and CKD decline, such as diabetes, obesity and hypertension.4, 5 While there is a paucity of aggregated data from community health centers that provide safety-net care, data from single institutions reinforce the idea that safety-net clinics play a central role in caring for individuals with CKD, particularly among younger, non-white individuals, who are at high risk of experiencing progression of CKD to ESRD.6, 7 Recent data from the San Francisco Health Network, the integrated public health care delivery system for San Francisco’s uninsured and underinsured population, for example, describe a CKD population in whom one-half is less than 60 years of age and one-fourth is younger than 50. This is in contrast to estimates from a nationally representative sample of US adults that find CKD to be relatively uncommon among individuals younger than 60 years.8 Among the San Francisco population with CKD, approximately 70% were members of nonwhite racial/ethnic groups, over 40% were uninsured or enrolled in Medicaid, and 72% were indigent, defined by an annual income <%15,000.9 Data from the National Kidney Foundation Kidney Early Evaluation Program (KEEP), a free community-based health screening program that targets populations at high risk for kidney disease, and the National Health and Nutrition Examination Survey (NHANES) suggest similar socio-demographic characteristics among nationally representative uninsured individuals with CKD,10, 11 the majority of whom report seeing a physician within the prior year, presumably from a safety-net provider.
Health outcomes among safety-net patients with CKD
Data from the 86,000 individuals who presented for a KEEP health screening between 2000 and 2011 demonstrate an increased risk of death among uninsured and publically insured individuals with and without CKD compared to those with private insurance. The uninsured population had much higher odds of death (adjusted odds ratio [AOR]=1.66, 95%CI 1.43–1.94) and the publically insured population had more than a 2-fold higher odds of death (AOR=2.37, 2.01–2.78) compared to those with private insurance. An increased risk of progression to ESRD among the uninsured and publically insured individuals with CKD and an eGFR > 30 ml/min/1.73m2 compared to those with private insurance was also noted (AOR=2.09, 1.31–3.35 and AOR=3.10, 1.92–5.00, respectively).10 Similarly, reported incidence rates of ESRD among Hispanic and non-Hispanic Whites were higher in the San Francisco safety-net compared to estimates from a similar geographical population insured by Kaiser Permanente Northern California around the same time period.6, 9
Contributions to adverse health outcomes among safety-net populations with CKD
While it is difficult to disentangle the patient-level, provider-level, and system-level contributions to adverse health outcomes among socially disadvantaged populations, it is clear that elements exist in each of these domains. At the patient level, non-traditional risk factors for CKD progression and mortality have been identified that may compound the increased risk already present from the high prevalence of diabetes, obesity and hypertension in these patient populations. At the provider level, suboptimal knowledge, competing priorities and risk factor management likely play a key role. And at the system-level, delivery of fragmented nephrology care from a paucity of specialists is likely contributing (Figure).
Figure.
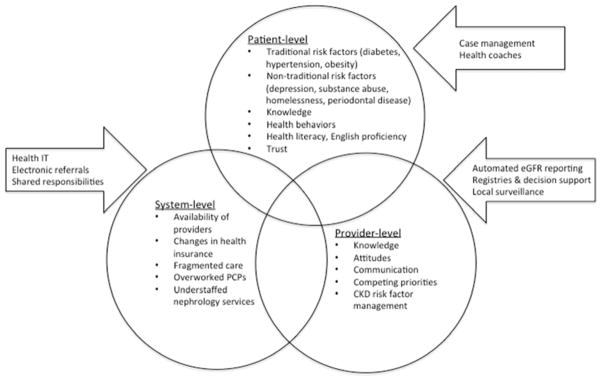
Barriers in access to high-quality CKD care in US safety-net health systems and current initiative that aim to address those barriers.
Patient-level factors
It is widely appreciated that traditional risk factors for CKD progression such as hypertension, diabetes and obesity are more prevalent among populations who receive care from safety-net settings.12 Exacerbating the issue, however, are highly prevalent, non-cardiovascular conditions like homelessness, depression and periodontal disease that may biologically contribute independently to CKD progression and mortality, as well as non-traditional behavioral and social factors.
Non-traditional biologic risk factors
In one study of low-income urban adults with CKD, homeless individuals experienced 28% higher risk of ESRD or death over a median follow-up of 2.6 years compared to housed counterparts, independent of socio-demographic variables, comorbid conditions and laboratory variables (aHR=1.28, 1.04–1.58).13 Of interest, this association differed by substance abuse status. Among adults without a history of substance abuse, risk of ESRD or death was higher among the homeless compared with housed adults (aHR=1.54, 1.18–2.03). Among adults with a history of substance abuse, there was no difference in ESRD or mortality by housing status, suggesting an independent relationship between substance abuse and ESRD/mortality. The nature of this relationship is not entirely clear, however. Several studies have demonstrated an increased risk of CKD progression and incident ESRD among individuals who self-report illicit drug use.14 But, one recent study only found a small association between cocaine use and CKD progression and did not found any association between methamphetamine or heroin use with CKD progression or development of ESRD.15 Less controversial is the contribution of depression to adverse health outcomes among individuals with CKD. Among veterans with CKD, presence of a major depressive episode has been independently associated with increased hospitalizations (aHR=1.90, 1.23–2.90) and progression to ESRD (aHR=3.51, 1.77–6.97), though not increased mortality (1.52, 0.53–4.34).16 Similar data have emerged from participants of the African American Study of Kidney Disease (AASK) cohort17. The plausible biologic mechanisms underlying the associations between homelessness, addiction and depression and adverse health outcomes may involve inflammation, compromised immunity, and platelet activation from altered serotonin levels.18–21 Similar mechanisms seem to link periodontal disease, highly prevalent in safety-net populations presumably due to lack of access to dental care,22 with prevalence of CKD among US adults23 (aOR=1.51, 1.13–2.02) and CKD progression24 among older Japanese adults (aOR=2.24, 1.05–4.79), independent of socioeconomic variables and co-morbid conditions.
Non-traditional behavioral risk factors
Behavioral factors also likely contribute to adverse outcomes in safety-net CKD populations. While robust longitudinal data are lacking, suboptimal patient knowledge,25 medication non-adherence,26 limited trust with providers/health systems,27 and poor health literacy,28 have been cross-sectionally associated with high prevalence of CKD and adverse cardiovascular health. Participation in poor health behaviors, including tobacco use,29 and sedentary lifestyles30, quite prevalent in this population, has been associated with CKD progression.
Provider-level factors
By definition, safety-net patients with CKD receive the majority of their care from limited networks of health care providers. Data from the nationally representative National Ambulatory Medical Care Surveys suggest that delivery of basic primary care is similar for individuals with private insurance, Medicaid and no insurance, the later two often representing patients cared for in safety-net settings. Specifically, length of primary care visit and receipt of preventive health counseling does not seem to differ by patient insurance status.31 However, while preventive primary care services may be similar across insurance status and thus health care settings, there is evidence to suggest that CKD awareness among providers and management of CKD risk factors are suboptimal in safety-net settings.
Under-recognition of CKD
Provider-level CKD awareness includes general recognition of CKD, understanding of its risk factors and associated complications, and knowledge/belief of patient management strategies. National estimates of CKD awareness among providers, including those that practice in safety-net settings, is low, ranging from 10–60%.32 Similarly, studies indicate that only 22%–30% of primary care providers are knowledgeable about CKD guidelines, regardless of practice setting. What may distinguish safety-net providers from their counterparts is the reason behind low recognition. Qualitative studies suggest that the need to care for competing and often-symptomatic chronic diseases and social issues relegates kidney disease lower on the priority list for safety-net providers.33
Suboptimal CKD risk factor management
Data from NHANES have demonstrated that insurance status is associated with suboptimal CKD management. In one study, using data from 1999–2006, uninsured persons with CKD were 40% less likely to receive hypertension treatment (AOR=0.61, 0.41–0.91) compared to those with private insurance and nearly 40% less likely than those with public insurance (AOR=0.51, 0.31–0.74). Similarly, uninsured individuals with CKD were less likely to receive an angiotensin converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) compared to those with private or public insurance (AOR=0.45, 0.27–0.76 and 0.45, 0.25–0.82, respectively).11 It is important to note, however, that uninsured individuals who participate in NHANES may not have access to medical care, so these statistics may not fairly depict the actual delivery of safety-net CKD care in the United States. Examining “real-world” delivery of safety-net CKD care is challenging, since CKD metrics are not routinely measured or reported by FQHCs nor are they part of the Healthcare Effectiveness Data and Information System (HEDIS) reporting requirements for health plans.34 However, a large study in 2007 examined the quality of diabetes and hypertension care among community health centers across the US. In that study, the authors found that over 70% of diabetic patients with proteinuria were prescribed an ACEi/ARB.35 These data were extended in a more recent study that demonstrated that primary care providers in FQHCs display greater adherence to established quality measures compared to primary care providers who work in private practice settings,36 particularly for measures that emphasize appropriate use of pharmaceuticals for coronary artery disease and congestive heart failure and appropriate use of screening tests. One would expect, then, that similar results would extend to care for all patients with CKD regardless of the etiology of kidney disease. While not extensive, studies from individual community health centers or systems provide a more nuanced picture of ambulatory CKD care delivery among safety-net populations. For example, among individuals with CKD in the San Francisco safety-net between 2003–2010, nearly 25% had uncontrolled blood pressure, defined by > 140/90 mmHg, compared to national estimates of 22%, a difference largely driven by individuals with moderate-severe CKD. Adjusted prevalence of uncontrolled blood pressure was 18% among those with CKD stages 1 and 2 compared to 22% nationally; adjusted prevalence of uncontrolled blood pressure was 28% among those with CKD stages 3 and 4, compared to 23% nationally.37 By contrast, data from 212 patients with and without CKD who receive care from one FQHC in Ingham County Michigan demonstrated that 62% of patients had uncontrolled blood pressure compared to national estimates of 50% in a similar population.38
System-level factors
Poor access to nephrology care
Despite these differences in estimates of blood pressure control, which may result from differences in study methodologies (using single vs. multiple BP measures per person, for example), sample sizes, and/or patient demographics, the aforementioned data paint a picture of a population of individuals with CKD at high risk of adverse health outcomes that is not receiving guideline-concordant CKD care. Limited access to nephrologists in safety-net health systems may be contributing to this problem.39 Optimal timing for nephrology referral remains uncertain, but nephrologist involvement in CKD care has been associated with slower CKD decline and more optimal blood pressure control and prescription of ACEi/ARB.40, 41 Nephrologists thus play an important role in early CKD care. Qualitative studies suggest that that patients who receive medical care in community health centers have difficulty accessing services that are not directly provided by the health center, such as specialty care.42 In a study of predictors of specialty referral, payor status was a significant determinant of a patient obtaining a specialty referral in a primary care setting, with uninsured status having nearly a 40% lower odds of referral than the privately insured (AOR=0.58, 0.41–0.82).43
Similarly, in a 2003 survey of all 101 medical directors of California’s federally qualified health centers, 85% reported that their uninsured patients “often” or “almost always” had problems obtaining specialty care and 40% reported that their Medicaid patients “often” or “almost always” had problems accessing specialists.44 Indeed, wait times for specialty appointments for patients in safety-net settings have been documented as long as 6–12 months.45
Fragmentation of health care delivery
Contributing to this issue is the inefficiency of the primary–specialty care interface46, 47 which relies on overworked primary care providers who must plead to have patients seen in an expedited fashion and a limited network of specialists, including nephrologists, who care for uninsured and Medicaid patients. The current referral system often results in duplicate testing and delayed diagnoses, leading to inefficient use of scare specialty resources48 and unnecessary costs. Furthermore, as patients gain and lose insurance based on temporary changes in employment and financial eligibility, continuity of care with generalist and specialty providers can be lost. This piece-meal coverage may translate into missed opportunities for CKD management.
Current initiatives to enhance safety-net CKD care
Current initiatives at improving safety-net CKD care mirror the national push for the delivery of high-access, high-quality, patient-centered nephrology care within the context of the Chronic Care Model. These initiatives can be grouped into three broad categories: expanding the diagnostic and management skills of primary care providers, creating comprehensive CKD management programs embedded in primary care which address patient-level factors, and leveraging technology to streamline the referral process and enhance patient access to nephrology expertise (Figure).
Automated eGFR reporting has improved recognition of CKD among vulnerable populations49 and has been associated with increased proteinuria quantification and increased nephrology referrals.50 Despite these improvements, however, identification of CKD remains suboptimal, suggesting that other approaches are needed. Disease registries, which are information platforms that enhance chronic disease management through targeted alerts, have been piloted in numerous systems across the United States to enhance health outcomes among complex patients. Often embedded within electronic medical records, their implementation has been associated with decreased glycosylated hemoglobin levels, systolic blood pressure and cholesterol levels among patients with diabetes51 and greater receipt of beta-blockers among patients with congestive heart failure.52 They have also shown some promise in enhancing CKD management in safety-net primary care. One CKD registry implemented in two urban, underserved primary care clinics in NY state, for example, was associated with increased diagnosis of CKD (from 21 to 79%, p<0.001) and decreased used of metformin and NSAIDS (p<0.001 for both).53 A similar registry in San Francisco aims to more efficiently enhance outcomes by empowering non-clinician members of the health care team in addition to primary care providers, to highlight gaps in CKD care during patient visits and to contact CKD patients who have fallen out of care.33 This approach, using standard orders to engage non-physicians in care delivery has improved immunization rates among non-CKD adults who receive care in the community clinics that comprise the Denver safety-net.54 Moving forward, CKD registries will be also tasked to provide local surveillance -- to identify individuals with CKD based on laboratory data, automatically track patient-level data over time and provide decision-support to all members of the health care team, allowing for proactive management of patients at point-of-care or via outreach.
Chronic disease management programs further expand on these concepts of patient identification and population health to include patient self-care elements. Disease management programs typically include the following components: population identification, evidence-based practice guidelines, collaborative practice models that embrace non-physician leaders, patient self-management education, process and outcomes measurement and routine feedback.55 Initially developed for patients with diabetes, disease management programs for other chronic diseases, such as congestive heart failure and CKD, have recently been piloted in safety-net clinics. For example, the Louisiana State University Health Care Services Division, which is the largest provider of health care to Louisiana’s uninsured citizens, achieved great success with their diabetes disease management program. Among patients with diabetes, the percentage of patients with glycosylated hemoglobin levels < 7% increased from 44 to 54% and the percentage with a renal assessment increased from 52 to 74% (p<0.05 for both comparisons). Similar increases were noted among the uninsured diabetic population, suggesting that an integrated approach to complex disease management can improve care among the most vulnerable individuals. A congestive heart failure program was subsequently added and demonstrated a reduction in racial and gender disparities with respect to CHF mortality.56 A CKD management program has recently been added to the LSU Health Care Services Division.57 Results and program evaluation are eagerly anticipated to identify elements that can be disseminated to other systems.
In an attempt to increase coordination among primary care and nephrology services and enhance CKD care delivery in the underserved population of Dallas County, Texas, Vazquez and colleagues are implementing a model of joint primary and nephrology care. This model relies on a robust, health information technology–enabled program that harnesses the electronic medical record to provide specific nephrology decision support to all providers in the system and monitor the delivery of evidence-based practice, such as ACEi/ARB administration and timely placement of vascular access.58 As such, this technology allows collaboration among clinicians to slow CKD progression and ensure optimal preparation for renal replacement therapy among a high-risk, traditionally underserved population. A different model of enhancing the primary care-nephrology care interface to enhance access to nephrology expertise relies on a “medical neighborhood” of nephrologists who can offer timely and efficient consultations, diagnostic services and needed treatments.59 Such a network requires a seamless exchange of information and a shared understanding among primary care clinicians and nephrologists about responsibility. While tenets of the medical neighborhood have been operationalized differently in large safety-net systems, such as those in Chicago, Los Angeles and San Francisco, they do share some similar characteristics, including referral guidelines that guide clinicians on initial diagnosis, management and pre-referral workup and bi-directional electronic communication among primary care providers and nephrologists.60, 61 This allows for a wide spectrum of virtual nephrology care, ranging from pre-consultative exchange, ensuring complete patient evaluations prior to a specialty clinic visit and rendering that visit most efficient, to virtual co-management by nephrologists on a one-time basis or on a longitudinal basis, simultaneously expanding scope of practice among PCPs and increasing access to nephrology expertise. While not specific to nephrology, such systems have improved access to specialty care, increased PCP and specialist satisfaction and enhanced communication among safety-net providers.61–63
Measuring the quality of CKD care
These differing approaches to enhance safety-net delivery of nephrology care are currently being tested. Results and best practices are eagerly anticipated prior to more widespread dissemination across other US safety-net systems. But how we determine the success of such initiatives remains uncertain. As previously mentioned, metrics for successful delivery of nephrology care have not been included in FQHC or HEDIS reporting requirements for health plans. More generally, there are few ambulatory measures that pertain to specialty care, with the exception of cardiovascular quality measures such as administration of a beta blocker after a myocardial infarction, or aspirin use.34 With health care reform, organizations and health care plans, in particular, are being held even more accountable for the quality of care they provide and the health outcomes of their populations. This will likely hold true for safety-net institutions and safety-net providers, even for those patients who remain uninsured. Identifying who is accountable for patients with CKD in fragmented safety-net systems is undoubtedly more challenging than in integrated health systems, because patients often access primary and specialty care sporadically and lack continuity of care. Nevertheless, current FQHC funding is contingent on reporting outcomes data and participating in quality improvement projects. Proposed metrics that can be used to evaluate the quality of ambulatory CKD care are outlined in Table 1. These include metrics from several different domains: prevention and counseling, monitoring and treatment, experience of care/patient-centeredness and access to specialty care. Consistent with the current goals of the federal FQHC funding programs, these measures should be reported among all populations, as well as among sub-groups to assess for disparities of care.
Table 1.
Proposed chronic kidney disease quality metrics, by domain.
| Quality metrics | Data source |
|---|---|
|
| |
| Prevention and screening | |
| Assessment of smoking status and cessation advice | EMR |
| Avoidance of NSAID prescription | EMR |
| Pneumovax | EMR |
| Hepatitis B vaccine among individuals with eGFR < 30ml/min/1.73m2 | EMR |
|
| |
| Monitoring and treatment | |
| Use of ACEi/ARB in patients with hypertension, proteinuria, diabetes | EMR |
| Lipid profile | EMR |
| Statin prescription | EMR |
| Assessment of anemia and iron studies | EMR |
| Assessment of Metabolic Bone Disorder parameters | EMR |
| Delivery of pre-ESRD education | Scheduling system |
|
| |
| Experience of care | |
| Patient care coordination perception | Patient satisfaction surveys |
|
| |
| Access to specialty care | |
| Time to next new nephrology appointment | Scheduling system |
| Availability of virtual nephrology consultation or co-management | EMR |
Abbreviations: EMR=electronic medical record; NSAID=non-steroidal anti-inflammatory drug; ESRD=end-stage renal disease
Fair comparisons of these proposed metrics and others across health care institutions will require appropriate case-mix adjustors. In addition to the traditional adjustors such as age, socioeconomic status, insurance status and co-morbid conditions, it will be imperative to include non-traditional case-mix adjustors that render safety-net nephrology care delivery that much more difficult. Percent of patients who speak a non-English language, have limited health literacy, have a marginal housing situation or are homeless, and percentage of patients who suffer from co-morbid mental illness, are some examples. Having a national CKD surveillance system that can report quality measures across diverse ambulatory health systems, including safety-nets, will enable the nephrology community to share best practices and identify the best way to translate scientific evidence into practice for all of patients with kidney disease.
Conclusion
Current data suggest that our most vulnerable populations with CKD are not receiving the nephrology care that they deserve. Several initiatives that show promise in improving safety-net CKD care delivery include those that enhance diagnostic and management skills of primary care providers, address patient-level barriers to adoption of healthy behaviors, and enhance patient access to virtual nephrology expertise. With health care reform underway and additional disruptive innovations expected, many other opportunities will arise. Uncovering better ways to translate scientific evidence into practice for patients with CKD is a formidable challenge in all environments. Let’s rise to that challenge to enhance health and quality of life and mitigate disparities of care among our most vulnerable patients with CKD.
Clinical Summary (2–4 bullets).
Individuals who rely on safety-net facilities for medical care shoulder a disproportionate burden of CKD and experience excess associated morbidity and mortality.
Several initiatives show promise in improving safety-net CKD care delivery: enhancing diagnostic and management skills of primary care providers; providing comprehensive care management programs led by non-physicians; leveraging technology to enhance patient access to virtual nephrology expertise
Uncovering better ways to translate scientific evidence into practice for vulnerable patients with CKD will require a national database of CKD quality measures across diverse ambulatory health systems, including safety-nets.
Acknowledgments
Funding sources:
DST is supported by 1K23DK094850 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). VG is support by 1K23DK093710-01A1 from the NIDDK and by the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation. Both receive support from the National Center for Advancing Translational Sciences at the National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004.
Footnotes
Conflict of Interest/Financial Disclosure: The authors have no conflicts of interest or financial disclosures to report.
The contents of this manuscript are solely the responsibility of the authors and do not represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lewin ME, Altman SH Institute of Medicine (U.S.). Committee on the Changing Market Managed Care and the Future Viability of Safety Net Providers. America’s health care safety net: intact but endangered. Washington, D.C: Institute of Medicine: National Academy Press; 2000. [Google Scholar]
- 2.Gaskin DJ, Hadley J. Population characteristics of markets of safety-net and non-safety- net hospitals. J Urban Health. 1999 Sep;76(3):351–370. doi: 10.1007/BF02345673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forrest CB, Whelan EM. Primary care safety-net delivery sites in the United States: A comparison of community health centers, hospital outpatient departments, and physicians’ offices. JAMA. 2000 Oct 25;284(16):2077–2083. doi: 10.1001/jama.284.16.2077. [DOI] [PubMed] [Google Scholar]
- 4.Rabi DM, Edwards AL, Southern DA, et al. Association of socio-economic status with diabetes prevalence and utilization of diabetes care services. BMC Health Serv Res. 2006;6:124. doi: 10.1186/1472-6963-6-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duggirala MK, Cuddihy RM, Cuddihy MT, et al. Predictors of blood pressure control in patients with diabetes and hypertension seen in primary care clinics. Am J Hypertens. 2005 Jun;18(6):833–838. doi: 10.1016/j.amjhyper.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Peralta CA, Shlipak MG, Fan D, et al. Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol. 2006 Oct;17(10):2892–2899. doi: 10.1681/ASN.2005101122. [DOI] [PubMed] [Google Scholar]
- 7.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003 Nov;14(11):2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 8.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007 Nov 7;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 9.Hall YN, Choi AI, Chertow GM, Bindman AB. Chronic kidney disease in the urban poor. Clin J Am Soc Nephrol. 2010 May;5(5):828–835. doi: 10.2215/CJN.09011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurkovitz CT, Li S, Norris KC, et al. Association between lack of health insurance and risk of death and ESRD: results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2013 Apr;61(4 Suppl 2):S24–32. doi: 10.1053/j.ajkd.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall YN, Rodriguez RA, Boyko EJ, Chertow GM, O’Hare AM. Characteristics of uninsured Americans with chronic kidney disease. J Gen Intern Med. 2009 Aug;24(8):917–922. doi: 10.1007/s11606-009-1028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall YN, Choi AI, Xu P, Smith NL, Boyko EJ. Predictors of end-stage renal disease in the urban poor. J Health Care Poor Underserved. 2013 Nov;24(4):1686–1700. doi: 10.1353/hpu.2013.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall YN, Choi AI, Himmelfarb J, Chertow GM, Bindman AB. Homelessness and CKD: a cohort study. Clin J Am Soc Nephrol. 2012 Jul;7(7):1094–1102. doi: 10.2215/CJN.00060112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perneger TV, Klag MJ, Whelton PK. Recreational drug use: a neglected risk factor for end-stage renal disease. Am J Kidney Dis. 2001 Jul;38(1):49–56. doi: 10.1053/ajkd.2001.25181. [DOI] [PubMed] [Google Scholar]
- 15.Vupputuri S, Batuman V, Muntner P, et al. The risk for mild kidney function decline associated with illicit drug use among hypertensive men. Am J Kidney Dis. 2004 Apr;43(4):629–635. doi: 10.1053/j.ajkd.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Hedayati SS, Minhajuddin AT, Afshar M, Toto RD, Trivedi MH, Rush AJ. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010 May 19;303(19):1946–1953. doi: 10.1001/jama.2010.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer MJ, Kimmel PL, Greene T, et al. Elevated depressive affect is associated with adverse cardiovascular outcomes among African Americans with chronic kidney disease. Kidney Int. 2011 Sep;80(6):670–678. doi: 10.1038/ki.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006 Jan;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Empana JP, Sykes DH, Luc G, et al. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2005 May 10;111(18):2299–2305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- 20.Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135–151. doi: 10.1007/7854_2012_211. [DOI] [PubMed] [Google Scholar]
- 21.Serebruany VL, Glassman AH, Malinin AI, et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation. 2003 Aug 26;108(8):939–944. doi: 10.1161/01.CIR.0000085163.21752.0A. [DOI] [PubMed] [Google Scholar]
- 22.Grubbs V, Plantinga LC, Tuot DS, Powe NR. Chronic kidney disease and use of dental services in a United States public healthcare system: a retrospective cohort study. BMC Nephrol. 2012;13:16. doi: 10.1186/1471-2369-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grubbs V, Plantinga LC, Crews DC, et al. Vulnerable populations and the association between periodontal and chronic kidney disease. Clin J Am Soc Nephrol. 2011 Apr;6(4):711–717. doi: 10.2215/CJN.08270910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasaki M, Taylor GW, Nesse W, Vissink A, Yoshihara A, Miyazaki H. Periodontal disease and decreased kidney function in Japanese elderly. Am J Kidney Dis. 2012 Feb;59(2):202–209. doi: 10.1053/j.ajkd.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 25.Tuot DS, Plantinga LC, Hsu CY, et al. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Nephrol. 2011 Aug;6(8):1838–1844. doi: 10.2215/CJN.00730111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muntner P, Judd SE, Krousel-Wood M, McClellan WM, Safford MM. Low medication adherence and hypertension control among adults with CKD: data from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis. 2010 Sep;56(3):447–457. doi: 10.1053/j.ajkd.2010.02.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulware LE, Cooper LA, Ratner LE, LaVeist TA, Powe NR. Race and trust in the health care system. Public Health Rep. 2003 Jul-Aug;118(4):358–365. doi: 10.1016/S0033-3549(04)50262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devraj R, Gordon EJ. Health literacy and kidney disease: toward a new line of research. Am J Kidney Dis. 2009 May;53(5):884–889. doi: 10.1053/j.ajkd.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Regalado M, Yang S, Wesson DE. Cigarette smoking is associated with augmented progression of renal insufficiency in severe essential hypertension. Am J Kidney Dis. 2000 Apr;35(4):687–694. doi: 10.1016/s0272-6386(00)70017-5. [DOI] [PubMed] [Google Scholar]
- 30.Toyama K, Sugiyama S, Oka H, Sumida H, Ogawa H. Exercise therapy correlates with improving renal function through modifying lipid metabolism in patients with cardiovascular disease and chronic kidney disease. J Cardiol. 2010 Sep;56(2):142–146. doi: 10.1016/j.jjcc.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Bruen BK, Ku L, Lu X, Shin P. No evidence that primary care physicians offer less care to Medicaid, community health center, or uninsured patients. Health Aff (Millwood) 2013 Sep;32(9):1624–1630. doi: 10.1377/hlthaff.2012.1300. [DOI] [PubMed] [Google Scholar]
- 32.Plantinga LC, Tuot DS, Powe NR. Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis. 2010 May;17(3):225–236. doi: 10.1053/j.ackd.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McBride D, Dohan D, Handley MA, Powe NR, Tuot DS. Developing a CKD Registry in Primary Care: Provider Attitudes and Input. Am J Kidney Dis. 2013 Nov 29; doi: 10.1053/j.ajkd.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Committee for Quality Assurance. [Accessed February 2014.];Measuring quality. Improving health care. http://www.ncqa.org/HEDISQualityMeasurement/HEDISMeasures/HEDIS2014.aspx.
- 35.Landon BE, Hicks LS, O’Malley AJ, et al. Improving the management of chronic disease at community health centers. N Engl J Med. 2007 Mar 1;356(9):921–934. doi: 10.1056/NEJMsa062860. [DOI] [PubMed] [Google Scholar]
- 36.Goldman LE, Chu PW, Tran H, Romano MJ, Stafford RS. Federally qualified health centers and private practice performance on ambulatory care measures. Am J Prev Med. 2012 Aug;43(2):142–149. doi: 10.1016/j.amepre.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuot DS, McCulloch CE, Hsu C-y, et al. Kidney Week. American Society of Nephrology; San Deigo: 2012. Blood pressure control among CKD patients in a public health system. [Google Scholar]
- 38.Olomu AB, Gourineni V, Huang JL, et al. Rate and predictors of blood pressure control in a federal qualified health center in Michigan: a huge concern? J Clin Hypertens (Greenwich) 2013 Apr;15(4):254–263. doi: 10.1111/jch.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper RA. There’s a shortage of specialists: is anyone listening? Acad Med. 2002 Aug;77(8):761–766. doi: 10.1097/00001888-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Jones C, Roderick P, Harris S, Rogerson M. Decline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney disease. Nephrol Dial Transplant. 2006 Aug;21(8):2133–2143. doi: 10.1093/ndt/gfl198. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Ramirez HR, Jalomo-Martinez B, Cortes-Sanabria L, et al. Renal function preservation in type 2 diabetes mellitus patients with early nephropathy: a comparative prospective cohort study between primary health care doctors and a nephrologist. Am J Kidney Dis. 2006 Jan;47(1):78–87. doi: 10.1053/j.ajkd.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Weissman JS, Moy E, Campbell EG, et al. Limits to the safety net: teaching hospital faculty report on their patients’ access to care. Health Aff (Millwood) 2003 Nov-Dec;22(6):156–166. doi: 10.1377/hlthaff.22.6.156. [DOI] [PubMed] [Google Scholar]
- 43.Forrest CB, Nutting PA, von Schrader S, Rohde C, Starfield B. Primary care physician specialty referral decision making: patient, physician, and health care system determinants. Med Decis Making. 2006 Jan-Feb;26(1):76–85. doi: 10.1177/0272989X05284110. [DOI] [PubMed] [Google Scholar]
- 44.Felt-Lisk S, McHugh M, Thomas M. Examining Access to Specialty Care for California’s Uninsured: Full report. 2004 Jun; [Google Scholar]
- 45.Felt-Lisk S, McHugh M, Howell E. Monitoring local safety-net providers: do they have adequate capacity? Health Aff (Millwood) 2002 Sep-Oct;21(5):277–283. doi: 10.1377/hlthaff.21.5.277. [DOI] [PubMed] [Google Scholar]
- 46.McPhee SJ, Lo B, Saika GY, Meltzer R. How good is communication between primary care physicians and subspecialty consultants? Arch Intern Med. 1984 Jun;144(6):1265–1268. [PubMed] [Google Scholar]
- 47.Cummins RO, Smith RW, Inui TS. Communication failure in primary care. Failure of consultants to provide follow-up information. Jama. 1980 Apr 25;243(16):1650–1652. [PubMed] [Google Scholar]
- 48.Gandhi TK, Sittig DF, Franklin M, Sussman AJ, Fairchild DG, Bates DW. Communication breakdown in the outpatient referral process. J Gen Intern Med. 2000 Sep;15(9):626–631. doi: 10.1046/j.1525-1497.2000.91119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plantinga LC, Tuot DS, Grubbs V, Hsu CY, Powe NR. Chronic kidney disease identification in a high-risk urban population: does automated eGFR reporting make a difference? J Urban Health. 2012 Dec;89(6):965–976. doi: 10.1007/s11524-012-9726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao MK, Morris CD, O’Malley JP, Davis MM, Mori M, Anderson S. Documentation and management of CKD in rural primary care. Clin J Am Soc Nephrol. 2013 May;8(5):739–748. doi: 10.2215/CJN.02410312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Georgiou A, Burns J, McKenzie S, Penn D, Flack J, Harris MF. Monitoring change in diabetes care using diabetes registers--experience from divisions of general practice. Aust Fam Physician. 2006 Jan-Feb;35(1–2):77–80. [PubMed] [Google Scholar]
- 52.Gheorghiade M, Albert NM, Curtis AB, et al. Medication dosing in outpatients with heart failure after implementation of a practice-based performance improvement intervention: findings from IMPROVE HF. Congest Heart Fail. 2012 Jan-Feb;18(1):9–17. doi: 10.1111/j.1751-7133.2011.00250.x. [DOI] [PubMed] [Google Scholar]
- 53.Fox CH, Swanson A, Kahn LS, Glaser K, Murray BM. Improving chronic kidney disease care in primary care practices: an upstate New York practice-based research network (UNYNET) study. J Am Board Fam Med. 2008 Nov-Dec;21(6):522–530. doi: 10.3122/jabfm.2008.06.080042. [DOI] [PubMed] [Google Scholar]
- 54.Swenson CJ, Appel A, Sheehan M, et al. Using information technology to improve adult immunization delivery in an integrated urban health system. Jt Comm J Qual Patient Saf. 2012 Jan;38(1):15–23. doi: 10.1016/s1553-7250(12)38003-3. [DOI] [PubMed] [Google Scholar]
- 55.Horswell R, Butler MK, Kaiser M, et al. Disease management programs for the underserved. Dis Manag. 2008 Jun;11(3):145–152. doi: 10.1089/dis.2007.0011. [DOI] [PubMed] [Google Scholar]
- 56.Hebert K, Lopez B, Horswell R, et al. The impact of a standardized disease management program on race/ethnicity and gender disparities in care and mortality. J Health Care Poor Underserved. 2010 Feb;21(1):264–276. doi: 10.1353/hpu.0.0243. [DOI] [PubMed] [Google Scholar]
- 57.Butler MK, Kaiser M, Johnson J, Besse J, Horswell R. Diabetes mellitus disease management in a safety net hospital system: translating evidence into practice. Popul Health Manag. 2010 Dec;13(6):319–324. doi: 10.1089/pop.2009.0078. [DOI] [PubMed] [Google Scholar]
- 58.Tuttle KR, Tuot DS, Corbett CL, Setter SM, Powe NR. Type 2 Translational Research for CKD. Clin J Am Soc Nephrol. 2013 Apr 25; doi: 10.2215/CJN.00130113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laine C. Welcome to the patient-centered medical neighborhood. Ann Intern Med. 2011 Jan 4;154(1):60. doi: 10.7326/0003-4819-154-1-201101040-00009. [DOI] [PubMed] [Google Scholar]
- 60.Patrick G, Hickner J. Four Models Bring Specialty Services to the Safety Net: Enhancing Scope of Practice and Referral Efficiency. California Healthcare Foundation; 2009. [Google Scholar]
- 61.Chen AH, Murphy EJ, Yee HF., Jr eReferral--a new model for integrated care. N Engl J Med. 2013 Jun 27;368(26):2450–2453. doi: 10.1056/NEJMp1215594. [DOI] [PubMed] [Google Scholar]
- 62.Kim Y, Chen AH, Keith E, Yee HF, Jr, Kushel MB. Not perfect, but better: primary care providers’ experiences with electronic referrals in a safety net health system. J Gen Intern Med. 2009 May;24(5):614–619. doi: 10.1007/s11606-009-0955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim-Hwang JE, Chen AH, Bell DS, Guzman D, Yee HF, Jr, Kushel MB. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010 Oct;25(10):1123–1128. doi: 10.1007/s11606-010-1402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


