Abstract
Chronic kidney disease (CKD) is a national public health problem that afflicts persons of all segments of society. While racial/ethnic disparities in advanced CKD including dialysis dependent populations have been well established, the finding of differences in CKD incidence, prevalence and progression across different socioeconomic groups and racial and ethnic strata has only recently started to receive significant attention. Socioeconomics may exert both interdependent and independent effects on CKD and its complications, and may confound racial and ethnic disparities. Socioeconomic constellations influence not only access to quality care for CKD risk factors and CKD treatment, but may mediate many of the cultural and environmental determinants of health that are becoming more widely recognized as impacting complex medical disorders. In this manuscript we have reviewed the available literature pertaining to the role of socioeconomic status and economic factors in both non-dialysis dependent CKD and end-stage-renal disease. Advancing our understanding of the role of socioeconomic factors in patients with or at risk for CKD can lead to improved strategies for disease prevention and management.
Keywords: poverty, chronic kidney disease, disparities, socio-economics, end-stage renal disease
INTRODUCTION
Chronic kidney disease (CKD) is a growing public health problem that has become recognized globally as an important cause of premature morbidity and mortality 1–3. Disparities in CKD may be related to many factors such as socioeconomic status (SES), gender, and race/ethnicity 4–6. Rostand and colleagues brought national attention to this issue for the first time in the early 1980’s when they reported a 4-fold higher race-specific risk for developing end stage renal disease (ESRD) among blacks in Jefferson County, Alabama, in comparison to their white counterparts 7. A consistently higher rate of ESRD has subsequently been noted among other racial/ethnic groups over the last 30 years 8,9. It should be noted that these high rates of ESRD occur despite similar or even lower prevalence rates of early stage CKD, reinforcing the need to better understand the multiple factors that conspire to influence progression to ESRD 9,10. The excess rate of ESRD among minorities not only levies a personal toll on affected families and communities, but the excess prevalence of ESRD accounts for nearly a third of the $45 billion (Medicare and non Medicare) a year in U.S. ESRD costs alone 9.
Whereas disparities in CKD prevalence and progression have generally been thought to be a function of racial/ethnic, gender or genetic differences influencing the prevalence and/or control of CKD risk factors such as diabetes and hypertension, the role of the social environment and economic conditions has recently gained greater attention as an important element in the pathway from CKD risk to the development and complications of CKD and ESRD 11. Indeed, the social environment has been cited as a key determinant in the persistence of health inequities in the U.S. Despite our recognized standing as a world leader in health technology and medical care, the U.S. ranks near last in preventable deaths among developed nations 12. Dr. Steven Schroeder, former president of the Robert Wood Johnson Foundation, argued that “since the less fortunate are disproportionately affected by actionable social determinants of health, we must focus on this population to improve the health of the American and concentrate our strategies on health behaviors, social factors, health care, and the environment” 13. This serves as a clear directive to establish greater social equity as part of a broad strategy to improve health outcomes among many vulnerable populations.
Theoretic Framework for Adverse Socioeconomic Status and Kidney Disease
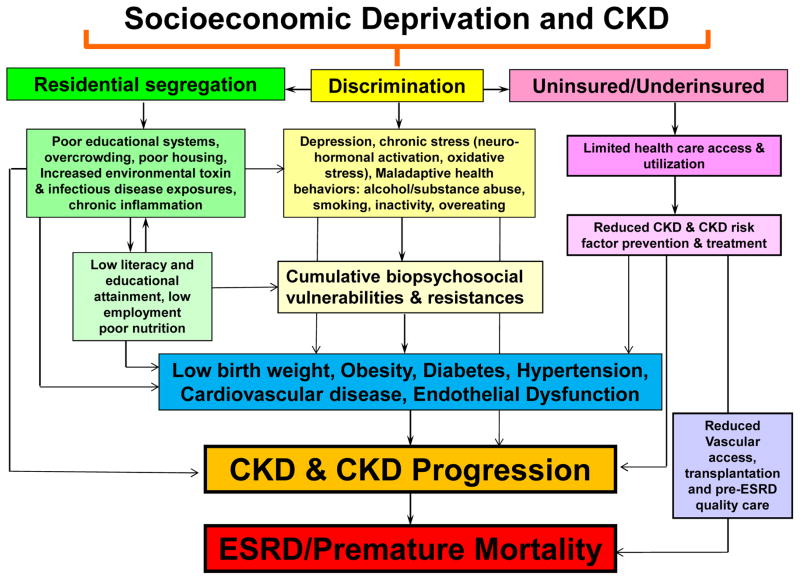
Socioeconomically disadvantaged populations across the globe exhibit a disproportionate burden of CKD often complicated by the inability to receive evidence based care leading to suboptimum clinical outcomes 2,14. A basic understanding of the vulnerabilities of the disadvantaged populations will facilitate the adaptation and adoption of the necessary policies to support kidney disease treatment and prevention guidelines 2. Moreover, the World Health Organization has identified three key tenets to improving health at a global level that each reinforce the impact of socioeconomic factors: 1) Improve the conditions of daily life, 2) Tackle the inequitable distribution of power, money, and resources – the structural drivers of those conditions of daily life – globally, nationally, and locally, and 3) Develop a workforce trained in the social determinants of health, and raise public awareness about the social determinants of health 15. The increasing impact of social factors and health behaviors has contributed to the growing CKD epidemic positioning the nephrology community to lead the charge and deal with the challenge of providing quality care in the setting of contrasting financial, and public health policies to control costs 16. A conceptual framework emphasizing the importance of socioeconomics as a mediator of key CKD prevention and treatment pathways highlights its vast impact on the CKD epidemic (figure 1). The figure shows that many of the determinants of CKD such as obesity, diabetes, hypertension and endothelial dysfunction, as well as chronic inflammation, neurohormonal activation and oxidative stress, may have their foundation in socioeconomic deprivation and its outcroppings or extensions. These include, but are not limited to discrimination and segregation, substandard living conditions, limited quality health care to the uninsured or underinsured, limited health literacy, poor educational systems and chronic stress that result in measureable and quantifiable pathologic factors that contribute to and enhance the development of CKD and eventually to ESRD and premature mortality 4,9,17–20.
Figure 1.
Conceptual model of the inter-relationship between socioeconomic deprivation and chronic kidney disease
Socioeconomic Class and Key Determinants of Health Values
The World Health Organization Commission on Social Determinants of Health has found that poor health of low income persons is directly related to the social gradient in health within and across countries caused by the unequal distribution of power, income, goods, and services, globally and nationally 21. Importantly they have noted that unequal and unfair social policies, poor economic arrangements, and bad politics conspire to cause much of the health inequity in the world. This has been seen dramatically for many years in infectious disease morbidity and mortality, and now more recently in chronic diseases such as cardiovascular disease, diabetes, CKD and others22. Table 1 highlights the influence of socioeconomic class including income on the context of patient specific needs, values, and preferences. An individual’s SES may actually considerably impact one’s perception of seemingly mundane matters such as food, education, language and time. Indeed, while these concepts may be apparent and easily recognizable in other social disciplines, their presence and implications may be lost or concealed to many in the medical arena. Therefore, an understanding of how SES may influence world-views is critical for health professionals to truly understand the diverse patients they care for and how to better connect with them to optimize the effectiveness of traditional health strategies and recommendations.
Table 1.
Socioeconomic Class and Values of Key Determinants of Health adapted from Payne and Blair 71
| POVERTY | MIDDLE CLASS | WEALTH | |
|---|---|---|---|
| FOOD | Key question: Did you have enough? Quantity important. |
Key question: Did you like it? Quality important. |
Key question: Was it presented well? Presentation important. |
| EDUCATION | Valued and revered as abstract but not as reality. | Crucial for climbing the success ladder and making money. | Necessary tradition for making and maintaining connections. |
| DESTINY | Believes in fate. Cannot do much to mitigate chance. | Believes in choice. Can change future with good choices now. | Noblesse oblige. |
| LANGUAGE | Casual register. Language is about survival. | Formal register. Language is about negotiation. | Formal register. Language is about networking. |
| FAMILY STRUCTURE | Tend to be matriarchal. | Tends to be patriarchal. | Depends on who has money. |
| WORLD VIEW | Sees the world in terms of local settings. | Sees the world in terms of national settings. | Sees the world in terms of international view. |
| TIME | Present most important. Decisions made for the moment based on feelings or survival. | Future most important. Decisions made against future ramifications. | Traditions and history most important. Decisions made partially on the basis of tradition and decorum |
Socioeconomic Status and Non-Dialysis-Dependent Chronic Kidney Disease
Several studies have highlighted a strong association between SES and the incidence, prevalence and complications of CKD 23–33. In an analysis of over 14,000 adults in the third National Health and Nutrition Examination Survey (NHANES III) we found the presence of poverty, defined as less than 200% federal poverty level (FPL), was associated with a 35% greater odds of prevalent microalbuminuria and a 78% greater odds of prevalent macroalbuminuria23. However, after adjusting for age, sex, race, education, obesity, hypertension, diabetes, reduced estimated glomerular filtration rate (eGFR), and medication use, the odds of prevalent microalbuminuria was less robust but still significant (18%; p<0.05), but the association with macroalbuminuria was no longer significant. Importantly, even after multiple statistical adjustments racial/ethnic differences in macroalbuminuria were more apparent among the subset of less affluent study participants than in those >200% FPL 23. Similarly, albuminuria was found to be associated with lower self-reported annual household income in over 22,500 adult participants 45 years and older in the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study, where Crews et al. also found that after multiple adjustments the self-reported annual household income <$20,000/year vs. >$75,000/year had a 1.34 greater odds of albumin to creatinine ratio (ACR) of 30–300mg/g and 2.36 odds of ACR >300mg/g for all participants and the relationship was more robust for blacks than whites, suggesting the effect of SES may be a determinant of racial disparities in albuminuria 24.
An analysis from the baseline examination data of the Jackson Heart Study assessed CKD status (albuminuria or eGFR <60 mL/min/1.73 m2) in over 3,400 African American adults living in the tricounty region of the Jackson, Mississippi, metropolitan area and found high SES participants (family income at least 3.5 times the FPL or having at least 1 undergraduate degree) was associated with a 41% lower odds of prevalent CKD than their less affluent counterparts 26. In a cohort of nearly 2,500 community-dwelling black and white adults age 30–64 years residing in Baltimore City, Maryland, stratified by SES (household income <125% FPL or higher) Crews and colleagues found low SES was independently associated with a 59% greater odds of CKD prevalence after adjusting for demographics, insurance status and comorbid disease, but there was no difference by race. However, when stratified by race, low SES was associated with CKD in African Americans, but not in whites, suggesting the role of SES to CKD may differ across racial/ethnic groups 27.
Similar to CKD prevalence, an increase in CKD incidence has also been associated with adverse SES. An assessment of CKD incidence among 5490 white and black residents with hypertension or diabetes enrolled in the Atherosclerosis Risk in Communities Study found that blacks had an increased risk for CKD compared to whites, of which 10% was explained by poorer access to health care and over 60% by demographic, socioeconomic, lifestyle and clinical factors 25.
An evaluation of a cross-sectional sample of U.S. adults that included over 16,000 adults who participated in the NHANES 1999–2006 found in adjusted analyses uninsured adults with non-dialysis dependent CKD were 40% less likely to be treated for their hypertension and 55% less likely to be receiving recommended therapy with angiotensin inhibitors compared to those with insurance coverage 28. The uninsured cohort was also more likely to be under the age of 50 years (62.8% vs. 23.0%, p <0.001) and nonwhite (58.7%, vs. 21.8%, p <0.001) compared with their insured counterparts 28. These findings reinforce some of the key pathways through which SES may mediate CKD progression and the public burden of ESRD. In addition, based on a Beck Depression score low SES as determined by unemployment and low income, as well as lower quality and satisfaction with life scale scores among 628 African Americans with hypertension and CKD was independently and significantly associated with a greater degree of depression, an important co-existing condition in persons with CKD 29.
The impact of individual or household income versus community poverty level on CKD outcomes is not clear. To investigate this issue McClellan et al. reported data from over 22,000 participants in the REGARDS cohort study in the Southeast U.S., finding household income (<$15,000) versus community poverty (≥25% of the community households were below the FPL) and found household income, but not community poverty, was independently associated with CKD (eGFR 10–59 ml/min/1.73 m2) prevalence 33. They also found that adjusting for household income attenuated, but did not fully account for the higher CKD prevalence in blacks compared with whites.
In summary, while low SES was strongly associated with albuminuria in unadjusted analyses, after adjusting for multiple factors such as demographic, clinical and laboratory variables, the association was much more modest. By contrast SES remained strongly associated with CKD as defined by reduced eGFR (<60 ml/min/m2) even after adjusting for multiple factors (table 2).
Table 2.
Selected studies of socioeconomics and chronic kidney disease
| Condition | Participants | Key Findings | Ref |
|---|---|---|---|
| Albuminuria | ~14,000 adults in national survey (NHANES) | <200% FPL was associated with a 35% greater odds of microalbuminuria (18% adjusted, p<0.05), and 78% greater odds of macroalbuminuria (NS after adjusted) | 23 |
| Albuminuria | 22,500 white and black adults ≥45 years in the Southeast U.S. (REGARDS cohort study) | After multiple adjustments persons with self-reported annual household income <$20,000/year vs. >$75,000/year had a 1.34 greater odds of albumin to creatinine ratio (ACR) of 30–300mg/g and 2.36 odds of ACR >300mg/g. | 24 |
| CKD Prevalence (albuminuria or eGFR <60) | 3,430 African American adults in Tri-county region of Jackson, MS | Family income ≥3.5 times FPL or at least 1 undergraduate degree was associated with a 41% lower odds of CKD vs. less affluent counterparts | 26 |
| CKD prevalence | 2,500 community-dwelling black and white adults age 30–64 years residing in Baltimore City, MD | After adjusting for demographics, insurance status and comorbid disease household income <125% vs ≥125% of FPL was associated with 59% greater adjusted odds of CKD. In stratified analyses the association was noted in African Americans, but not in whites. | 27 |
| CKD Prevalence (eGFR <60) | 736 African Americans > 65 years at multiple sites across the nation | Income <$8000/year vs. >$35,000/year was associated with a 3 times greater odds of CKD. There was no association between genetic African ancestry and kidney function | 40 |
| CKD incidence | 5490 white and black residents with diabetes or hypertension at multiple sites across the nation | Blacks had increased risk for CKD compared to whites; 10% was explained by lesser access to health care and over 60% was explained by demographic, socioeconomic, lifestyle and clinical factors. | 25 |
| Blood pressure control in CKD | Over 16,000 adults in a national survey NHANES 1999–2006 | Uninsured persons with non-dialysis dependent CKD were 40% less likely to be treated for their hypertension compared to insured persons. | 28 |
| CKD prevalence (eGFR 10–59) | 628 African American adults at multiple sites across the nation | Low SES as determined by unemployment and low income associated with a greater degree of depression | 29 |
| CKD prevalence (eGFR 10–59) | ~22,000 Black and White adults ≥45 years in the Southeast U.S. (REGARDS) | Household income (<$15,000) but not community poverty (≥ 25% of the community households below FPL) was independently associated with CKD | 33 |
Chronic kidney disease – CKD; estimated glomerular filtration rate – eGFR in mL/min/1.73 m2; federal poverty level – FPL; end stage renal disease – ESRD; socioeconomic status – SES; National Health and Nutrition Examination Survey – NHANES; REasons for Geographic And Racial Differences in Stroke Study – REGARDS; National Kidney Foundation Kidney Early Evaluation Program – KEEP
Socioeconomic Status and End-Stage Renal Disease
Similar to non-dialysis-dependent CKD, several studies have highlighted a strong association between SES and the incidence, prevalence and complications of ESRD 34–38. In an analysis of 79,943 black and white participants in the Southern Community Cohort Study, Lipworth et al. found low income (income below vs. above $15,000) was associated with a 50% increased risk of ESRD and that the 3.5 fold increase in black-white ESRD incidence was attenuated, but not eliminated, after controlling for known risk factors in a cohort closely matched by socioeconomics 34. Like low income, homelessness might impact ESRD risk. Hall et al. also examined time to ESRD and death in over 15,000 urban and mostly poor adults (73% with annual income <$15,000) with CKD utilizing a public health safety-net system and found that racial/ethnic minorities had a 2.2–4.0 fold higher risk of progression to ESRD compared with white persons with CKD, which was not explained by lower relative mortality, which could increase their likelihood of progressing to ESRD 35. In addition to many urban adults with low SES being more likely to be under- or un-insured, they also are at greater risk of homelessness. When Hall and co-workers re-examined this urban and mostly poor cohort they found that 858 adults were homeless. The homeless group was younger, disproportionately male, and uninsured, and not only did they suffer from far higher rates of depression and substance abuse compared to adults with stable housing (P <0.001), but over a follow-up period of nearly 3 years had a 80% crude and 28% adjusted higher risk of ESRD or death suggesting homeless adults with CKD suffer from increased CKD morbidity and mortality 32.
Of 86,588 adults younger than 65 years in the National Kidney Foundation’s Kidney Early Evaluation Program which screened persons at risk for CKD (history of diabetes or hypertension, or family history of CKD), Jurkovitz et al. found uninsured participants were 82% (adjusted) more likely than privately insured participants to die and 72% (adjusted) more likely to develop ESRD 39. Thus, lack of insurance, which is more common among low SES persons, is an independent risk factor for early death and ESRD in this high risk population 39. While health insurance was associated with improved survival in this cohort, having a primary care provider or nephrologist did not affect the risk of survival, suggesting the need to explore the connection between insurance, primary care access and outcomes in persons at high risk of or with CKD 31.
The impact of income on outcomes for patients with ESRD, who are largely relieved of structural and insurance barriers to care due to the Medicare ESRD program, is poorly understood. In a cohort of over 3,000 ESRD patients Garg et al. reported higher neighborhood income was associated with decreased mortality and an increased likelihood of placement on the renal transplant waiting list 36. The presence of private insurance coverage in addition to Medicare improved rates of listing for transplantation in a graded manner, but had no effect on socioeconomic disparities in mortality, suggesting greater health benefits can attenuate financial barriers to transplantation in low-income patients 36. The effects of low SES could also be mediated through structural barriers to care. While an assessment of distance from patient residence to transplant center did not predict placement on the transplant waitlist in over 35,000 subjects in ESRD Network 6 (Georgia, North Carolina, or South Carolina), increasing neighborhood poverty was associated in a gradated manner with greater likelihood of decreased placement on the transplant waitlist for all patients, but the effect was even greater in blacks than in whites 37. When Volkova and co-workers explored the contribution of neighborhood poverty to racial disparity in ESRD incidence by examining census tract level neighborhood poverty in over 34,000 patients in ESRD Network 6 they found census tract was strongly associated with higher ESRD incidence for both blacks and whites 38. Increasing levels of census tract poverty was associated with a greater disparity in ESRD rates between blacks and whites, while census tracts with lesser poverty had more similar ESRD rates by race suggesting an interaction between race and poverty 38.
In summary, low SES was strongly associated with risk for ESRD progression and or death even after adjusting for multiple factors such as demographic, clinical and laboratory variables suggesting a strong link between social and economic deprivation and progression to severe kidney disease and premature death (table 3).
Table 3.
Selected studies of socioeconomics and end stage renal disease
| Condition | Participants | Key Findings | Ref |
|---|---|---|---|
| Risk of ESRD | 79,943 black and white participants in the Southern Community Cohort Study | Income below vs. above $15,000 was associated with a 50% increased risk of ESRD. A 3.5 fold increase in black-white ESRD incidence was markedly attenuated, after controlling for known risk factors in a SES matched cohort | 34 |
| Risk of ESRD or death | 15,000 urban, mostly poor adults with CKD in the San Francisco Community Health Network | In this low SES cohort (73% < $15,000/year) racial/ethnic minorities had a 2.2–4.0 fold higher risk of progression to ESRD compared with white persons with CKD | 35 |
| Risk of ESRD or death | 15,000 urban, mostly poor adults with CKD in the San Francisco Community Health Network | In this low SES cohort (73% < $15,000/year), a subgroup of 858 homeless persons followed over ~3 years. had a 80% crude and 28% adjusted higher risk of ESRD or death vs. adults with stable housing. They also suffered from higher rates of depression and substance abuse (P < 0.001). | 32 |
| Risk of ESRD or death | 86,588 adults <65 years in a national CKD screening program (KEEP) | Uninsured participants with or at risk for CKD were 82% (adjusted) more likely than privately insured participants to die and 72% (adjusted) more likely to develop ESRD. | 39 |
| Risk of death | 138,331 adults <65 years in a national CKD screening program (KEEP) | The lack of having a primary care provider or nephrologist was not associated with increased risk for death in persons with or at risk for CKD (unlike being uninsured – ref 39) | 31 |
| Risk of death or placed on transplant list | 3,000 ESRD patients | Higher neighborhood income was associated with decreased mortality and an increased likelihood of placement on the renal transplant waiting list. | 36 |
| Risk of placement on the transplant waitlist | 35,000 ESRD patients in Network 6 (Georgia, North Carolina, or South Carolina) | Distance from patient residence to transplant center did not predict placement on the transplant waitlist. Increasing neighborhood poverty was associated with greater likelihood of decreased placement on the transplant waitlist and the effect was even greater in blacks than in whites | 37 |
| Risk of ESRD | 34,000 patients in ESRD Network 6 (Georgia, North Carolina, or South Carolina) | Neighborhood poverty (census track level) was strongly associated with higher ESRD incidence for both blacks and whites. Increasing levels of census track poverty was associated with a greater disparity in ESRD rates between blacks and whites, suggesting an interaction between race and poverty | 38 |
| Risk of ESRD incidence, kidney transplant waiting list, and kidney transplantation | 408,535 adults aged 20 to 64 from national administrative dataset (Medicaid) | Low-income nonelderly adults covered by states with Medicaid broader coverage (closer to the projected impact of ACA) had a 1.8% lower ESRD incidence for each additional 10% of the low-income nonelderly population covered by Medicaid. There was also a reduction in gaps to access to care between those with private insurance and those with expanded Medicaid in access to peritoneal dialysis, kidney transplant waiting list, and kidney transplantation. | 59 |
Chronic kidney disease – CKD; end stage renal disease – ESRD; socioeconomic status – SES; federal poverty level – FPL; National Health and Nutrition Examination Survey – NHANES; REasons for Geographic And Racial Differences in Stroke Study – REGARDS; National Kidney Foundation Kidney Early Evaluation Program – KEEP
Socioeconomic Status and Genetic Risk Factors
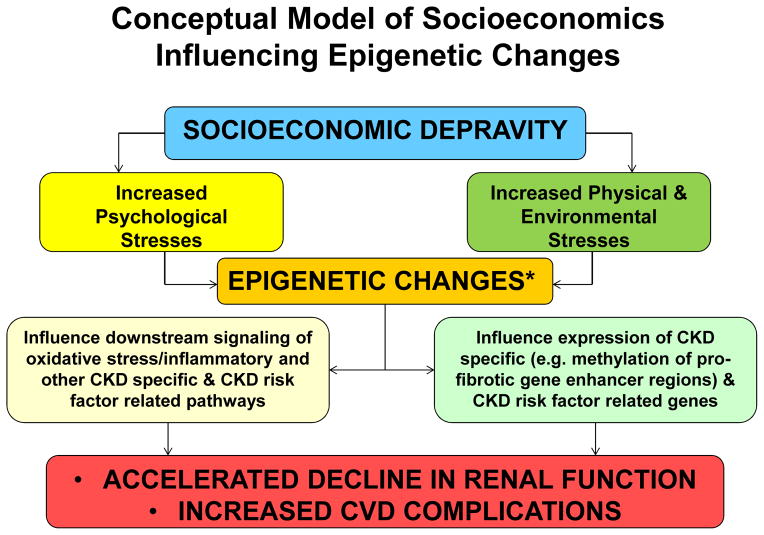
Among 736 African Americans participating in the Cardiovascular Health Study who were aged >65 years a low income (<$8000/year vs. >$35,000/year) was associated with a 3-fold greater odds of prevalent CKD assessed by cystatin C or serum creatinine based eGFR, while there was no association between genetic African ancestry and CKD, suggesting a strong influence of social and environmental factors 40. While African ancestry alone has not been shown to increase the risk of CKD, recent studies have identified that persons with two allelic variants of the APOL1 gene which is particularly prevalent in African Americans is associated with increased risk of progression to ESRD in the presence of CKD such as hypertension, diabetes, and HIV related CKD as well as focal glomerulosclerosis 41–43. These APOL1 gene variants are associated with protection of trypansomiasis and are most highly prevalent in western Africa where they are estimated to have developed 4,000 years ago, well prior to the original migration of humans from Africa to Europe, but well before the transatlantic slave trade leading to the high prevalence in African Americans 44. Importantly socioeconomic stresses can lead to neurohormonal and/or epigenetic changes that could adversely impact CKD45 as well as CKD risk factors such as blood pressure, metabolic pathways, oxidative stress, inflammatory mediators and/or other signaling factors 46–49. Given the emerging evidence of epigenetic alterations contributing to CKD and ESRD risk,45,50 it is conceptually plausible that socioeconomic depravity can contribute to not only societal and health system level inequities in care leading to adverse outcomes (figure 1), but to epigenetic changes that can influence the expression of CKD and CKD risk factor genes and signaling factors (figure 2). The reversible nature of the epigenetic changes51 gives a unique opportunity to halt or even reverse socioeconomic induced epigenetic disease processes through targeted social interventions or to create targeted interventions to attenuate the biologic alteration of low SES while social changes, which often take generations to occur, are being implemented.
Figure 2.
Conceptual model of socioeconomics influencing epigenetic changes
* As well as neurohormonal changes
CONCLUSION
The increasing rates of poverty and exposure to adverse social determinants of health, both in the U.S. and globally is reaching a level of crisis 13,52. The nephrology community and related stakeholders should unite in a strategic effort to address the clinical, financial, and public policy issues that will enable the delivery of appropriate CKD care to low socioeconomic and other vulnerable patient populations 16. The Affordable Care Act (ACA) has dramatically increased the number of low-income nonelderly adults eligible for insurance overage including Medicaid,53 and may have favorable overarching consequences on mitigating prior disparities. A recent survey from Barcellos et al. found that lower income persons (100–250% FPL) in comparison to higher income persons (≥400% FPL) were 31% less likely to score above the median on ACA knowledge, and 54% less likely to score above the median on health insurance knowledge suggesting low SES can adversely impact understanding of the ACA and may limit its success 54. In addition to extending health insurance coverage, the ACA of 2010 allowed introduction of bundled payments for a range of services, proposed the creation of accountable care organizations (ACOs), and established the Centers for Medicare and Medicaid Innovation to test new care delivery and payment models aimed to improve quality of care and contain costs with many of the demonstration programs being introduced in the nephrology community 55,56. The implications of ACA for CKD are substantial given the tremendous role that the nephrology community has played in piloting key ACA demonstration programs 55,57,58. To gain a sense of the potential impact of extending health insurance coverage, Kurella-Tamura et al. found that low-income nonelderly adults covered by states with Medicaid broader coverage (closer to the projected impact of ACA) had a significant decrease in ESRD incidence (1.8% for each additional 10% of the low-income nonelderly population covered by Medicaid) and a reduction in gaps to access to care between those with private insurance and those with Medicaid in access to peritoneal dialysis, kidney transplant waiting list, and kidney transplantation 59. The ACA may set the stage for not only more available care but more structured medical care systems which can help improve renal outcomes 60. However, education of the potential benefits of the ACA directed towards lower income persons will need to be enhanced 54.
An important challenge for the nephrology community as well as the broader medical community is to rethink how we might improve each element that impacts the health outcomes we are trying to achieve, and not just those limited to a procedure or prescription. Increased awareness of social and environmental factors that contribute to CKD disparities must be followed by cost effective policies to improve CKD/ESRD prevention and care, especially in the setting of increasing diversity and increasing disparities in wealth and educational attainment 4,9,60–62. As health care providers, we can directly address many of the factors crucial for closing the CKD/ESRD disparities gap, and while other factors may seem beyond our reach, we should not turn a blind eye to those elements of institutionalized racism entrenched within the fabric of our society, such as social injustice and human indifference 11,61. Examples of evidence-based initiatives to mitigate untoward effects of socioeconomic deprivation include expanding awareness of CKD in vulnerable communities and high-risk individuals such as through the National Kidney Foundation Early Evaluation Program or mobile clinics63–66 and implementing strategies to increase health literacy even among low-educational groups with or at risk for CKD with use of videos and/or novellas,67,68 the use of social support such as social networks,19 and primary intervention strategies including the use of lay health workers and patient navigators to address CKD and CKD risk factors ranging from diabetes to childhood chronic diseases63,66,69,70. Finally, we should not miss the opportunity to learn important lessons as we strive to advance the necessary policies to improve social welfare and health outcomes, as the existence of health inequities provides unique, unrecognized opportunities for understanding biologic, environmental, sociocultural, and health care system factors that can lead to improved clinical outcomes 4,5,9,60,61.
Acknowledgments
Financial support: Supported in part by National Institutes of Health grants U54MD007598 (S.B.N.,K.C.N.), UL1TR000124 (K.C.N.), P30AG021684 (K.C.N.), and P20-MD000182 (K.C.N.); a Burnham and Hubrecht Endowment (S.B.N.); and K24-DK091419 Q5 and R01-DK078106 (K.K.Z.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ. Chronic kidney disease: common, harmful and treatable–World Kidney Day 2007. American journal of nephrology. 2007;27(1):108–112. doi: 10.1159/000099801. [DOI] [PubMed] [Google Scholar]
- 2.Martins D, Agodoa L, Norris K. Kidney disease in disadvantaged populations. International journal of nephrology. 2012;2012:469265. doi: 10.1155/2012/469265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007 Nov 7;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 4.Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol. 2008 Jul;19(7):1261–1270. doi: 10.1681/ASN.2008030276. [DOI] [PubMed] [Google Scholar]
- 5.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney international. 2005 Sep;68(3):914–924. doi: 10.1111/j.1523-1755.2005.00485.x. [DOI] [PubMed] [Google Scholar]
- 6.Powe NR. To have and have not: health and health care disparities in chronic kidney disease. Kidney Int. 2003;64:763–772. doi: 10.1046/j.1523-1755.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- 7.Rostand SG, Kirk KA, Rutsky EA, Pate BA. Racial differences in the incidence of treatment for end-stage renal disease. N Engl J Med. 1982 May 27;306(21):1276–1279. doi: 10.1056/NEJM198205273062106. [DOI] [PubMed] [Google Scholar]
- 8.USRDS; Health NIo, editor. United States Renal Data System 2013 annual data report: Atlas of chronic kidney disease and end stage-renal disease in the United States. Bethesda, MD: 2013. [Google Scholar]
- 9.Nicholas SB, Kalantar-Zadeh K, Norris KC. Racial disparities in kidney disease outcomes. Seminars in nephrology. 2013 Sep;33(5):409–415. doi: 10.1016/j.semnephrol.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003 Nov;14(11):2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 11.Bruce MA, Beech BM, Sims M, et al. Social environmental stressors, psychological factors, and kidney disease. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2009 Apr;57(4):583–589. doi: 10.231/JIM.0b013e31819dbb91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolte E, McKee CM. Measuring the health of nations: updating an earlier analysis. Health affairs (Project Hope) 2008 Jan-Feb;27(1):58–71. doi: 10.1377/hlthaff.27.1.58. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder SA. Shattuck Lecture. We can do better--improving the health of the American people. The New England journal of medicine. 2007 Sep 20;357(12):1221–1228. doi: 10.1056/NEJMsa073350. [DOI] [PubMed] [Google Scholar]
- 14.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013 Jul 20;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 15.Marmot M, Friel S, Bell R, Houweling TA, Taylor S. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008 Nov 8;372(9650):1661–1669. doi: 10.1016/S0140-6736(08)61690-6. [DOI] [PubMed] [Google Scholar]
- 16.Rettig RA, Norris K, Nissenson AR. Chronic kidney disease in the United States: a public policy imperative. Clinical journal of the American Society of Nephrology : CJASN. 2008 Nov;3(6):1902–1910. doi: 10.2215/CJN.02330508. [DOI] [PubMed] [Google Scholar]
- 17.Calderon JL, Zadshir A, Norris K. A survey of kidney disease and risk-factor information on the World Wide Web. MedGenMed : Medscape general medicine. 2004;6(4):3. [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser SD, Roderick PJ, Casey M, Taal MW, Yuen HM, Nutbeam D. Prevalence and associations of limited health literacy in chronic kidney disease: a systematic review. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013 Jan;28(1):129–137. doi: 10.1093/ndt/gfs371. [DOI] [PubMed] [Google Scholar]
- 19.Lora CM, Gordon EJ, Sharp LK, Fischer MJ, Gerber BS, Lash JP. Progression of CKD in Hispanics: potential roles of health literacy, acculturation, and social support. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011 Aug;58(2):282–290. doi: 10.1053/j.ajkd.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricardo AC, Yang W, Lora CM, et al. Limited health literacy is associated with low glomerular filtration in the Chronic Renal Insufficiency Cohort (CRIC) study. Clinical nephrology. 2014 Jan;81(1):30–37. doi: 10.5414/CN108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marmot M, Friel S, Bell R, Houweling TA, Taylor S. Closing the gap in a generation: health equity through action on the social determinants of health. The Lancet. 2008;372(9650):1661–1669. doi: 10.1016/S0140-6736(08)61690-6. [DOI] [PubMed] [Google Scholar]
- 22.Isaacs SL, Schroeder SA. Class - the ignored determinant of the nation’s health. The New England journal of medicine. 2004 Sep 9;351(11):1137–1142. doi: 10.1056/NEJMsb040329. [DOI] [PubMed] [Google Scholar]
- 23.Martins D, Tareen N, Zadshir A, et al. The association of poverty with the prevalence of albuminuria: data from the Third National Health and Nutrition Examination Survey (NHANES III) American journal of kidney diseases : the official journal of the National Kidney Foundation. 2006 Jun;47(6):965–971. doi: 10.1053/j.ajkd.2006.02.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crews DC, McClellan WM, Shoham DA, et al. Low income and albuminuria among REGARDS (Reasons for Geographic and Racial Differences in Stroke) study participants. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012 Nov;60(5):779–786. doi: 10.1053/j.ajkd.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans K, Coresh J, Bash LD, et al. Race differences in access to health care and disparities in incident chronic kidney disease in the US. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011 Mar;26(3):899–908. doi: 10.1093/ndt/gfq473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruce MA, Beech BM, Crook ED, et al. Association of socioeconomic status and CKD among African Americans: the Jackson Heart Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010 Jun;55(6):1001–1008. doi: 10.1053/j.ajkd.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010 Jun;55(6):992–1000. doi: 10.1053/j.ajkd.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall YN, Rodriguez RA, Boyko EJ, Chertow GM, O’Hare AM. Characteristics of uninsured Americans with chronic kidney disease. Journal of general internal medicine. 2009 Aug;24(8):917–922. doi: 10.1007/s11606-009-1028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer MJ, Kimmel PL, Greene T, et al. Sociodemographic factors contribute to the depressive affect among African Americans with chronic kidney disease. Kidney international. 2010 Jun;77(11):1010–1019. doi: 10.1038/ki.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurkovitz CT, Elliott D, Li S, et al. Physician utilization, risk-factor control, and CKD progression among participants in the Kidney Early Evaluation Program (KEEP) American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012 Mar;59(3 Suppl 2):S24–33. doi: 10.1053/j.ajkd.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saab G, Chen SC, Li S, et al. Association of physician care with mortality in Kidney Early Evaluation Program (KEEP) participants. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012 Mar;59(3 Suppl 2):S34–39. doi: 10.1053/j.ajkd.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall YN, Choi AI, Himmelfarb J, Chertow GM, Bindman AB. Homelessness and CKD: a cohort study. Clinical journal of the American Society of Nephrology : CJASN. 2012 Jul;7(7):1094–1102. doi: 10.2215/CJN.00060112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClellan WM, Newsome BB, McClure LA, et al. Poverty and racial disparities in kidney disease: the REGARDS study. American journal of nephrology. 2010;32(1):38–46. doi: 10.1159/000313883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipworth L, Mumma MT, Cavanaugh KL, et al. Incidence and predictors of end stage renal disease among low-income blacks and whites. PloS one. 2012;7(10):e48407. doi: 10.1371/journal.pone.0048407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall YN, Choi AI, Chertow GM, Bindman AB. Chronic kidney disease in the urban poor. Clinical journal of the American Society of Nephrology : CJASN. 2010 May;5(5):828–835. doi: 10.2215/CJN.09011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg PP, Diener-West M, Powe NR. Income-based disparities in outcomes for patients with chronic kidney disease. Seminars in nephrology. 2001 Jul;21(4):377–385. doi: 10.1053/snep.2001.23764. [DOI] [PubMed] [Google Scholar]
- 37.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM. Neighborhood poverty and racial disparities in kidney transplant waitlisting. Journal of the American Society of Nephrology : JASN. 2009 Jun;20(6):1333–1340. doi: 10.1681/ASN.2008030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volkova N, McClellan W, Klein M, et al. Neighborhood poverty and racial differences in ESRD incidence. Journal of the American Society of Nephrology : JASN. 2008 Feb;19(2):356–364. doi: 10.1681/ASN.2006080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jurkovitz CT, Li S, Norris KC, et al. Association between lack of health insurance and risk of death and ESRD: results from the Kidney Early Evaluation Program (KEEP) American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013 Apr;61(4 Suppl 2):S24–32. doi: 10.1053/j.ajkd.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peralta CA, Ziv E, Katz R, et al. African ancestry, socioeconomic status, and kidney function in elderly African Americans: a genetic admixture analysis. Journal of the American Society of Nephrology : JASN. 2006 Dec;17(12):3491–3496. doi: 10.1681/ASN.2006050493. [DOI] [PubMed] [Google Scholar]
- 41.Freedman BI. APOL1 and nephropathy progression in populations of African ancestry. Seminars in nephrology. 2013 Sep;33(5):425–432. doi: 10.1016/j.semnephrol.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wasser WG, Tzur S, Wolday D, et al. Population genetics of chronic kidney disease: the evolving story of APOL1. Journal of nephrology. 2012 Sep-Oct;25(5):603–618. doi: 10.5301/jn.5000179. [DOI] [PubMed] [Google Scholar]
- 43.Parsa A, Kao WH, Xie D, et al. APOL1 Risk Variants, Race, and Progression of Chronic Kidney Disease. The New England journal of medicine. 2013 Nov 9; doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman DJ, Pollak MR. Genetics of kidney failure and the evolving story of APOL1. The Journal of clinical investigation. 2011 Sep;121(9):3367–3374. doi: 10.1172/JCI46263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wing MR, Devaney JM, Joffe MM, et al. DNA methylation profile associated with rapid decline in kidney function: findings from the CRIC study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014 Apr;29(4):864–872. doi: 10.1093/ndt/gft537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America. 2004 Dec 7;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McEwen BS. Protective and damaging effects of stress mediators. The New England journal of medicine. 1998 Jan 15;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 48.Lam LL, Emberly E, Fraser HB, et al. Factors underlying variable DNA methylation in a human community cohort. Proceedings of the National Academy of Sciences of the United States of America. 2012 Oct 16;109( Suppl 2):17253–17260. doi: 10.1073/pnas.1121249109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loi M, Del Savio L, Stupka E. Social Epigenetics and Equality of Opportunity. Public health ethics. 2013 Jul;6(2):142–153. doi: 10.1093/phe/pht019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ko YA, Mohtat D, Suzuki M, et al. Cytosine methylation changes in enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome biology. 2013;14(10):R108. doi: 10.1186/gb-2013-14-10-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dwivedi RS, Herman JG, McCaffrey TA, Raj DS. Beyond genetics: epigenetic code in chronic kidney disease. Kidney international. 2011 Jan;79(1):23–32. doi: 10.1038/ki.2010.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.WHO. World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. 2013 [Google Scholar]
- 53.Hill SC, Abdus S, Hudson JL, Selden TM. Adults In The Income Range For The Affordable Care Act’s Medicaid Expansion Are Healthier Than Pre-ACA Enrollees. Health affairs (Project Hope) 2014 Apr;33(4):691–699. doi: 10.1377/hlthaff.2013.0743. [DOI] [PubMed] [Google Scholar]
- 54.Barcellos SH, Wuppermann AC, Carman KG, et al. Preparedness of americans for the affordable care act. Proceedings of the National Academy of Sciences of the United States of America. 2014 Apr 15;111(15):5497–5502. doi: 10.1073/pnas.1320488111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watnick S, Weiner DE, Shaffer R, Inrig J, Moe S, Mehrotra R. Comparing mandated health care reforms: the Affordable Care Act, accountable care organizations, and the Medicare ESRD program. Clinical journal of the American Society of Nephrology : CJASN. 2012 Sep;7(9):1535–1543. doi: 10.2215/CJN.01220212. [DOI] [PubMed] [Google Scholar]
- 56.Maddux FW, McMurray S, Nissenson AR. Toward population management in an integrated care model. Clinical journal of the American Society of Nephrology : CJASN. 2013 Apr;8(4):694–700. doi: 10.2215/CJN.09050912. [DOI] [PubMed] [Google Scholar]
- 57.Nissenson AR, Maddux FW, Velez RL, Mayne TJ, Parks J. Accountable care organizations and ESRD: the time has come. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012 May;59(5):724–733. doi: 10.1053/j.ajkd.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Hamm LL, Hostetter TH, Shaffer RN. Considering an integrated nephrology care delivery model: six principles for quality. Clinical journal of the American Society of Nephrology : CJASN. 2013 Apr;8(4):682–686. doi: 10.2215/CJN.04460512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurella-Tamura M, Goldstein BA, Hall YN, Mitani AA, Winkelmayer WC. State Medicaid Coverage, ESRD Incidence, and Access to Care. Journal of the American Society of Nephrology : JASN. 2014 Mar 20; doi: 10.1681/ASN.2013060658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Powe NR. Let’s get serious about racial and ethnic disparities. Journal of the American Society of Nephrology : JASN. 2008 Jul;19(7):1271–1275. doi: 10.1681/ASN.2008040358. [DOI] [PubMed] [Google Scholar]
- 61.Norris K, Nissenson A. Racial disparities in chronic kidney disease: tragedy, opportunity, or both? Clinical journal of the American Society of Nephrology : CJASN. 2008 Mar;3(2):314–316. doi: 10.2215/CJN.00370108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi AI, Weekley CC, Chen SC, et al. Association of educational attainment with chronic disease and mortality: the Kidney Early Evaluation Program (KEEP) American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011 Aug;58(2):228–234. doi: 10.1053/j.ajkd.2011.02.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collins AJ, Gilbertson DT, Snyder JJ, Chen SC, Foley RN. Chronic kidney disease awareness, screening and prevention: rationale for the design of a public education program. Nephrology (Carlton, Vic) 2010 Jun;15( Suppl 2):37–42. doi: 10.1111/j.1440-1797.2010.01312.x. [DOI] [PubMed] [Google Scholar]
- 64.Saab G, Whaley-Connell AT, McCullough PA, Bakris GL. CKD awareness in the United States: the Kidney Early Evaluation Program (KEEP) American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008 Aug;52(2):382–383. doi: 10.1053/j.ajkd.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 65.Whaley-Connell A, Shlipak MG, Inker LA, et al. Awareness of kidney disease and relationship to end-stage renal disease and mortality. The American journal of medicine. 2012 Jul;125(7):661–669. doi: 10.1016/j.amjmed.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gutierrez-Padilla JA, Mendoza-Garcia M, Plascencia-Perez S, et al. Screening for CKD and cardiovascular disease risk factors using mobile clinics in Jalisco, Mexico. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010 Mar;55(3):474–484. doi: 10.1053/j.ajkd.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 67.Calderon JL, Shaheen M, Hays RD, Fleming ES, Norris KC, Baker RS. Improving Diabetes Health Literacy by Animation. The Diabetes educator. 2014 Mar 27; doi: 10.1177/0145721714527518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldstein K, Briggs M, Oleynik V, et al. Using digital media to promote kidney disease education. Advances in chronic kidney disease. 2013 Jul;20(4):364–369. doi: 10.1053/j.ackd.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Congdon HB, Eldridge BH, Truong HA. Development and implementation of a navigator-facilitated care coordination algorithm to improve clinical outcomes of underserved Latino patients with uncontrolled diabetes. Journal of health care for the poor and underserved. 2013 Nov;24(4):1604–1613. doi: 10.1353/hpu.2013.0181. [DOI] [PubMed] [Google Scholar]
- 70.Raphael JL, Rueda A, Lion KC, Giordano TP. The role of lay health workers in pediatric chronic disease: a systematic review. Academic pediatrics. 2013 Sep-Oct;13(5):408–420. doi: 10.1016/j.acap.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Payne RK, Blair T. A framework for understanding poverty. 2005 aha! Process. [Google Scholar]