Abstract
Environmental exposures are a potential trigger of chronic pulmonary graft-versus-host disease (pGVHD) after successful recovery from hematopoietic cell transplant (HCT). We hypothesized that inhalations of LPS, a prototypic environmental stimulus, trigger pGVHD via increased pulmonary recruitment of donor-derived antigen-presenting cells (APCs) through the C-C motif ligand 2 (CCL2)–C-C motif receptor 2 (CCR2) chemokine axis. B10.BR(H2k) and C57BL/6(H2b) mice underwent allogeneic (Allo) or syngeneic (Syn) HCT with wild-type (WT) C57BL/6, CCL2−/−, or CCR2−/− donors. After 4 weeks, recipient mice received daily inhaled LPS for 5 days and were killed at multiple time points. Allo mice exposed to repeated inhaled LPS developed prominent lymphocytic bronchiolitis, similar to human pGVHD. The increase in pulmonary T cells in Allo mice after LPS exposures was accompanied by increased CCL2, CCR2, and Type-1 T-helper cytokines as well as by monocytes and monocyte-derived dendritic cells (moDCs) compared with Syn and nontransplanted controls. Using CCL2−/− donors leads to a significant decrease in lung DCs but to only mildly reduced CD4 T cells. Using CCR2−/− donors significantly reduces lung DCs and moDCs but does not change T cells. CCL2 or CCR2 deficiency does not alter pGVHD pathology but increases airway hyperreactivity and IL-5 or IL-13 cytokines. Our results show that hematopoietic donor-derived CCL2 and CCR2 regulate recruitment of APCs to the Allo lung after LPS exposure. Although they do not alter pathologic pGVHD, their absence is associated with increased airway hyperreactivity and IL-5 and IL-13 cytokines. These results suggest that the APC changes that result from CCL2–CCR2 blockade may have unexpected effects on T cell differentiation and physiologic outcomes in HCT.
Keywords: graft-versus-host disease, lipopolysaccharide, dendritic cells, C-C motif ligand 2, C-C motif receptor 2
Clinical Relevance
Pulmonary graft-versus-host disease is a major source of morbidity and mortality in recipients of hematopoietic cell transplant. We show that during this disease process, C-C motif ligand 2 (CCL2) and C-C motif receptor 2 (CCR2) mediate recruitment of dendritic cells but do not prevent lymphocytic inflammation. CCL2 and CCR2 deficiency increase airway hyperreactivity, which argues for careful assessment of physiologic side effects before future clinical intervention upon this signaling axis.
Pulmonary complications after hematopoietic cell transplant (HCT) are common, poorly understood, understudied, and difficult to treat, leading to calls for further research in this area from the National Institutes of Health, the American Thoracic Society, and European organizations (1–3). Despite advances made in acute graft-versus-host disease (GVHD) research (4), it remains unclear why some patients recover well from HCT and later develop chronic pulmonary GVHD (pGVHD). pGVHD is characterized by lung T cell infiltration, lymphocytic bronchiolitis, and later-onset intraluminal airway fibrosis with progressive airflow obstruction (1).
pGVHD differs from GVHD in other organs in its time course, its pathology, and its resistance to immunosuppressive therapeutics, including anti–T cell strategies. Evidence suggests that a key reason for the unique characteristics of pGVHD is the constant pulmonary exposure to inflammatory stimuli not seen by other organs. The resulting low-grade lung inflammation appears to trigger or exacerbate the development of pGVHD. The specific mechanism by which this occurs is not known, although increased antigen presentation has been brought forth as a potential mediating factor. Based on studies of skin and gastrointestinal GVHD, recipient antigen-presenting cells (APCs) are thought to contribute to acute GVHD by presenting alloantigen to donor T cells, leading to subsequent T cell expansion in target organs (4). However, donor APCs and donor APC–T cell interactions have not been studied in the context of pGVHD.
Important recent advances in pulmonary immunology have taught us that there are multiple subsets of APCs in the lung, including subspecialized dendritic cells (DCs), which are highly efficient at antigen presentation. These DCs are not resident to the lung but rather are monocyte derived and recruited to the lung and pulmonary lymph nodes via chemokine binding to their C-C motif receptor 2 (CCR2) (5, 6). Although several chemokines bind CCR2 and cause lymph node monocyte recruitment, monocyte chemoattractant protein-1 (MCP-1)/C-C motif ligand 2 (CCL2) appears to be the primary chemokine responsible for tissue monocyte recruitment. The recruited CCR2-positive monocyte-derived DCs (moDCs) have been shown to be very effective T cell stimulators and potentiators of Type 1 T-helper (Th1) adaptive responses (6).
Although CCL2 and CCR2 appear to regulate acute GVHD in animal models (7–9), their role in chronic GVHD has not been addressed. To study the features of chronic pGVHD, we have established a novel model system in which mice recover from allogeneic HCT (Allo mice) and then are challenged with repeated exposures to inhaled LPS (iLPS) for 1 week, replicating clinically relevant workplace or domestic exposures. Allo mice challenged with iLPS develop prominent lymphocytic bronchiolitis, similar to human pGVHD (10, 11). In this present study, we demonstrate that the lungs of Allo mice after iLPS have markedly elevated CCL2 levels and exaggerated recruitment of donor moDCs, as compared with syngeneic controls. We further show that HCT using CCL2- or CCR2-deficient donors decreases the DC influx without preventing the development of lymphocytic bronchiolitis at a cellular or histologic level. Instead, donor CCL2 or CCR2 deficiency negatively affects pulmonary physiology with augmented airway hyperreactivity (AHR) and an increase in IL-5 and IL-13 cytokines.
Materials and Methods
Murine HCT and LPS Exposures
Male, 8- to 10-week-old, C57Bl/6(H2b) or B10.BR(H2k) recipient mice underwent HCT with 4 × 106 bone marrow cells and 1 × 106 splenocytes (10) from C57Bl/6 wild-type (WT), CCL2−/−, or CCR2−/− donors (6). Four weeks after HCT, mice were exposed to 4.5 μg/m3 LPS aerosol (12, 13) for 2.5 h/d for five consecutive days. Mice were killed without LPS exposure, at 4 hours after 1 LPS exposure, or at 4 hours, 24 hours, 72 hours, or 7 days after the fifth LPS exposure. Further details are provided in the online supplement.
Bronchoalveolar Lavage and Lung Tissue Analysis
After performing bronchoalveloar lavage (BAL), the smallest right accessory lung lobe was used for RNA analysis, the remaining right lung for flow cytometry, and the left lung for pathology. Cytokine analysis was performed on the BAL supernatant using specific CCL2, IL-4, IL-5, and IL-13 ELISA kits (R&D Systems, Minneapolis, MN) and mouse 23-plex and 9-plex cytokine assays that included detection of IFN-γ, IL-12p40, and IL-12p70 (Bio-Rad Laboratories, Hercules, CA). RNA transcripts were measured using Taqman probe-and-primer combinations for the Th1-specific transcription factor T-box-expressed-in-T cells (T-bet), CCL2, CCR2, IL-12b, CD103, and endogenous β-actin. Further details are provided in the online supplement.
Flow Cytometry
The lymphocyte-staining panel included FITC-conjugated anti-mouse CD3, PE-Cy7–conjugated anti-mouse CD8, and APC-Cy7–conjugated anti-mouse CD4 (BioLegend, San Diego, CA). The myeloid cell–staining panel included FITC-conjugated anti-mouse MHCII, PE-conjugated anti-mouse Ly6G, APC-Cy7–conjugated anti-mouse Gr1(Ly6G/Ly6C) (BD Biosciences, San Jose, CA), APC-conjugated anti-mouse CD11c (eBioscience, San Diego, CA), PE-Cy7–conjugated anti-mouse CD11b, and PerCP-Cy5.5–conjugated anti-mouse CD45 (Biolegend). The intracellular cytokine-staining panel included FITC-conjugated anti-mouse CD3, PerCP-Cy5.5–conjugated anti-mouse CD4 (Biolegend), APC-Cy7–conjugated anti-mouse-CD8, PE-conjugated anti-mouse IL-5, and APC-conjugated anti-mouse IFN-γ (eBioscience). Flow cytometry analysis was performed using FlowJo software (Tree Star Inc., Ashland, OR). A singlet gate was used to exclude cell aggregates, followed by an all-cell gate to exclude small debris and dead cells and a CD45+ cell gate to define all white blood cells. Myeloid cell gating was done based on previously published studies (5, 6) defining inflammatory monocytes as Ly6G−CD11c−MHCII−CD11b+SSCsmallGr1+ cells, DCs as Ly6G−CD11c+MHCIIhigh, and moDCs as Ly6G−CD11c+MHCIIhighCD11b+Gr1+. Cell percentages are expressed as percentage of all cells and converted to absolute numbers by multiplying by live-cell counts.
Airway Physiology Measurements
Lung physiology measurements were performed 72 hours after LPS exposures using the flexiVent mechanical ventilator and data acquisition system (SCIREQ, Montreal, Quebec, Canada). Further details are provided in the online supplement.
DC and T Cell Coculture
T cells from naive B10.BR mice were cocultured with lung DCs from nontransplanted (NT) LPS-exposed C57BL/6 WT, CCL2−/−, or CCR2−/− mice. After 5 days of coculture, cells were stimulated, and intracellular IL-5 was measured by flow cytometry. Further details are provided in the online supplement.
Statistical Analysis
Data are expressed as means ± SEM. Individual comparisons between groups at each time point were performed using a Student’s t test. Curves for airway resistance and dynamic compliance in response to increasing doses of methacholine were compared using a two-way ANOVA for repeated measures analysis. Throughout the graphs, star (*) and plus sign (+) indicate a P value of < 0.05. For knockout experiments, (*) refers to the comparison between CCR2−/− and WT, and (+) refers to the comparison between CCL2−/− and WT.
Results
iLPS Potentiates pGVHD in Mice after Allogeneic HCT
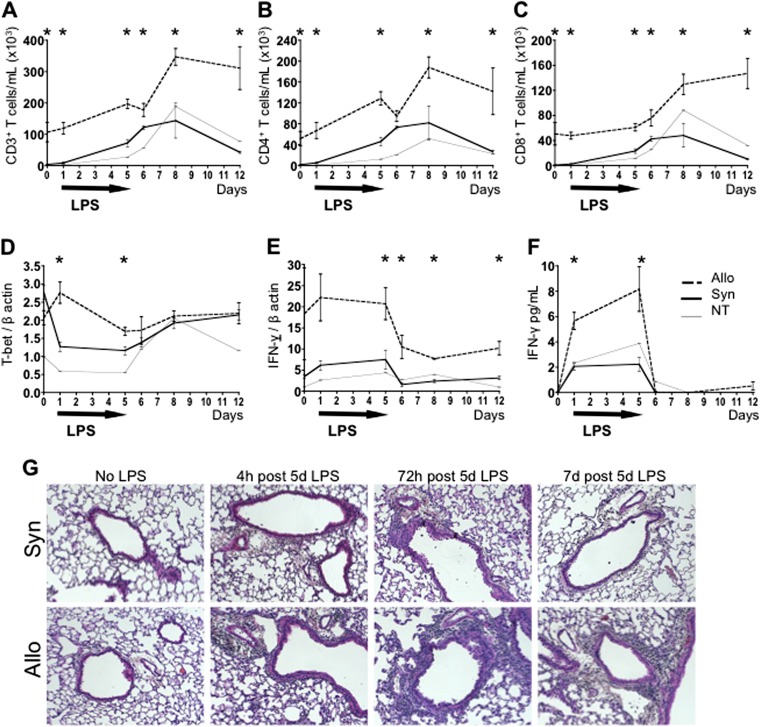
Mice that recover from allogeneic HCT (Allo) or syngeneic HCT (Syn) have only minimal pulmonary pathology at baseline. The pulmonary response of Allo, Syn, and NT mice to subacute exposures to aerosolized LPS was studied at 4 hours after one LPS exposure and at 4 hours, 24 hours, 72 hours, and 7 days after five daily LPS exposures. The BAL flow cytometric analysis is shown and parallels data from lung tissue (lung tissue data not shown). In response to subacute exposures to daily aerosolized LPS, Allo mice demonstrate a significantly stronger lymphocytic inflammatory response compared with Syn and NT mice at all time points and as far out as 1 week after the LPS exposures (Figure 1). This inflammatory response in Allo mice is consistent with features of pGVHD, with increased numbers of pulmonary CD4 and CD8 T cells (Figures 1A–1C). Elevated transcript levels of T-bet (Figure 1D) and a strong IFN-γ production (Figures 1E and 1F) in the lungs are also seen after LPS exposures, suggesting Th1 polarization. However, IFN-γ levels drop rapidly when LPS administration is stopped on Day 6, despite increased T cell accumulation. This is likely due to the fact that, whereas acute LPS exposure stimulates robust IL-12 production, continued exposure leads to APC exhaustion and the decreased production of IL-12 (14). In addition, other inflammatory mediators induced by LPS, such as NOS2 (15), which we also find to be up-regulated in lungs of Allo mice exposed to LPS (data not shown), have been demonstrated to inhibit T cell IFN-γ production. The associated pathologic findings in Allo mice exposed to LPS include pulmonary perivascular mononuclear infiltrates and lymphocytic bronchiolitis (Figure 1G).
Figure 1.
Inhaled LPS potentiates pulmonary graft-versus-host disease (pGVHD) in mice after allogeneic hematopoietic cell transplant (HCT). Mice received an allogeneic HCT (Allo), syngeneic HCT (Syn), or no HCT (nontransplanted [NT]). Allo, Syn, and NT mice underwent daily exposures to aerosolized LPS for 5 days. The LPS exposures were staggered to kill mice without LPS exposures, at 4 hours after one LPS exposure, and at 4 hours, 24 hours, 72 hours, and 7 days after five daily LPS exposures. (A–C) T cells were measured in the bronchoalveolar lavage (BAL) by flow cytometry. LPS exposures led to an increase in BAL total CD3+ T cells (A), CD4+ T cells (B), and CD8+ T cells (C) in Allo mice as compared with Syn or NT mice. (D and E) the Th1-specific transcription factor T-box-expressed-in-T cells (T-bet) and IFN-γ mRNA transcripts, measured in whole lung tissue, are elevated in the lungs of Allo mice as compared with Syn or NT mice. (F) IFN-γ BAL protein levels, as measured by ELISA, are elevated in Allo mice compared with Syn and NT controls. (G) Representative lung histology images show lymphocytic bronchiolitis and perivascular lymphocytic infiltrates in Allo mice 4 hours, 72 hours, and 7 days after 5 days of LPS exposures (hematoxylin and eosin; original magnification: ×100). Syn mice at these time points have evidence of much milder lymphocytic inflammation. Allo and Syn mice not exposed to LPS have minimal lung inflammation on histology. Quantitative data represent the average ± SEM. *P < 0.05. Data have been replicated in three independent experiments.
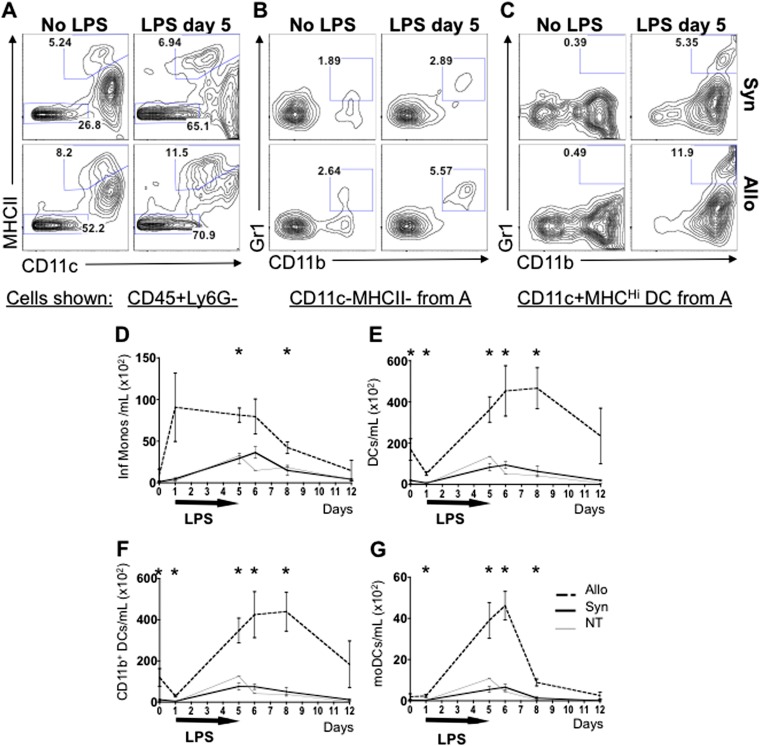
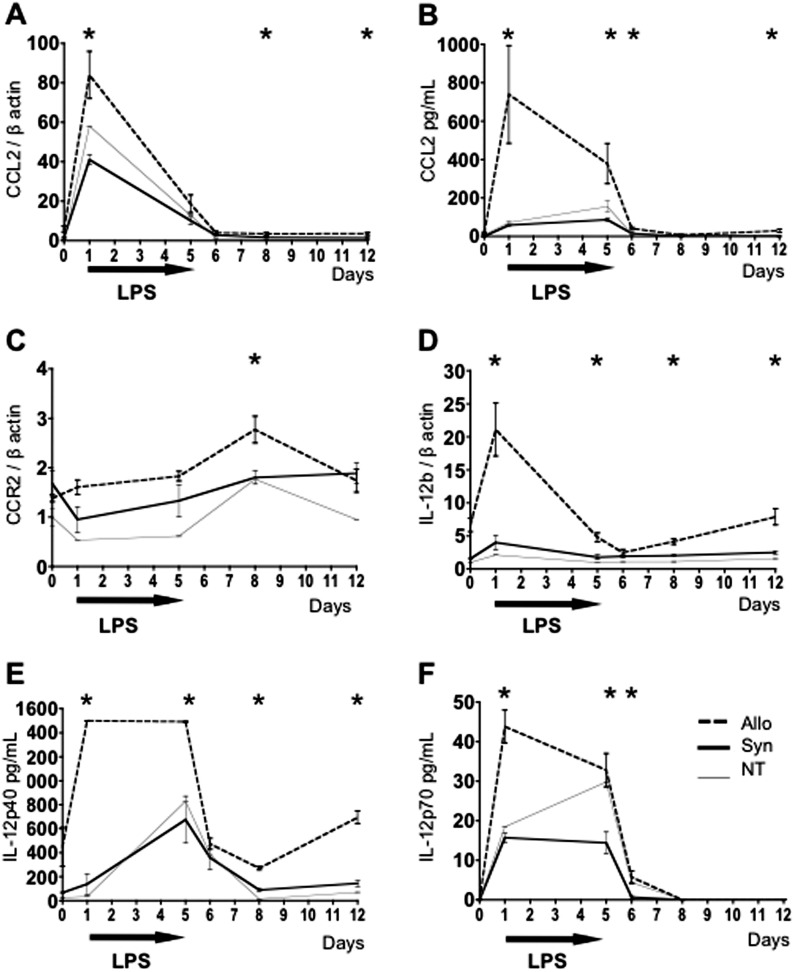
iLPS Leads to Expansion of Pulmonary Inflammatory Monocytes and moDCs in Mice after Allogeneic HCT with Up-regulation of CCL2, CCR2, and IL-12
Detailed flow cytometric analysis of APC subsets in the lungs of Allo mice reveals an increased presence of inflammatory APCs compared with Syn and NT mice (Figures 2A–2C). At this time point, > 95% of lung myeloid cells in Allo mice are donor derived (see Figure E1 in the online supplement). CD11b+CD11c− inflammatory monocytes that express high levels of Gr1 are significantly more abundant after LPS in the lungs of Allo mice (Figures 2B and 2D). CD11c+MHCII+High DCs are also present in high numbers (Figures 2A and 2E), and moDCs that coexpress CD11b and Gr1 (Figures 2C and 2G) are increased compared with controls. Total CD11b+ DCs (Figures 2C and 2F), a population of DCs that is thought to result from the loss of the Gr1 surface markers by moDCs (5), is also increased after Allo HCT. Based on recent publications showing that CCR2-expressing inflammatory monocytes get recruited into the lung via a CCL2 chemokine gradient and give rise to moDCs (6), we investigated this signaling pathway in our model. Increased levels of CCL2 transcript are present in the lung tissue (Figure 3A), and protein is elevated in the BAL (Figure 3B) of Allo mice after LPS exposure when compared with Syn and NT controls. Furthermore, the CCR2 transcript is increased in lungs of Allo mice 72 hours after LPS exposure (Figure 3C). Additionally, the main cytokine produced by Th1-polarizing moDCs, IL-12, is found in significantly higher levels in Allo mice after LPS exposure compared with Syn and NT controls (Figures 3D–3F). Consistent with our IFN-γ data, IL-12 production falls off rapidly when LPS administration is stopped despite increasing accumulation of moDCs, suggesting that APC exhaustion or inhibition by other inflammatory mediators reduces IL-12 expression in the absence of its being driven by LPS exposures (14). Overall, these data indicate that subacute exposures to iLPS in mice after allogeneic HCT lead to exaggerated pulmonary recruitment of inflammatory monocytes. These monocytes have previously been shown to express CCR2 and to differentiate into moDCs that confer a Th1-polarizing phenotype to the adaptive T cell response (6).
Figure 2.
Allogeneically transplanted mice exposed to inhaled LPS have increased pulmonary inflammatory monocytes and dendritic cells (DCs) compared with syngeneic or nontransplanted mice. Mice underwent an allogeneic HCT (Allo), syngeneic HCT (Syn), or no HCT (nontransplanted or NT) with subsequent daily exposures to aerosolized LPS for 5 days. Mice were killed without LPS exposure, at 4 hours after one LPS exposure, and at 4 hours, 24 hours, 72 hours, and 7 days after five daily LPS exposures. (A–C) Representative BAL cell flow cytometry plots are shown for Allo and Syn mice without LPS and 4 hours after five daily LPS exposures. (A) CD45+Ly6G− nonneutrophil leukocytes are shown. The highest percentage of CD11c+MHCIIHigh DCs is found in the Allo LPS-exposed mice as compared with Allo LPS-unexposed and Syn mice. (B) Among CD11c−MHCII− cells, the CD11b+ monocytes have the highest expression of Gr1 in the Allo LPS-exposed group. (C) When evaluating the Gr1 and CD11b expression on the DC population, the Allo LPS-exposed mice have the highest percentage of CD11b+ and of Gr1+CD11b+ double-positive DCs. (D–G) Total numbers of BAL monocyte and DC subsets are graphed. Allo mice exposed to LPS have higher absolute numbers of inflammatory Gr1+CD11b+ monocytes (D), DCs (E), total CD11b+ DC (F), and Gr1+CD11b+ monocyte-derived DCs (moDCs) (G) as compared with Syn and NT controls. Data represent the average ± SEM. *P < 0.05. Data have been replicated in two independent experiments.
Figure 3.
Allogeneically transplanted mice exposed to inhaled LPS have increased pulmonary C-C motif ligand 2 (CCL2), C-C motif receptor 2 (CCR2), and IL-12. Mice underwent an allogeneic HCT (Allo), syngeneic HCT (Syn), or no HCT (NT) with subsequent daily exposures to aerosolized LPS for 5 days. Mice were killed without LPS exposures, at 4 hours after one LPS exposure, and at 4 hours, 24 hours, 72 hours, and 7 days after five daily LPS exposures. Whole lung tissue mRNA transcript and BAL protein levels are shown. Allo mice exposed to LPS have increased CCL2 transcript in the lung tissue (A) and increased CCL2 protein in the BAL (B), compared with Syn and NT controls. Allo mice also have increased CCR2 (C) and IL-12b transcripts (D) in the lung tissue and increased IL-12 protein in the BAL (both the homodimer p40 [E] and heterodimer p70 [F]). Data represent the average ± SEM. *P < 0.05. Data have been replicated in two independent experiments.
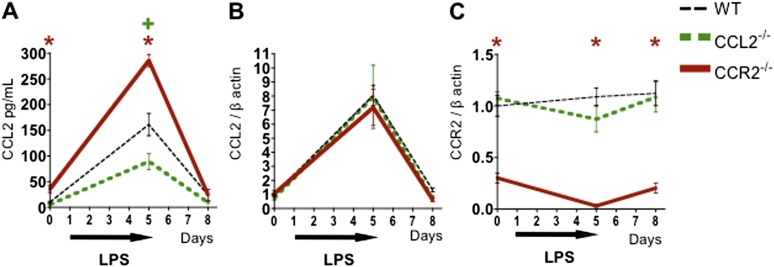
In Allo Mice Exposed to LPS, Absence of CCL2 or CCR2 on Donor Hematopoietic Cells Leads to Decreased Detection of Pulmonary CCL2 and CCR2, Respectively
Based on the above data, we postulated that inflammatory moDCs recruited to the lung during environmental exposures in Allo mice lead to increased T cell activation, recruitment, and expansion, with consequent exacerbation of pGVHD pathology and physiology. To evaluate whether CCL2 and/or CCR2 mediate recruitment of moDCs and generation of pGVHD in mice after HCT and iLPS, we performed experiments using donor mice deficient in CCL2 or CCR2. Absence of the CCL2 gene in donor mice leads to a significant decrease in CCL2 protein in the BAL of Allo mice exposed to iLPS (Figure 4A) with no change in the lung tissue CCL2 or CCR2 transcripts (Figures 4B and 4C). This suggests that a significant component of the CCL2 produced in the lungs of Allo mice after LPS exposure is produced by airspace donor-derived hematopoietic cells (e.g., macrophages) (Figure 4A). However, other structural lung cells also contribute to CCL2 production, which is thus not completely abrogated in these experiments (Figures 4A and 4B). Allo mice transplanted with donor cells deficient in CCR2 have significantly decreased CCR2 transcript levels in the lung tissue (Figure 4C) with an increase in CCL2 protein levels in the BAL (Figure 4A), consistent with compensatory changes previously described in CCR2-deficient mice (16–18).
Figure 4.
Allogeneic HCT mice transplanted with CCL2−/− or CCR2−/− donors have reduced pulmonary CCL2 and CCR2, respectively. Mice underwent an allogeneic HCT using wild-type (WT), CCL2−/−, or CCR2−/− donors and were killed without LPS exposure and at 4 and 72 hours after 5 days of daily LPS exposures. Allo mice transplanted with CCL2−/− donors have reduced CCL2 protein in their BAL (A) but have unchanged CCL2 (B) and CCR2 transcripts (C) in the lung tissue. Allo mice transplanted with CCR2−/− donors have elevated CCL2 protein in their BAL (A), unchanged CCL2 transcripts in the lung tissue (B), and reduced CCR2 transcript in the lung (C). Data represent the average ± SEM and have been replicated in two independent experiments. +P < 0.05 comparing CCL2−/− group with WT. *P < 0.05 comparing CCR2−/− group with WT.
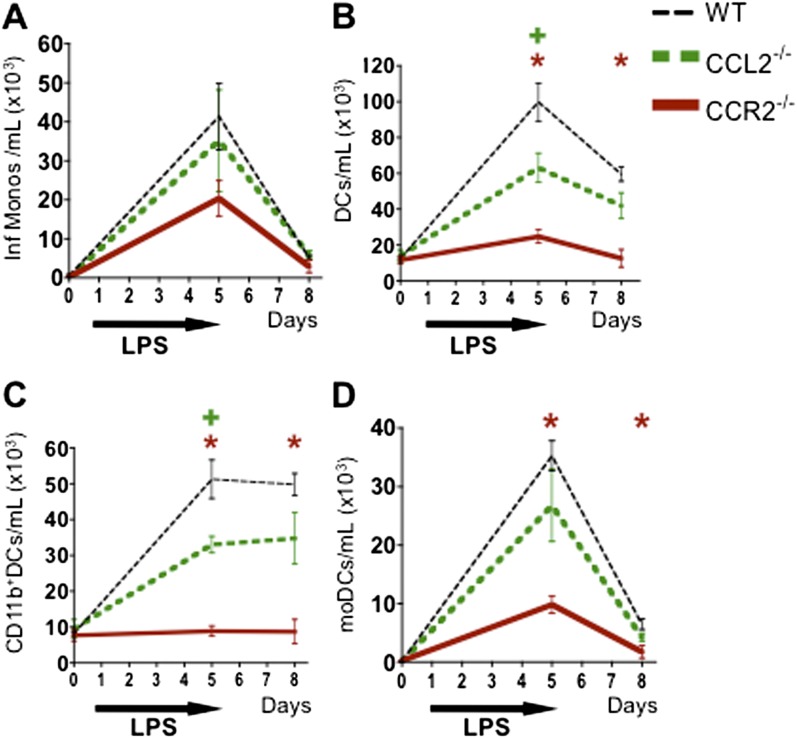
Absence of CCL2 or CCR2 on Donor Hematopoietic Cells Leads to a Decrease in Pulmonary DCs in Allo Mice after LPS Exposure
LPS-exposed Allo mice transplanted with CCL2- or CCR2-deficient donor cells have no significant reduction in inflammatory monocytes at the time points studied (Figure 5A) but display reduced expansion of DCs (Figure 5B) and total CD11b+ DCs (Figure 5C) compared with the WT group. This finding suggests that inflammatory monocyte recruitment can be mediated by CCL2 produced by recipient cells or by CCR2 ligands other than CCL2 but that donor-derived CCL2 contributes to DC expansion. In contrast, LPS-exposed Allo mice transplanted with CCR2-deficient donor cells display reductions in all DC subsets (Figures 5B–5D), consistent with a requirement for CCR2 on donor cells for DC localization and/or differentiation.
Figure 5.
Allogeneic HCT mice transplanted with CCL2−/− or CCR2−/− donors have reduced pulmonary DCs. Mice underwent an allogeneic HCT using WT, CCL2−/−, or CCR2−/− donors and were killed without LPS and at 4 and 72 hours after 5 days of daily LPS exposures. BAL cells were analyzed via flow cytometry. (A) Allo mice transplanted with either CCL2−/− or CCR2−/− donors have only a trend toward reduced inflammatory monocytes in their BAL. (B and C) Allo mice transplanted with CCL2−/− donors have a significant reduction in overall DC (B) and CD11b+ DC (C), but not in moDC numbers (C). (B–D) Allo mice transplanted with CCR2−/− donors have a significant reduction in the overall DC, CD11b+ DC, and moDC numbers in their BAL. Data represent the average ± SEM and have been replicated in two independent experiments. +P < 0.05 comparing CCL2−/− group with WT. *P < 0.05 comparing CCR2−/− group with WT.
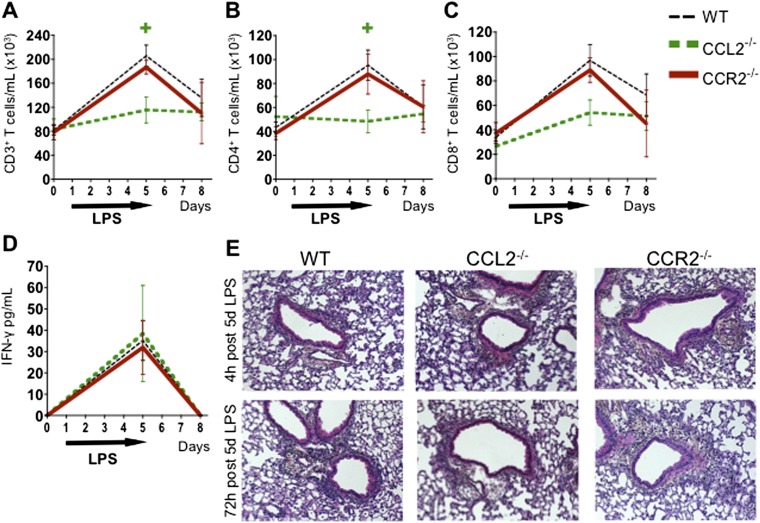
Absence of CCL2 but Not CCR2 on Donor Hematopoietic Cells Leads to a Mild T Cell Decrease, but Neither Gene Deficiency Abrogates pGVHD Pathology or IFN-γ Production
Allo mice transplanted with CCL2-deficient donors have a significant reduction in CD4 T cells at 4 hours after five LPS exposures, but this decrease is no longer evident at 72 hours after LPS exposure (Figures 6A and 6B). There is no significant difference in CD8 T cells in Allo mice transplanted with CCL2-deficient donors (Figure 6C). Allo mice transplanted with CCR2-deficient donors have no change in T cells at any time point (Figures 6A–6C). Neither CCL2 deficiency nor CCR2 deficiency in donor cells changes IFN-γ levels in the BAL (Figure 6D) or the overall pGVHD pathology at 4 and 72 hours after 5 days of LPS exposure (Figure 6E). In summary, neither CCL2 nor CCR2 deficiency abrogates pGVHD, although CCL2 deficiency is associated with a mild CD4 T cell reduction at an early time point.
Figure 6.
Allogeneic HCT mice transplanted with CCL2−/− but not CCR2−/− donors have a mild reduction in CD3 and CD4 T cells, although neither gene deficiency changes CD8 T cells, IFN-γ, or pGVHD pathology. Mice underwent an allogeneic HCT using WT, CCL2−/−, or CCR2−/− donors and were killed without LPS exposure and at 4 and 72 hours after 5 days of daily LPS exposures. BAL cells were analyzed via flow cytometry and pulmonary histology was assessed. (A and B) Allo mice transplanted with CCL2−/− but not CCR2−/− donors have a reduction in CD3 (A) and CD4 T cells (B) at 4 hours after 5 days of LPS exposure. (C and D) Neither CCL2 nor CCR2 deficiency leads to CD8 T cell reduction (C) or decrease in IFN-γ protein (D) in the BAL. (E) Representative lung histology images are shown (hematoxylin and eosin; original magnification: ×100). There is no significant change in pGVHD pathology, including perivascular and peribronchiolar lymphocytic infiltrates, in Allo mice transplanted with CCL2−/− or CCR2−/− donors. Quantitative data represent the average ± SEM and have been replicated in two independent experiments. +P < 0.05 comparing CCL2−/− group with WT. *P < 0.05 comparing CCR2−/− group with WT.
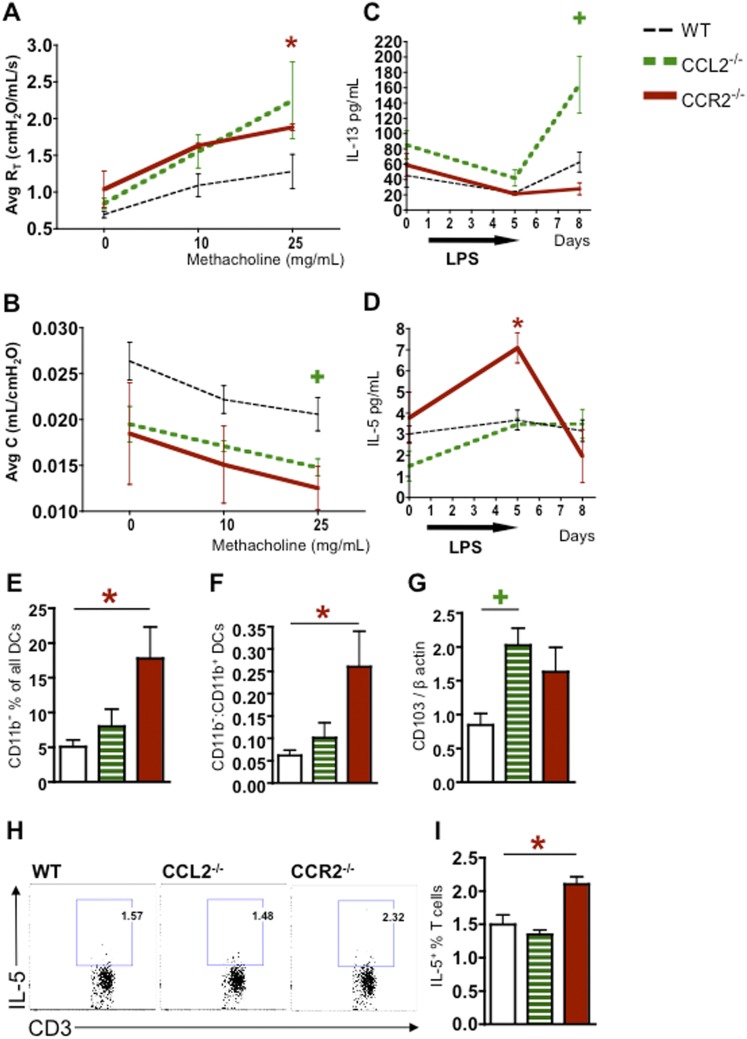
Absence of CCL2 or CCR2 on Donor Hematopoietic Cells Increases AHR in Allo Mice Exposed to LPS with Up-regulation of IL-13 or IL-5 and Increased Th2-Promoting DCs
Although the overall pathology is not different in the knockout groups, we postulated that CCR2 or CCL2 deficiency would improve lung physiology, and we therefore evaluated airway resistance and compliance in our model with CCR2- and CCL2-deficient donors. Contrary to our expectations, 72 hours after LPS exposures, Allo mice transplanted with CCL2- or CCR2-deficient donors show higher airway resistance (Figure 7A) and lower airway dynamic compliance (Figure 7B) in response to methacholine, as compared with WT controls. Static lung compliance is not significantly different between the groups (data not shown), suggesting that the decrease in dynamic compliance is due to airway disease and not due to lung parenchymal tissue changes. These physiologic changes suggest a process of airway remodeling that occurs in the setting of donor CCL2 or CCR2 deficiency, although no increase in airway fibrosis was seen on pathologic analysis and no change in collagen levels was observed (data not shown).
Figure 7.
Allogeneic HCT mice transplanted with CCL2−/− or CCR2−/− donors have increased AHR, IL-13 or IL-5 cytokines, and Th2-promoting DCs after LPS exposures. Mice underwent an allogeneic HCT using WT, CCL2−/−, or CCR2−/− donors and were killed without LPS exposure and at 4 and 72 hours after 5 days of daily LPS exposures. Airway resistance and compliance in response to methacholine were measured in vivo before mice were killed, 72 hours after LPS exposures. (A) In Allo mice exposed to LPS, donor CCL2 or CCR2 deficiency leads to increased dynamic airway resistance in response to methacholine (P < 0.05 when comparing WT and CCR2−/−). (B) In Allo mice, donor CCR2 or CCL2 deficiency leads to decreased dynamic lung compliance in response to methacholine (P < 0.05 when comparing WT and CCL2−/−). (C) Allo mice transplanted with CCL2−/− donors have increased IL-13 protein in their BAL at 72 hours after LPS exposures. (D) Mice transplanted with CCR2−/− donors have increased IL-5 protein in their BAL at 4 hours after LPS exposures. (E and F) Allo mice transplanted with CCR2−/− donors have an elevated proportion of CD11b− DCs as a percentage of all DCs (E) and an increased ratio of CD11b− to CD11b+ DC (F) compared with WT at 72 hours after LPS exposures. (G) Allo mice transplanted with CCL2−/− donors have increased levels of the CD103 transcript in the lungs compared with WT at 72 hours after LPS exposures. (H and I) Lung DCs were isolated from WT, CCL2−/−, or CCR2−/− LPS-exposed mice and cocultured with allogeneic T cells. The resulting T cells were analyzed by flow cytometry, and intracellular IL-5 was measured. (H) Representative flow diagrams show IL-5+ T cells after coculture with DCs from WT, CCL2−/−, or CCR2−/− mice. (I) Allogeneic T cells cocultured with DCs from CCR2−/− mice, but not CCL2−/− mice, result in higher numbers of IL-5+ T cells compared with WT controls. Data represent the average ± SEM. +P < 0.05 comparing CCL2−/− group with WT. *P < 0.05 comparing CCR2−/− group with WT.
To better understand the mechanisms of these AHR responses, we wondered whether cytokines that have been ascribed to Th2 differentiation and involved in AHR in asthma models might be implicated in our findings. Indeed, Allo mice transplanted with CCL2-deficient donors have increased IL-13 in their BAL after LPS exposure (Figure 7C), whereas Allo mice transplanted with CCR2-deficient donors have increased IL-5 levels (Figure 7D). IL-4 levels are not different between the groups (data not shown).
We then assessed whether CCL2 or CCR2 deficiency in our studies may change the DC populations toward an increase in Th2-potentiating DCs. CD103+ DCs, which are for the most part all CD11b−, have been studied in models of allergic asthma and have been found to have a strong capacity to polarize CD4 T cells toward the Th2 phenotype (19). We therefore assessed the presence of CD11b− DCs in Allo mice transplanted with CCL2- or CCR2-deficient donors, 72 hours after 5 days of LPS exposures. CD11b− DCs constitute a higher proportion of all DCs (Figure 7E), with a higher CD11b− to CD11b+ DC ratio (Figure 7F), in the CCR2-deficient group compared with WT. Additionally, the CD103 transcript is elevated in the CCL2-deficient group compared with WT (Figure 7G). This suggests that CD103+/CD11b− Th2-promoting DCs are relatively more abundant in LPS-exposed Allo mice transplanted with CCL2- or CCR2-deficient donor cells compared with WT controls.
DCs from LPS-Exposed Lungs of CCR2−/− Mice Change T Cell Differentiation toward Increased IL-5 Production
We sought to evaluate whether DC populations that expand in response to LPS in the setting of CCL2 or CCR2 deficiency differentially stimulate allogeneic T cells. DCs were isolated from WT, CCL2-deficient, and CCR2-deficient mice 24 hours after five daily LPS exposures. Compared with WT controls, the lungs from CCR2−/− and CCL2−/− mice have lower proportions of Gr1+CD11b+ moDCs and CCR2−/− mice also have slightly lower Gr1−CD11b+ DCs (Figure E2). More pertinently, the percentage of CD11b− DCs is markedly higher in CCR2−/− and only slightly elevated in CCL2−/− as compared with WT lungs (Figure E2). Allogeneic T cells cocultured with DCs from CCR2−/− mice produce more IL-5 compared with cocultures with DCs from WT mice (Figures 7H and 7I). This suggests that CCR2 deficiency leads to a shift toward populations of CD11b− DCs that preferentially promote proliferation of IL-5–expressing T cells. There is a trend toward increased IL-5+ CD4 and IL-5+ CD8 T cells, but the differences are not statistically significant, likely due to low T cell numbers. Therefore, we cannot comment specifically on the effect of CD11b− DCs on Th2 versus Tc2 cells, although it is plausible that they have effects on both. The DCs from CCL2−/− mice did not increase IL-5 production by T cells (Figures 7H and 7I), presumably due to lower proportions of CD11b− DCs compared with the CCR2−/− mice. Additionally, production of IFN-γ by T cells is not different between the groups (data not shown), which is likely due to the large number of Th1-polarizing CD11b+ DCs present regardless of CCR2 or CCL2 deficiency. Overall, these results show that decreasing recruitment of CCR2+ cells to the lung results in a pulmonary DC population that has a higher propensity to stimulate IL-5 production by T cells.
Discussion
In this investigation, we show that subacute, environmentally triggered pGVHD is accompanied by up-regulation of the CCL2–CCR2 chemokine axis and expansion of pulmonary moDCs. However, the absence of CCR2 or CCL2 in donor-derived hematopoietic cells does not prevent pGVHD despite a decrease in pulmonary DCs. Instead, CCR2 or CCL2 deficiency worsens AHR with up-regulation of IL-13 or IL-5.
We demonstrate that CCR2 mediates pulmonary recruitment of donor moDCs to the lungs of Allo mice in response to iLPS. This is consistent with prior non–transplant-related studies showing that CCR2 is important for recruitment of moDCs to inflamed lungs after influenza infection (5, 6). Nevertheless, ours is the first report of this phenomenon after HCT at a delayed time point where the majority of pulmonary APCs are donor derived. Our findings stand in contrast to acute GVHD, where tissue host APCs interact with donor T cells (4). A study of post-HCT idiopathic pneumonia syndrome, which is a form acute pGVHD, showed that CCR2 expression on host cells did not affect pulmonary APC numbers or development of lung disease (7).
Donor CCL2 deficiency leads to reduced pulmonary DCs at an early post-iLPS time point but does so to a lesser degree compared with CCR2 deficiency in our experiments. Previous studies have reported that the source of CCL2 is the hematopoietic compartment in other transplant settings (20–22). However, our data show that the use of CCL2-deficient HCT donors leads to only a partial reduction in the overall pulmonary CCL2 levels, which likely accounts for the reduced effect on DC recruitment.
Contrary to our expectations, reduction of the post-LPS influx of DCs does not translate into ameliorated pGVHD as measured by lymphocytic inflammation and AHR. In fact, the T cell expansion appears to be independent of DCs and moDCs. This may explain the disconnect between the early peak (4 h after first LPS exposure) in IFN-γ and IL-12 proteins and the later peak in DC and moDC numbers (4–72 h after five LPS exposures). It is possible that other cell populations, such as macrophages or neutrophils, contribute to cytokine production. Our analyses have not shown differences in macrophage or neutrophil numbers between the groups studied, but cell-specific cytokine production and cell function will need to be given additional attention in future experiments to better elucidate their roles.
We cannot exclude the possibility that T cell stimulation and proliferation occur in lymphoid organs and not in the lung, which could represent another explanation as to why reduced DC numbers in the lung do not equate to reduced T cell numbers. However, we have not been able to adequately study lymph nodes in our Allo mice because all lymphoid structures are atrophied and extremely difficult to identify due to low-grade chronic GVHD, a phenomenon that has been well described in the literature (23). Our limited analysis of lymph node cell populations have not shown any major differences in DCs or T cells between WT, CCR2−/−, and CCL2−/− groups (data not shown). We have assessed spleen cell populations and also found low cell numbers in all Allo spleens and no significant changes between the WT, CCR2−/−, and CCL2−/− groups studied (data not shown). Given the lymphoid organ atrophy, we believe that, in the context of pGVHD, DC–T cell interactions may occur in the lung itself. This would be consistent with findings from the mouse model of orthotopic lung transplantation, where rejection occurs in the absence of secondary lymphoid structures and where DC–T cell interactions have been identified within the lung allograft (16, 24).
Our body of data indicates that LPS-mediated T cell expansion may occur independent of APC stimulation. In fact, the CCL2 chemokine may be exerting its effect directly on T cell receptors. This would explain the small reduction in pulmonary CD4 T cells in the Allo group transplanted with CCL2-deficient donors. In the experiments using CCR2-deficient donors, we demonstrate a concurrent compensatory increase in CCL2, which suggests a potential CCL2-mediated, CCR2-independent mechanism of T cell recruitment proposed in other publications (25–27). Compensatory CCL2 up-regulation does not appear to take place in acute GVHD based on the literature, perhaps due to the shorter timeline of the experiments or their lack of environmental stimuli. This would explain why donor CCR2 deficiency reduced pulmonary T cell recruitment to the lung in a model of acute idiopathic pneumonia syndrome (8) and why CCR2 was found to mediate systemic disease in a model of acute CD8-induced GVHD (9).
In addition to not ameliorating pGVHD pathology, donor CCR2 and CCL2 deficiency in our experiments led to a modest increase in AHR in allogeneic HCT mice exposed to iLPS. This increase in AHR correlates with an up-regulation of IL-13 in the CCL2-deficient group and IL-5 in the CCR2-deficient group. Given the published literature on Th2 cytokines, such as IL-13 and IL-5, mediating AHR in asthma models (28), we further investigated whether CCR2 and CCL2 deficiency in our experiments promote a Th2 environment. We found an increase in the proportion of CD11b− DCs in the setting of CCR2 deficiency and elevated CD103 transcript levels in CCL2 deficiency. This suggests that Allo mice transplanted with CCR2−/− or CCL2−/− may have a relative increase in pulmonary CD11b−/CD103+ DCs, which have been described in the literature as potentiators of Th2 differentiation (19). We also showed that nontransplanted CCR2−/− mice after LPS exposure have a higher proportion of lung CD11b− DCs, and their DCs have a stronger propensity to stimulate in vitro IL-5 production by allogeneic T cells compared with WT controls. In contrast, DCs from CCL2−/− LPS-exposed mice have a minimal increase in the proportion of CD11b− DCs, and these DCs do not change IL-5 production by allogeneic T cells in vitro. These in vitro data are consistent with our in vivo findings that CCR2 deficiency has a stronger effect, compared with CCL2 deficiency, on decreasing DCs and moDCs, increasing the proportion of CD11b− DCs, and augmenting IL-5. In light of this, it is interesting that CCL2 deficiency has a similar effect on AHR as CCR2, and we suspect that this is due to the contribution of other mechanisms that we have not explored in this study, such as direct effects of CCL2 on T cell recruitment and polarization. Additionally, although we think that a plausible mechanism of increased AHR in the setting of donor CCR2 or CCL2 deficiency includes relative increases in DCs that polarize T cells toward IL-5 and IL-13 production, we cannot exclude the possibility that these cytokines are produced by other cells in vivo.
The increase in AHR and IL-5 with CCR2-deficient donors is consistent with several studies showing that CCR2 deficiency increases AHR in nontransplant settings. One study focused on an OVA allergy model using multiple repeat exposures to aerosolized OVA (29), and the other used an aspergillus sensitization and live conidia exposure model (30). Both of these studies showed up-regulation of the pulmonary IL-5 cytokine in the CCR2-knockout mice. Other studies in the literature showed divergent results and demonstrated that CCR2 and CCL2 in fact mediate AHR, mostly in models with more acute allergen exposures (31–36). It follows that the effects of CCR2 and CCL2 on AHR depend on the context under study and that the chronicity and type of environmental exposures likely play important roles.
In conclusion, our study demonstrates that subacute iLPS-potentiated pGVHD proceeds independently of the CCL2–CCR2 signaling axis. Despite reductions in select populations of recruited lung APCs, the overall immunological and histopathological features of pGVHD occur unabated in the setting of CCL2 or CCR2 deficiency. Additionally, donor CCL2 and CCR2 deficiency, likely through the alteration of the lung APC profile and up-regulation of IL-5 and IL-13, lead to more AHR. Blockade of CCL2 or CCR2 in transplantation needs to be further studied before speculating on its clinical usefulness.
Acknowledgments
Acknowledgments
The authors thank Mr. Robert F. Beasley for help with lung DC isolation. Flow cytometric sorting was performed in the Duke Human Vaccine Institute Research Flow Cytometry Shared Resource Facility (Durham, NC) supported by National Institutes of Health grant S10RR019145.
Footnotes
This work was supported by National Institutes of Health/National Heart, Lung and Blood Institute grant 1P50-HL084917–01 (SCCOR) (T.M., K.M.G., S.M.P.), by National Institutes of Health grant 1 K24 HL91140–01A2 (S.M.P.), and by a Parker B. Francis Fellowship Program Award (T.M.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0451OC on June 12, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA. 2009;302:306–314. doi: 10.1001/jama.2009.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panoskaltsis-Mortari A, Griese M, Madtes DK, Belperio JA, Haddad IY, Folz RJ, Cooke KR American Thoracic Society Committee on Idiopathic Pneumonia Syndrome. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183:1262–1279. doi: 10.1164/rccm.2007-413ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi SW, Levine JE, Ferrara JL. Pathogenesis and management of graft-versus-host disease. Immunol Allergy Clin North Am. 2010;30:75–101. doi: 10.1016/j.iac.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 6.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panoskaltsis-Mortari A, Hermanson JR, Taras E, Wangensteen OD, Charo IF, Rollins BJ, Blazar BR. Post-BMT lung injury occurs independently of the expression of CCL2 or its receptor, CCR2, on host cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L284–L292. doi: 10.1152/ajplung.00154.2003. [DOI] [PubMed] [Google Scholar]
- 8.Hildebrandt GC, Duffner UA, Olkiewicz KM, Corrion LA, Willmarth NE, Williams DL, Clouthier SG, Hogaboam CM, Reddy PR, Moore BB, et al. A critical role for CCR2/MCP-1 interactions in the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood. 2004;103:2417–2426. doi: 10.1182/blood-2003-08-2708. [DOI] [PubMed] [Google Scholar]
- 9.Terwey TH, Kim TD, Kochman AA, Hubbard VM, Lu S, Zakrzewski JL, Ramirez-Montagut T, Eng JM, Muriglan SJ, Heller G, et al. CCR2 is required for CD8-induced graft-versus-host disease. Blood. 2005;106:3322–3330. doi: 10.1182/blood-2005-05-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garantziotis S, Palmer SM, Snyder LD, Ganous T, Chen BJ, Wang T, Cook DN, Schwartz DA. Alloimmune lung injury induced by local innate immune activation through inhaled lipopolysaccharide. Transplantation. 2007;84:1012–1019. doi: 10.1097/01.tp.0000286040.85007.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinu T, Kinnier CV, Gowdy KM, Kelly FL, Snyder LD, Jiang D, Foster WM, Garantziotis S, Belperio JA, Noble PW, et al. Innate immune activation potentiates alloimmune lung disease independent of chemokine (C-X-C motif) receptor 3. J Heart Lung Transplant. 2011;30:717–725. doi: 10.1016/j.healun.2011.01.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savov JD, Brass DM, Berman KG, McElvania E, Schwartz DA. Fibrinolysis in LPS-induced chronic airway disease. Am J Physiol Lung Cell Mol Physiol. 2003;285:L940–L948. doi: 10.1152/ajplung.00102.2003. [DOI] [PubMed] [Google Scholar]
- 13.Savov JD, Brass DM, Lawson BL, McElvania-Tekippe E, Walker JK, Schwartz DA. Toll-like receptor 4 antagonist (E5564) prevents the chronic airway response to inhaled lipopolysaccharide. Am J Physiol Lung Cell Mol Physiol. 2005;289:L329–L337. doi: 10.1152/ajplung.00014.2005. [DOI] [PubMed] [Google Scholar]
- 14.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 15.Xiong H, Zhu C, Li F, Hegazi R, He K, Babyatsky M, Bauer AJ, Plevy SE. Inhibition of interleukin-12 p40 transcription and NF-kappaB activation by nitric oxide in murine macrophages and dendritic cells. J Biol Chem. 2004;279:10776–10783. doi: 10.1074/jbc.M313416200. [DOI] [PubMed] [Google Scholar]
- 16.Gelman AE, Okazaki M, Sugimoto S, Li W, Kornfeld CG, Lai J, Richardson SB, Kreisel FH, Huang HJ, Tietjens JR, et al. CCR2 regulates monocyte recruitment as well as CD4 T1 allorecognition after lung transplantation. Am J Transplant. 2010;10:1189–1199. doi: 10.1111/j.1600-6143.2010.03101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wareing MD, Lyon A, Inglis C, Giannoni F, Charo I, Sarawar SR. Chemokine regulation of the inflammatory response to a low-dose influenza infection in CCR2-/- mice. J Leukoc Biol. 2007;81:793–801. doi: 10.1189/jlb.0506299. [DOI] [PubMed] [Google Scholar]
- 18.Maus UA, Wellmann S, Hampl C, Kuziel WA, Srivastava M, Mack M, Everhart MB, Blackwell TS, Christman JW, Schlöndorff D, et al. CCR2-positive monocytes recruited to inflamed lungs downregulate local CCL2 chemokine levels. Am J Physiol Lung Cell Mol Physiol. 2005;288:L350–L358. doi: 10.1152/ajplung.00061.2004. [DOI] [PubMed] [Google Scholar]
- 19.Nakano H, Free ME, Whitehead GS, Maruoka S, Wilson RH, Nakano K, Cook DN. Pulmonary CD103(+) dendritic cells prime Th2 responses to inhaled allergens. Mucosal Immunol. 2012;5:53–65. doi: 10.1038/mi.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakanashi Y, Takeya M, Yoshimura T, Feng L, Morioka T, Takahashi K. Kinetics of macrophage subpopulations and expression of monocyte chemoattractant protein-1 (MCP-1) in bleomycin-induced lung injury of rats studied by a novel monoclonal antibody against rat MCP-1. J Leukoc Biol. 1994;56:741–750. doi: 10.1002/jlb.56.6.741. [DOI] [PubMed] [Google Scholar]
- 21.Krüger B, Schröppel B, Ashkan R, Marder B, Zülke C, Murphy B, Krämer BK, Fischereder M. A Monocyte chemoattractant protein-1 (MCP-1) polymorphism and outcome after renal transplantation. J Am Soc Nephrol. 2002;13:2585–2589. doi: 10.1097/01.asn.0000031701.53792.54. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann I, Fischereder M, Böhmig GA, Regele H, Exner M, Raith M, Weiss N, Segerer S. The source matters: no impact of the CCL2/MCP-1-1-2518G polymorphism of the donor on renal allograft outcome during the first year after transplantation. Transplant Proc. 2008;40:3359–3361. doi: 10.1016/j.transproceed.2008.08.126. [DOI] [PubMed] [Google Scholar]
- 23.Dulude G, Roy DC, Perreault C. The effect of graft-versus-host disease on T cell production and homeostasis. J Exp Med. 1999;189:1329–1342. doi: 10.1084/jem.189.8.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelman AE, Li W, Richardson SB, Zinselmeyer BH, Lai J, Okazaki M, Kornfeld CG, Kreisel FH, Sugimoto S, Tietjens JR, et al. Cutting edge: acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182:3969–3973. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marra F, Romanelli RG, Giannini C, Failli P, Pastacaldi S, Arrighi MC, Pinzani M, Laffi G, Montalto P, Gentilini P. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999;29:140–148. doi: 10.1002/hep.510290107. [DOI] [PubMed] [Google Scholar]
- 26.Zhang T, Somasundaram R, Berencsi K, Caputo L, Gimotty P, Rani P, Guerry D, Swoboda R, Herlyn D. Migration of cytotoxic T lymphocytes toward melanoma cells in three-dimensional organotypic culture is dependent on CCL2 and CCR4. Eur J Immunol. 2006;36:457–467. doi: 10.1002/eji.200526208. [DOI] [PubMed] [Google Scholar]
- 27.Schecter AD, Berman AB, Yi L, Ma H, Daly CM, Soejima K, Rollins BJ, Charo IF, Taubman MB. MCP-1-dependent signaling in CCR2(-/-) aortic smooth muscle cells. J Leukoc Biol. 2004;75:1079–1085. doi: 10.1189/jlb.0903421. [DOI] [PubMed] [Google Scholar]
- 28.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y, Sung Ss, Kuziel WA, Feldman S, Fu SM, Rose CE., Jr Enhanced airway Th2 response after allergen challenge in mice deficient in CC chemokine receptor-2 (CCR2) J Immunol. 2001;166:5183–5192. doi: 10.4049/jimmunol.166.8.5183. [DOI] [PubMed] [Google Scholar]
- 30.Blease K, Mehrad B, Standiford TJ, Lukacs NW, Gosling J, Boring L, Charo IF, Kunkel SL, Hogaboam CM. Enhanced pulmonary allergic responses to Aspergillus in CCR2-/- mice. J Immunol. 2000;165:2603–2611. doi: 10.4049/jimmunol.165.5.2603. [DOI] [PubMed] [Google Scholar]
- 31.Campbell EM, Charo IF, Kunkel SL, Strieter RM, Boring L, Gosling J, Lukacs NW. Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2-/- mice: the role of mast cells. J Immunol. 1999;163:2160–2167. [PubMed] [Google Scholar]
- 32.MacLean JA, De Sanctis GT, Ackerman KG, Drazen JM, Sauty A, DeHaan E, Green FH, Charo IF, Luster AD. CC chemokine receptor-2 is not essential for the development of antigen-induced pulmonary eosinophilia and airway hyperresponsiveness. J Immunol. 2000;165:6568–6575. doi: 10.4049/jimmunol.165.11.6568. [DOI] [PubMed] [Google Scholar]
- 33.Koth LL, Rodriguez MW, Bernstein XL, Chan S, Huang X, Charo IF, Rollins BJ, Erle DJ. Aspergillus antigen induces robust Th2 cytokine production, inflammation, airway hyperreactivity and fibrosis in the absence of MCP-1 or CCR2. Respir Res. 2004;5:12. doi: 10.1186/1465-9921-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalo JA, Lloyd CM, Wen D, Albar JP, Wells TN, Proudfoot A, Martinez-A C, Dorf M, Bjerke T, Coyle AJ, et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188:157–167. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukacs NW, Strieter RM, Warmington K, Lincoln P, Chensue SW, Kunkel SL. Differential recruitment of leukocyte populations and alteration of airway hyperreactivity by C-C family chemokines in allergic airway inflammation. J Immunol. 1997;158:4398–4404. [PubMed] [Google Scholar]
- 36.Blease K, Mehrad B, Lukacs NW, Kunkel SL, Standiford TJ, Hogaboam CM. Antifungal and airway remodeling roles for murine monocyte chemoattractant protein-1/CCL2 during pulmonary exposure to Asperigillus fumigatus conidia. J Immunol. 2001;166:1832–1842. doi: 10.4049/jimmunol.166.3.1832. [DOI] [PubMed] [Google Scholar]