Abstract
Ciliary beating is important for effective mucociliary clearance. Soluble adenylyl cyclase (sAC) regulates ciliary beating, and a roughly 50-kD sAC variant is expressed in axonemes. Normal human bronchial epithelial (NHBE) cells express multiple sAC splice variants: full-length sAC; variants with catalytic domain 1 (C1) deletions; and variants with partial C1. One variant, sACex5v2-ex12v2, contains two alternative splices creating new exons 5 (ex5v2) and 12 (ex12v2), encoding a roughly 45-kD protein. It is therefore similar in size to ciliary sAC. The variant increases in expression upon ciliogenesis during differentiation at the air–liquid interface. When expressed in NHBE cells, this variant was targeted to cilia. Exons 5v2–7 were important for ciliary targeting, whereas exons 2–4 prevented it. In vitro, cytoplasmic sACex2-ex12v2 (containing C1 and C2) was the only variant producing cAMP. Ciliary sACex5v2-ex12v2 was not catalytically active. Airway epithelial cells isolated from wild-type mice revealed sAC-dependent ciliary beat frequency (CBF) regulation, analogous to NHBE cells: CBF rescue from HCO3−/CO2–mediated intracellular acidification was sensitive to the sAC inhibitor, KH7. Compared with wild type, sAC C2 knockout (KO) mice revealed lower CBF baseline, and the HCO3−/CO2–mediated CBF decrease was not inhibited by KH7, confirming lack of functional sAC. Human sACex5v2-ex12v2 was targeted to cilia and sACex2-ex12v2 to the cytoplasm in these KO mice. Introduction of the ciliary sACex5v2-ex12v2 variant, but not the cytoplasmic sACex2-ex12v2, restored functional sAC activity in C2 KO mice. Thus, we show, for the first time, a mammalian axonemal targeting sequence that localizes a sAC variant to cilia to regulate CBF.
Keywords: adenylyl cyclase, alternative splicing, cilia, protein targeting, cAMP
Clinical Relevance
This work describes the novel finding that the local source for cAMP in cilia is a soluble adenylyl cyclase variant that is specifically targeted to the axoneme. This could present a novel target for interventions in airway diseases with ciliary dysmotility.
Cilia are important for effective mucociliary clearance, as demonstrated by patients with primary ciliary dyskinesia. This disease is characterized by a variety of ciliary defects that lead to ineffective beating patterns or total absence of ciliary beating. As a consequence, the patients develop significant lung disease with associated morbidity and mortality. cAMP is important for regulating flagellar and ciliary beating, and is produced by transmembrane adenylyl cyclase (tmAC) and soluble adenylyl cyclase (sAC) (1–8). Given the intracellular diffusion restrictions for cAMP in airway epithelial cells (9), cAMP needs to be produced close to its target, but no tmAC has been identified in ciliary membranes (10). We have shown that sAC is expressed in ciliated cells from human bronchial epithelia (6), and that it regulates ciliary beat frequency (CBF). As opposed to full-length sAC (sACfl) that is roughly 180 kD, the specific ciliary form was roughly 50 kD in size (6). The original sAC preparation purified from rat testes revealed 180- and 50-kD proteins (11). Full-length, testicular sAC (180 kD) contains two catalytic domains, known as catalytic domain 1 (C1) and C2. Both catalytic domains are required for adenylyl cyclase (AC) activity (12).
The 50-kD protein is produced by alternative splicing, skipping rat exon 12 (human exon 13) that shifts the reading frame to introduce an early stop codon in rat exon 13 (13). This 50-kD form was named truncated sAC (sACt) and contains both catalytic domains common to ACs. The catalytic activity of rat sACt is 20 times higher than sACfl (14). More alternatively spliced variants of sAC were reported in different organisms and tissues (12, 15), which indicates that sAC messenger RNA (mRNA) undergoes extensive alternative splicing.
Although a C1 domain knockout (KO) mouse was found to be deficient in testicular sAC activity (16), brain tissue from this KO mouse was found to have normal sAC activity. This activity was due to somatic sAC, a variant protein with only a C2 domain that is translated from an mRNA transcribed from a different promoter skipping exons 2, 3, and 4 and starting translation in murine exon 6 (15). Other C2-only sAC variants have also been reported in several species. These data suggest that C2-only variants expressed in somatic tissues may interact with a C1 domain–containing protein to provide the required second catalytic domain to make an enzymatically functional AC.
In normal human bronchial epithelial (NHBE) cells, we reported three different, alternatively spliced transcripts of human sAC (6). Two of these transcripts introduce a new open reading frame, initiating within a retained intron and predicting proteins containing only part of C1 (6). In Western blots, three proteins of roughly 180 kD, roughly 75 kD, and roughly 50 kD were detected by an sAC antibody targeting the unique epitope encoded at the N terminus of these splice variants. The roughly 50-kD variant was specifically localized to cilia. Given that the antibody may recognize proteins with only complete C2 domains, we expressed alternatively spliced variants of sAC in NHBE cells and measured catalytic activity of some in vitro and their functional activity in vivo in C2 KO mice. Here, we show that, in addition to sACfl, splice variants in airway epithelial cells can be classified into two major groups, each of which has a complete C2 domain, but an incomplete C1. Although these variants are not catalytically active in transfected HEK293T cells, nor in transduced NHBE cells, after immunoprecipitation, one of them, sACex5v2-ex12v2, is targeted specifically to cilia and restores sAC-mediated CBF regulation in bronchial epithelia from sAC C2 KO mice. Thus, we identified several splice variants of sAC that localize differently in airway epithelial cells, including the first mammalian axonemal targeting of a protein, and we provide further evidence for the importance of sAC in regulating ciliary beating by producing local cAMP.
Materials and Methods
Primary Cell Culture
Primary NHBE cells were isolated as previously described (6). Tracheas from wild-type (WT) and sAC C2 KO mice (17) were isolated and plated on collagen IV–coated T-clear Transwell filters (Corning, Corning, NY) in the presence of Y27632, a Rho-associated protein kinase inhibitor (18, 19).
RNA Isolation, RT-PCR, and Cloning of RT-PCR Products
mRNAs were isolated and PCR reactions performed using specific primers (Table 1). PCR fragments were sequenced after cloning at the Oncogenomic Core Facility of the University of Miami Miller School of Medicine.
Table 1.
Primer Sequences for PCR
| Primer Pairs for RT-PCR | 5′ Position | Sequence |
|---|---|---|
| F exon2 | 386 | 5′-TCCCCAGAGCGACCCTTTATG-3′ |
| R exon33 | 5,068 | 5′-GTTTACCCTGCCTGCTACAAT-3′ |
| F exon2A | 379 | 5′-ACATTTCTCCCCAGAGCGACCCT-3′ |
| R exon32A | 4,916 | 5′-GCCGCAAGGTGTGTTCAGGA-3′ |
| F exon2L | 387 | 5′-CCCCAGAGCGACCCTTTATG-3′ |
| R exon27 | 4,184 | 5′-CCACGATTTCAATGCCCTC-3′ |
| R exon23 | 3,515 | 5′-GGGCCAGAGGCAAGATG-3′ |
| F exon2 | 387 | 5′-CCCCAGAGCGACCCTTTA-3′ |
| R exon20 | 2,779 | 5′-TTGGTGGGAAAGTCTCATGCTA-3′ |
| F exon2 | 320 | 5′-TTCCAGGACTGGCCCATAGTCAGAA-3′ |
| R exon18 | 2,502 | 5′-AATGGAATCCCACAGCTTCCCTCC-3′ |
| R exon13 | 1,716 | 5′-TGGCGTGCCTCATCTGCAACA-3′ |
| F exon5v2 | 372 | 5′-GGCATGTCTCTCTCTGAAGGT-3′ |
| R exon6 | 618 | 5′-GTCCACTGCCTGACCAATCA-3′ |
Definition of abbreviations: F, forward primer; R, reverse primer.
5′ position of primer sequence on the cDNA of human full-length soluble adenylyl cyclase.
Real-Time PCR
Total RNA was extracted from air–liquid interface (ALI) cultured NHBE cells at different times. Sybr green real-time PCR was performed with the last primer pair in Table 1, annealed at 56°C for 40 cycles.
Cloning of N-Terminal Hemagglutinin and C-Terminal Flag–Tagged sAC Variants into Lentivirus Vectors
DNAs encoding human sAC variants with N-terminal hemagglutinin (HA) and C-terminal Flag tags were generated by PCR (primer pairs in Table 2) and cloned into the pCDH-EF1-MCS-T2A-copGFP (CD526A-1; System Bioscience, Mountain View, CA) lentivirus vector. sACfl was amplified using a human clone from Origene (CMV6-sAC-DDK, RC214876; Origene, Rockville, MD).
Table 2.
Primer Pairs Sequence Cloning
| Primer | Sequence |
|---|---|
| sACex5v2-ex12v2 first F: | 5′-CGATGTGCCGGATTATGCTATGTCTCTCTCTGAAGG-3′ |
| sACex5v2-ex12v2 first R: | 5′-CGTCATCCTTGTAATCTTCTGGACTCAGCCTTG-3′ |
| sACex5v2-ex12v2 second F: | 5′-CGCTCTAGAGCCACCATGTATCCATACGATGTGCCGGATTAT-3′ |
| sACex5v2-ex12v2 second R: | 5′-CGCGGATCCCTTATCGTCGTCATCCTTGTAATC-3′ |
| sACex2-ex5 first F: | 5′-GATGTGCCGGATTATGCTATGAACACTCCAAAAGAAG-3′ |
| sACex2-xe5 (NheI) R: | 5′-GGGGCTAGCAGTGCATCACCTGCAAATTTC-3′ |
| sACex2-ex5 second F: | 5′-CGCTCTAGAGCCACCATGTATCCATACGATGTGCCGGATTAT-3′ |
| sACfl first F: | 5′-CGATGTGCCGGATTATGCTATGAACACTCCAAAAGAAGA-3′ |
| sACfl second F: | 5′-CGCTCTAGAGCCACCATGTATCCATACGATGTGCCGGATTAT-3′ |
| sACfl R: | 5′-CGCGGATCCCTTATCGTCGTCATCCTTGTAATC-3′ |
| sACex2-ex7 F: | 5′-CGCTCTAGAGCCACCATGTATCCATACGATGTGCCGGATTAT-3′ |
| sACex2-ex7 first R: | 5′-CGTCATCCTTGTAATCGGAGACTCTGGGACAC-3′ |
| sACex2-ex7 second R: | 5′-CGCGGATCCCTTATCGTCGTCATCCTTGTAATC-3′ |
Definition of abbreviations: F, forward primer; R, reverse primer; sAC, soluble adenylyl cyclase; sACfl, full-length sAC.
Bold letters indicate hemagglutinin tag sequence; bold italic letters indicate flag tag sequence; underlined letters indicate restriction site sequences.
Lentivirus Production and Infection of Human and Mouse Epithelial Cells
Third generation, replication-deficient, human immunodeficiency virus–pseudotyped lentiviruses were packaged in HEK293T cells.
Western Blot
HEK293T cells were transfected with recombinant sAC variants. At 48 hours after transfection, cells were lysed. Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Blotting used mouse anti-sAC R21 antibody (1:1,000) and chemiluminescence for detection. The membrane was stripped and reprobed with mouse anti–β-actin antibody (1:5,000; Sigma-Aldrich, St. Louis, MO).
In Vitro AC Activity Assays
HEK293T cells transfected with sAC variants were lysed, and 25 μg of protein was assayed in 200 mM Tris-HCl, pH 7.5, 20 mM creatine phosphate, 3 mM dithiothreitol, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 100 U/ml phosphocreatine kinase, 2.5 mM ATP, protease inhibitors in the presence or absence of 40 mM NaHCO3 with or without 50 μM KH7. cAMP was measured with the Correlate-EIA Direct cAMP Enzyme Immunoassay Kit (Enzo Life Science, Farmingdale, NY). Cell lysates from differentiated NHBE cells infected with sAC lentivirus constructs were precleared with protein G sepharose 4 fast flow (GE Health, Pittsburgh, PA) and incubated with 2.5 μg flag antibody per sample by rotating overnight at 4°C.
Cytospin and Immunofluorescence Staining
Fully differentiated cells were gently trypsinized. Cells were fixed with 4% formaldehyde followed by permeabilization with 0.1% TritonX-100. Slides were incubated overnight with a monoclonal mouse HA antibody (1:500; Cell Signaling, Danvers, MA) and rabbit anti–acetylated tubulin (1:800, Cell Signaling) at 4°C. Secondary antibody (goat) was coupled to Alexa 555 (1:1,000; Invitrogen, Grand Island, NY) for HA and Alexa 647 (1:2,000; Invitrogen) for acetylated tubulin.
CBF and Statistics
Fully differentiated mouse bronchial epithelial cells were mounted in a closed chamber (RC20H; Warner Instruments, Hamden, CT) and apically perfused (6). CBF was measured with a Nikon E600fn microscope (Nikon, Melville, NY) using a 63× water immersion objective as previously described (6). Data were analyzed using Prism (GraphPad Software, Inc., La Jolla, CA). Multiple groups were compared by one-way ANOVA followed by Newman Keuls test. Two groups were compared by Student’s t test.
Results
Identification of Alternatively Spliced Transcripts of sAC in NHBE Cells
Three alternatively spliced transcripts of sAC, one that represented the intron 4–to–exon 5 splice previously identified in the sACfl mRNA, and two that retain portions of the 3′ end of what was previously identified as intron 4 to create new versions of exon 5 (ex5v2 and ex5v3), were identified in NHBE cells using primers specific for exons 3 and 6 (6). Among these three transcripts, two introduce in-frame stop codons upstream of an in-frame translation start codon, 16 bases upstream of the originally identified exon 5 and add the amino acid sequence MSLSE to the N terminus, encoding a protein that has an incomplete C1 and a complete C2. Using an extensive RT-PCR approach with different combinations of specific primers through 33 exons of human sAC, alternatively spliced transcripts were investigated in fully differentiated NHBE cells.
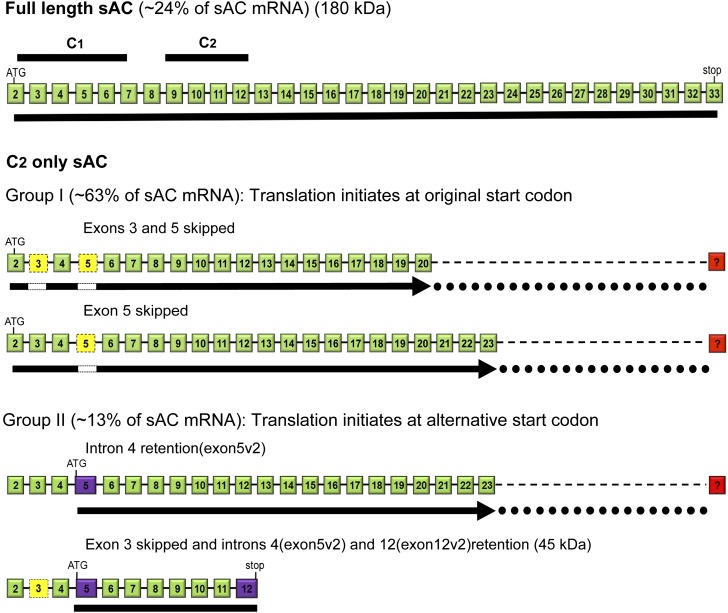
Sequencing analysis of RT-PCR products indicated that C1 was the major region of alternative splicing. From over 80 sequencing results using various combinations of exon-specific primers (Table 1) and 8 different human lung donors, alternatively spliced variants could be assigned to sACfl or C2-only sAC. The latter could be divided into two groups: group 1 containing variants that initiated at the original start codon with C1 disruptions or deletions (i.e., skipping exon 5 or exons 3 and 5), and group 2 containing variants that initiated translation from a new start codon produced by an alternative splice that retains nucleotides from the 3′ end of previously annotated intron 4 inserting an in-frame termination codon and a new translation start codon. Given our expression data and the previous Western blots, sequences of group 2 are expressed, and we therefore labeled the new exon 5 created by this splice variant exon 5v2. One of these variants stopped at a premature stop codon in a second intron retention in what was previously thought to be intron 12, now called exon 12v2 (sACex5v2-ex12v2, Figure 1). The calculated molecular size of protein encoded from this variant is roughly 45 kD, close to the molecular size of the form identified in cilia using an anti-sAC antibody that recognizes N-terminal peptide encoded from the unique start site encoded by exon 5v2.
Figure 1.
Groups of alternatively spliced soluble adenylyl cyclase (sAC) transcripts identified in normal human bronchial epithelial (NHBE) cells. The exon compositions of alternatively spliced sAC messenger RNAs (mRNAs) are diagrammed showing the included exons (green boxes), excluded exons (yellow boxes), and newly identified exons (containing previously thought intron sequences; purple boxes). Top: Full-length sAC (sACfl) mRNA containing coding exons from 2 to 33 is shown with the locations of the two catalytic domains (C1 and C2; black bars above their coding exons 2–7 and 9–12, respectively). Full-length or C1- and C2-containing splice variants represent roughly 24% of the found forms. The black line below the mRNA indicates the open reading frame. Alternatively spliced mRNAs were identified by RT-PCR with primers from exon 2 to exons 20 or 23. Group 1 contains mRNAs with an open reading frame that initiates at the same ATG as the full-length form, but skips exons within the C1 coding region, and thus do not contain a complete C1, but maintain the correct reading through C2, as indicated by the black arrow below, and initiate at the original start codon. Group 1 variants made up roughly 63% of the found forms. The dotted black line indicates the putative remainder of the mRNA. Group 2 contains transcripts that retain a portion of previously annotated intron 4 (now exon 5v2), introducing a stop codon and new translation initiation codon that is in frame with the sAC coding sequence, and thus deletes roughly 50% of C1 and has a complete C2. Group 2 variants made up roughly 13% of the found forms. One of these mRNAs (bottom) also retains a portion of what was formerly annotated as intron 12 (new exon 12v2), introducing a new stop codon and encoding a roughly 45-kD protein.
Except for sACfl, all alternatively spliced variants do not have a complete C1, but retain a complete C2. This is similar to the mouse somatic sAC isoform found in Sacytm1Lex/Sacytm1Lex KO mice, which also only contains C2, except that transcription of this mRNA initiates from a new promoter upstream of exon 5 and initiates translation from an ATG codon in exon 6 (15).
Expression of Recombinant sAC Variants in HEK and NHBE Cells
To localize different sAC variants in NHBE cells and test for catalytic activity, we cloned several sAC variants with N-terminal HA and C-terminal flag tags into the pCDH-EF1-T2A-copGFP lentivirus expression vector. The sACex5v2-ex12v2 cDNA was chosen because its calculated molecular weight is close to ciliary sAC. For comparison, tagged constructs of sACfl, exon 2 to exon 12v2 (C1 and C2, consistent with sACt), and exon 2 to intron 7 (C1-only–containing sAC) were used. Total cell protein lysates of HEK293T cells transiently transfected with HA-sACex5v2-ex12v2-flag, HA-sACex2-ex12v2-flag, HA-sACex2-in7-flag, and HA-sACfl-flag were used for Western blots with the sAC R21 antibody (20) to confirm the size and amount of the expected proteins (Figure 2A). Then, undifferentiated NHBE cells were infected with lentiviruses expressing the same variants from the EF1 promoter. After full differentiation, heterologously expressed sAC variants in NHBE cells were immunoprecipitated using a flag antibody. Precipitates were loaded on an SDS-PAGE gel and blotted with biotinylated R21 antibody and streptavidin to confirm proper protein sizes (Figure 2B).
Figure 2.
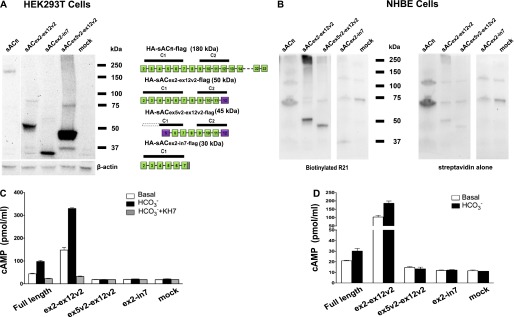
In vitro catalytic activity of recombinant sAC variants expressed in HEK293T and NHBE cells. (A) Lentivirus expression plasmids for sACfl and several variants shown on the right were transiently transfected into HEK293T cells. After 3 days, whole-cell protein lysates were prepared and equal amounts were separated via SDS-PAGE and blotted to a polyvinylidene difluoride membrane. The membrane was probed with anti–sAC R21. The blot was stripped and reprobed for β-actin as a loading control. (B) Undifferentiated NHBE cells were infected with lentiviruses, driving the expression of N-terminal hemagglutinin (HA)– and C-terminal flag–tagged human sACfl and several variants (shown on the left). Cells were redifferentiated using air–liquid interface (ALI) conditions. Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors and immunoprecipitated with an antibody to flag (cell lysates alone had insufficient sAC activity for analysis). Aliquots of the immunoprecipitated protein were run on SDS-PAGE, blotted to a nylon membrane, probed with a biotinylated anti–sAC R21 followed by horseradish peroxidase–streptavidin. The blot was stripped and reprobed with streptavidin to identify background streptavidin-binding proteins. (C) In vitro cyclase activity measured using a cAMP assay of cell lysates of transfected HEK293T cells. White bars show the basal level of activity; black bars show activity in the presence of 40 mM HCO3−, and gray bars show activity in the presence of 40 mM HCO3− plus 50 μM KH7. Activity was normalized to the expression levels of sAC variants using the Western blot data (A). Data are shown as mean (± SEM) from three experiments done as triplicates. (D) In vitro cyclase activity measured using a cAMP assay of infected NHBE after immunoprecipitation with mouse flag antibody–coated sepharose beads (cell lysates could not be used, because expression levels were insufficient to reliably measure cyclase activity). White bars show the basal level of activity and black bars show activity in the presence of 40 mM HCO3−. KH7 couldn’t be used because of interference with detergent. Activity was normalized to the expression levels of sAC variants using the Western blot data (B). Data are shown as mean (± SEM) from three experiments done as triplicates.
In Vitro AC Activity of Different Variants
HEK293T cell lysates expressing HA-sACfl-flag, HA-sACex2-ex12v2-flag, HA-sACex5v2-ex12v2-flag, and HA-sACex2-in7-flag were tested in vitro for catalytic AC activity. Cell extracts with the HA-sACex5v2-ex12v2-flag variant and HA-sACex2-in7-flag showed minimal AC activity, consistent with previous observations (12). sACt (HA-sACex2-ex12v2-flag) and sACfl demonstrated significant catalytic activity, shown by HCO3− stimulation and inhibition by 50 μM KH7 (Figure 2C), a specific sAC inhibitor (21). The catalytic activity of sACex2-ex12v2 was roughly 30 times higher than that of sACfl, consistent with previous reports (14). These data indicate that both complete C1 and C2 domains are required for in vitro sAC activity.
Total cell protein lysates from fully differentiated NHBE cells expressing these variants did not reveal sAC activity. Therefore, we used immunoprecipitation to concentrate the sAC variants from these cells for activity measurements. Cyclase activity of the immunoprecipitated variants was similar to the results obtained with lysates from HEK293T cells (Figure 2D). KH7 sensitivity was not tested, because the detergents in the immunoprecipitation buffer hamper its ability to inhibit sAC (15). HA-sACex2-ex12v2-flag was again roughly 15 times more active than sACfl. Neither the sACex5v2-ex12v2 variant (incomplete C1 and complete C2) nor the sACex2-in7 variant (C1 only) was active under these conditions, indicating again that both complete C1 and C2 domains are required for catalytic activity of sAC in vitro. Because the sACex5v2-ex12v2 variant is regulating physiological processes in airway epithelial cells, such as CBF (see subsequent text), it is catalytically active and the immunoprecipitation procedure must not have pulled down the helper proteins necessary for its activity.
Subcellular Localization of Recombinant sAC Variants in NHBE Cells
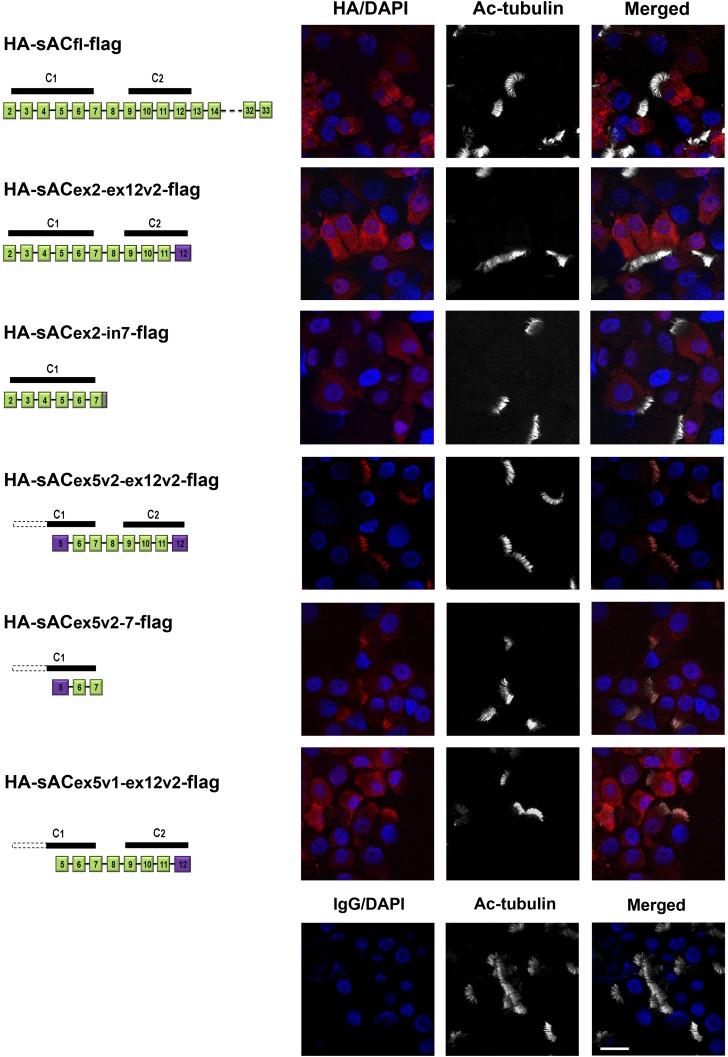
Next, we examined subcellular localization of sAC variants in fully differentiated NHBE cells expressing HA-sACfl-flag, HA-sACex2-ex12v2-flag, HA-sACex2-in7-flag, HA-sACex5v2-ex12v2-flag, HA-sACex5v2–7-flag, and HA-sACex5-ex12v2-flag (Figure 3). Although HA-sACfl-flag, HA-sACex2-ex12v2-flag, and HA-sACex2-in7-flag remained cytoplasmic, HA-sACex5v2-ex12v2-flag was found almost exclusively in cilia (Figure 3). Interestingly, a construction that does not have the MSLSE, HA-sACex5v1-ex12v2-flag was also localized to cilia although some was seen in the cytoplasm. sACfl, a C1 only variant (sACex2-in7) and one with C1 and C2 (sACex2-ex12v2) were not detected in cilia, but found exclusively in the cytoplasm. These data may suggest that the MSLSE N terminus, encoded from sequences previously thought to be intronic, may be important for ciliary targeting; however, deleting this sequence did not eliminate ciliary presence, suggesting that other sAC sequences in exons 5, 6, and 7 contribute to ciliary targeting, but only when they are near the N terminus, as the presence of exons 2–4 prevents ciliary localization.
Figure 3.
Localization of sAC isoforms in NHBE cells. Lentivirus constructs expressing different portions of sAC with an N-terminal HA and C-terminal flag tag were used to infect undifferentiated NHBE cells. After the cells were differentiated using ALI conditions, the location of the expressed sAC was determined in cytospin preparations using HA antibodies and immunofluorescence, shown to the right of each construct. These are representative images of each construct tested in cells from at least three donors. Left: diagrams of sAC constructs expressed in NHBE cells. Green boxes represent the coding exons, purple boxes indicate newly classified exons (containing previously thought intron sequences), and the gray box shows an added intron stop codon. White dashed bars indicate the missing catalytic domain, and black bars represent the contained catalytic domains. Right panels: Immunofluorescence of cytospin preparations of fully differentiated NHBE cells infected with lentiviruses expressing different sAC variants, stained with mouse anti-HA antibody (red), cilia with rabbit anti–acetylated tubulin (Ac-tubulin) antibody (white), and 4′,6-diamidino-2-phenylindole (DAPI) for nuclei (blue). HA-sACfl-flag, HA-sACex2-ex12v2-flag, and HA-sACex2-in7-flag are localized in the cytoplasm of ciliated cells. HA-sACex5v2-ex12v2-flag and HA-sACex5v2-in7-flag are localized to cilia. HA-sACex5-ex12v2-flag is found in both cilia and cytoplasm. Lower right panels: NHBE cells infected with the lentivirus vector with no insert served as a negative staining control. Scale bar, 20 μm.
Localization of sACex5v2-ex12v2 and sACex2-ex12v2 Isoforms in sAC C2 KO Mouse Airway Epithelial Cells
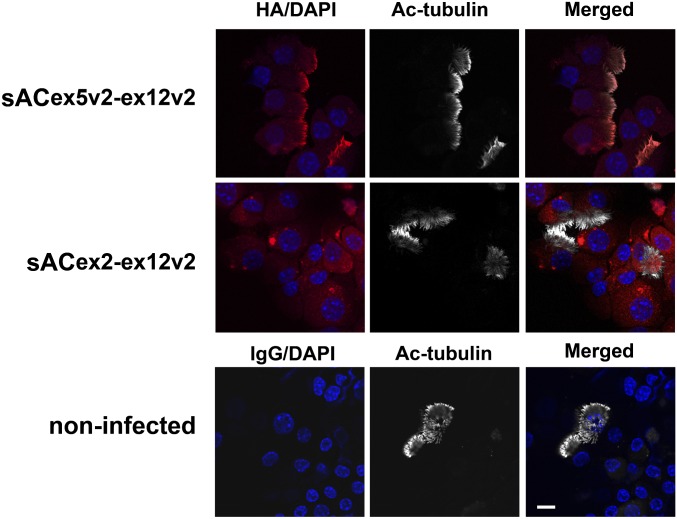
To confirm that HA-sACex5v2-ex12v2-flag localizes to cilia and HA-sACex2-ex12v2-flag to the cytosol in C2 KO mouse airway epithelial cells, we immunostained infected cells with an HA antibody. Analogous to human cells, HA-sACex5v2-ex12v2-flag was indeed found mainly in cilia and HA-sACex2-ex12v2-flag in the cytosol in C2 KO mice (Figure 4).
Figure 4.
Localization of sAC variants in C2 knockout (KO) murine airway epithelial cells. HA-sACex5v2-ex12v2-flag or HA-sACex2-ex12v2-flag were infected into undifferentiated sAC C2 KO murine airway epithelial cells. Cytospin preparations were made from fully differentiated airway epithelial cells and stained with an HA antibody (red). Cilia were identified with an acetylated tubulin (Ac-tubulin) antibody (white). DAPI shows nuclei (blue). These are representative of two or more independent experiments. Upper panels: the HA-sACex5v2-ex12v2-flag variant is localized to cilia, analogous to NHBE cells. Middle panels: the HA-sACex2-ex12v2-flag variant is not localized to cilia, but remains in the cytoplasm, again analogous to NHBE cells. Lower panels: noninfected C2 KO murine cells stained with mouse IgG antibody. Scale bars, 10 μm.
The observation that the human sACex5v2-ex12v2 isoform was targeted to cilia in mouse tracheal epithelial cells suggested that mouse cells use a similar mechanism, and may express a similar isoform. RT-PCR using primers in exons 2 and 6 identified two splice variants in the mouse tracheal epithelial cell RNA. One splice variant retaining a portion of intron 4 was identified, similar to the human sACex5v2-ex12v2 splice, but with a longer retained portion (199 bases). In addition, there is a single base deletion within the MSLSE coding sequence that shifts reading out of frame with sAC, thereby moving the initiation of sAC translation to the next in-frame ATG sequence, which is located in exon 6, basically representing somatic AC (15). These data suggest that murine ciliary localization domains are encoded in exons 6–7, when these are expressed near the N-terminal part of the protein.
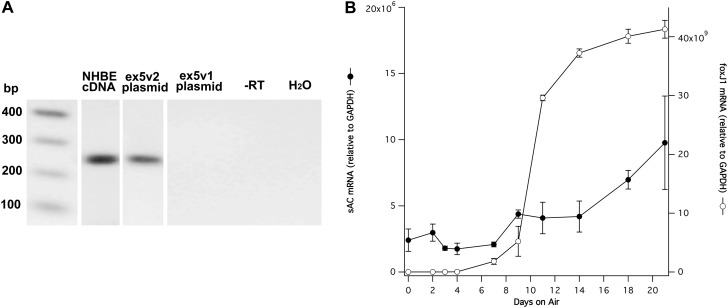
We also examined the expression of the sACex5v2-ex12v2 splice variant mRNA during NHBE cell differentiation by quantitative RT-PCR using a forward primer in the retained intron 4 of sACex5v2-ex12v2 mRNA, a reverse primer in exon 6, and RNA isolated from NHBE cells at different times during differentiation (Figure 5). FoxJ1 mRNA expression was used as a marker for ciliated cell differentiation. The results show that sACex5v2-ex12v2 variant mRNA is expressed in undifferentiated cells (Day 0 on air), and begins to increase at the same time ciliated cell differentiation begins—9 days on air, as indicated by the increase in FoxJ1 expression. The level of sACex5v2-ex12v2 shows about a threefold increase over undifferentiated cells after 21 days on air. These data are consistent with the hypothesis that sACex5v2-ex12v2 mRNA is expressed at higher levels in ciliated cells.
Figure 5.
Expression of ex5v2 containing sAC variants during differentiation. (A) Agarose gel analysis of PCR products using ex5v2-specific primers (see Table 1, last pair). Lane 2, cDNA from fully differentiated NHBE cells; lane 3, a plasmid with ex5v2 variant; lane 4, a plasmid with ex5v1 variant (i.e., without the retained intron 4 sequence); lane 5, with no reverse transcriptase; lane 6, with H2O only. The expected 266-bp band is observed in the human cell cDNA and ex5v2 plasmid, but not in the ex5v1 plasmid, indicating that the primers are specific for the ex5v2 splice variant. DNA size markers are in lane 1. (B) Graph of the ex5v2 expression (closed circles) during NHBE cell differentiation relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA measured by SYBR green quantitative RT-PCR. The level of FoxJ1 mRNA (open circles) is shown as a marker for ciliated cell differentiation. The amount of ex5v2 mRNA increases roughly threefold during NHBE cell differentiation. Data are shown as mean (± SEM).
sAC-Dependent CBF Regulation Is Rescued by HA-sACex5v2-ex12v2-Flag in Airway Epithelial Cells from C2 KO Mice
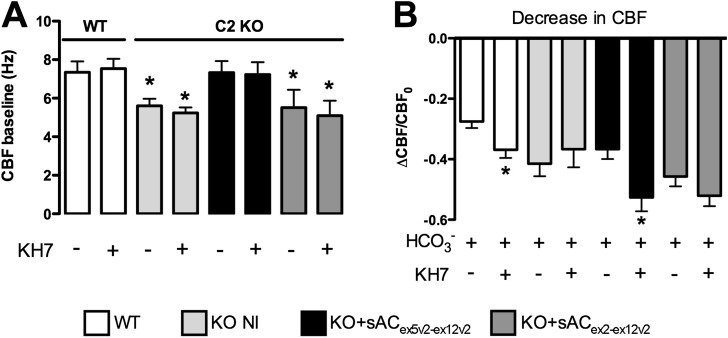
HA-sACex2-ex12v2-flag (complete C1 and C2) or HA-sACex5v2-ex12v2-flag (incomplete C1 and complete C2) were infected into murine sAC C2 KO airway epithelial cells. CBF was measured in fully differentiated cells on Transwell membranes mounted in a closed chamber, perfused apically first with Hepes-buffered Hanks’ balanced salt solution with and without KH7 (25 μM), and then with 25 mM HCO3/5% CO2 with and without KH7 (25 μM). Baseline CBF in WT mice was not sensitive to KH7, but C2 KO cells had lower CBF baselines (Figure 6A). In control WT cells, CBF decreased from baseline upon 25 mM HCO3−/5% CO2 perfusion, due to cytosolic acidification from the rapid CO2 diffusion into the cells (Figure 6B). CBF decreased even further when 25 mM HCO3−/5% CO2 was perfused together with 25 μM KH7, confirming that sAC activity in WT cells regulates CBF (6). In contrast, CBF was insensitive to KH7 in C2 KO cells (i.e., CBF decreases due to acidification in response to HCO3− perfusion alone were not different from decreases upon KH7 addition [Figure 6B]). These data demonstrate that functional ciliary sAC activity was absent in C2 KO cells.
Figure 6.
Ciliary beat frequency (CBF) in C2 KO mouse airway epithelial cells expressing sAC variants. Depicted here are the baseline and ΔCBF values from C2 KO mice upon HCO3− exposure in the presence or absence of KH7, a specific sAC inhibitor. Data are shown as mean (± SEM) from at least eight cell culture experiments seeded from two or more different animal tracheas. Left panel shows CBF baselines, and right panel shows ΔCBF in response to HCO3− with and without 25 μM KH7. *Statistically significant difference to pooled wild-type (WT) CBF baselines or to CBF decreases upon exposure to HCO3− in the absence of KH7. Even though KH7 doesn’t influence baseline CBF in WT cells, C2 KO cells (KO + not infected [NI]) or C2 KO cells infected with HA-sACex2-ex12v2-flag had a lower baseline CBF than WT and HA-sACex5v2-ex12v2-flag–infected cells (A). (B) quantitative comparisons of CBF decreases as a fraction of baseline in WT and sAC C2 KO mouse airway epithelial cells. In sAC C2 KO cells, there was no difference between the two perfusates (± KH7), again indicating absence of sAC activity. However, infection with the HA-sACex5v2-ex12v2-flag variant rescued WT CBF behavior, indicating restored sAC activity for CBF regulation. It is of note that the in vitro active form of sAC (HA-sACex2-ex12v2-flag) did not rescue CBF, indicating that sAC has to be present in cilia for proper regulation.
When C2 KO cells were infected with the HA-sACex5v2-ex12v2-flag, but not with the HA-sACex2-ex12v2-flag construct, baseline CBF was restored, as was the CBF response to CO2/HCO3−, indicating that sAC activity was restored in C2 KO cilia only when infected with the HA-sACex5v2-ex12v2-flag variant (Figure 6).
Discussion
Alternative splicing, a mechanism to produce a diverse proteome from single genes, gives rise to splice variants that can produce similar proteins, but with different functions, and that have even been implicated in diseases (22). Several different alternatively spliced variants of sAC have been reported in different tissues and organisms (12, 13, 15, 23). This suggests that extensive alternative splicing occurs with sAC.
No complete analysis of alternative splicing of sAC in human exists, and only limited information is available on tissue-specific distribution of sAC splice variants. We started a systematic compilation of the alternative splice forms of sAC in human bronchial epithelial cells. We found splice variants either corresponding to sACfl or sAC variants containing only full C2 domains. A similar variant with only C2 was thought to define murine somatic sAC isoforms, and was found specifically in brain, transcribed from an alternate promoter within intron 5 and a start codon in exon 6 (15). Searching the Genbank sequence databases lead to the identification of a large number of splice variants with predicted coding regions that encode sAC isoforms without a complete C1 domain, but with a complete C2 domain. These are predicted in a variety of species, including human, cattle, rodent, manatee, and walrus, suggesting some physiological relevance to these isoforms due to conservation throughout evolution. It is interesting to note that the in-frame MSLSE coding sequence in the retained portion of intron 4 is conserved in primates, but is altered by a single base deletion in rodents and other species, suggesting that this sAC isoform may have a unique function in primates.
On the other hand, a sAC variant without a complete C1 did not have AC activity when heterologously expressed in insect cells (12). Consistent with this finding, our variants without complete C1 had no detectable cyclase activity in vitro. However, at least one of these variants was functional in cells: when localized to cilia in C2 KO mouse airway epithelial cells, it rescued sAC-dependent beat regulation. The structures of tmAC and an sAC-like bacterial cyclase (24, 25), along with homology alignments and modeling, reveal that mammalian nucleotidyl cyclases are active as dimers of two catalytic units, which can be found in three distinct modular arrangements (reviewed in Refs. 26, 27). The bacterial sAC-like cyclase and transmembrane guanylyl cyclases are active as homodimers of proteins containing a single catalytic domain. Soluble guanylyl cyclases (sGC) are active as heterodimers between two distinct proteins (sGCα and sGCβ), each containing a single catalytic domain. tmACs and the well characterized sAC isoforms (sACt and sACfl) are active due to intramolecular “dimerization” between two related, but distinct, C domains (C1a and C2a in tmACs; C1 and C2 in sAC isoforms). In the heterodimeric cyclases (whether they are intermolecular heterodimers or intramolecular “heterodimers”), nucleotide selectivity (guanylyl versus adenylyl) is defined by amino acid residues in only one of the C domains; the other C domain contributes catalytic residues, but no nucleotide specifying interactions. In sACt, C1 provides catalytic residues, whereas C2 defines specificity for ATP over GTP; in tmACs, C1a is catalytic and C2a defines specificity for ATP; in sGC, the α subunit is catalytic, whereas β is responsible for GTP selectivity. The sAC C2 isoforms identified here do not possess all the residues necessary for both nucleotide specificity and catalysis, consistent with our inability to recover cyclase activity in vitro. It is tempting to hypothesize that they may heterodimerize with yet-unidentified C1-only–containing sAC isoforms, C1a-containing tmAC isoforms (28), or sGCα subunits.
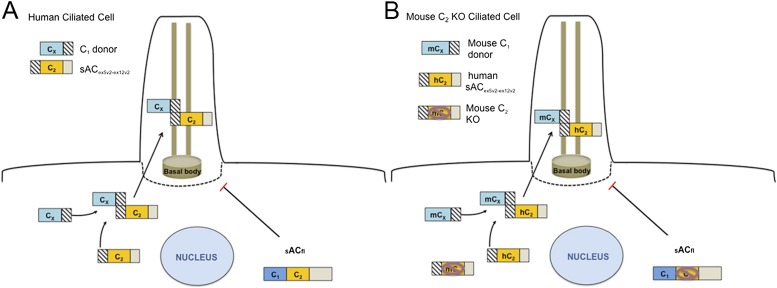
Previous data suggested that multiple forms of sAC exist in airway epithelia, and that one roughly 50-kD form localizes specifically to cilia. An antibody made against an interesting peptide sequence, SLSEGDALLA, present at the N terminus, recognized this latter form. This sequence targets part of sequences previously annotated as intron 4 and the start of exon 5, now called exon 5v2, both of which are in the sACex5v2-ex12v2 splice variant. This splice variant has a calculated molecular weight of roughly 45 kD, similar to the ciliary variant detected by Western blotting. Our experiments also indicate that splice variants that initiate translation within the retained intron 4 are targeted to cilia, suggesting that MSLSEGDALLA may act as part of a ciliary targeting sequence (CTS), even though exons 5–7 may also be important. A few CTSs have been identified for transmembrane ciliary proteins, including rhodopsin (29), fibrocystin (30), and polycystin-2 (31), but not for nontransmembrane proteins, like sAC (requiring axonemal targeting). The mechanism of trafficking membrane proteins to cilia is hypothesized to be via vesicular targeting and crossing the diffusion barriers (32). The identification of a CTS for a nonmembrane ciliary protein provides a new clue to understanding the targeting of nonmembrane proteins to cilia (Figure 7). In addition, because sAC is localized to many different locations in the cell, these observations suggest that other alternative splices may target to other cellular locations.
Figure 7.
Model of ciliary sAC (A) Human model depicts sACex5v2-ex12v2 interaction with a C1 donor that is targeted to cilia to regulate CBF in airway epithelial cells. Targeting depends on appropriate sequences: exons 5v2–7 only will allow ciliary expression; if exons 2–4 are present, as in sACfl, no ciliary localization occurs. The putative associating sites between the unknown C1 donor and sACex5v2-ex12v2 are purely speculative. (B) Rescue of sAC-mediated regulation of CBF in C2 KO mice using the human sACex5v2-ex12v2.
We reported that sAC is involved in CBF regulation of NHBE cells in response to changing HCO3− and CO2 (6). Here, we also show that mouse airway epithelial cells possess the same regulatory mechanisms. However, cells from C2 KO mice lost their ability to regulate CBF in an sAC-dependent manner, providing strong evidence that the HCO3−- and CO2-mediated changes in CBF are truly mediated by sAC. In C2 KO mice, this regulation is restored by the HA-sACex5v2-ex12v2-flag variant that is localized to cilia. On the other hand, the cytosolic variant, even though showing strong AC activity in vitro, did not rescue CBF regulation. Given our knowledge on cyclase activity, it is highly likely that the cytosolic form produced cAMP, even though we cannot completely rule out that the form was inactive in the cell (we don’t have direct cAMP measurements). If active, as suspected, cAMP production by sAC must be close to its target, and its cAMP product might not be able to diffuse freely into cilia.
In summary, we present a map of alternatively spliced transcripts of sAC in NHBE cells. Most of them contain only a part of C1, but a complete C2. One of these variants is specifically localized to cilia, suggesting a previously unappreciated axonemal targeting mechanism. Even though many of the investigated incomplete C1 variants are not active in vitro, the ciliary variant rescues CBF regulation by sAC in C2 KO mice, and thus gives credence to previous findings that sAC variants with incomplete C1 can possibly associate with helper proteins to become active ACs in the cell. Further work will be needed to identify such proteins.
Footnotes
This work was supported by University of Miami Life Alliance Organ Recovery Agency, and the Analytical Imaging Core at the University of Miami. This work was funded by the NIH HL-060644, HL-089399 (M.S.) and the Flight Attendants Medical Research Institute (FAMRI) (M.S. and N.F.).
Originally Published in Press as DOI: 10.1165/rcmb.2013-0542OC on May 29, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Di Benedetto G, Magnus CJ, Gray PT, Mehta A. Calcium regulation of ciliary beat frequency in human respiratory epithelium in vitro. J Physiol. 1991;439:103–113. doi: 10.1113/jphysiol.1991.sp018659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmid A, Bai G, Schmid N, Zaccolo M, Ostrowski LE, Conner GE, Fregien N, Salathe M. Real-time analysis of cAMP-mediated regulation of ciliary motility in single primary human airway epithelial cells. J Cell Sci. 2006;119:4176–4186. doi: 10.1242/jcs.03181. [DOI] [PubMed] [Google Scholar]
- 3.Schmid A, Sutto Z, Schmid N, Novak L, Ivonnet P, Horvath G, Conner G, Fregien N, Salathe M. Decreased soluble adenylyl cyclase activity in cystic fibrosis is related to defective apical bicarbonate exchange and affects ciliary beat frequency regulation. J Biol Chem. 2010;285:29998–30007. doi: 10.1074/jbc.M110.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frohock JI, Wijkstrom-Frei C, Salathe M. Effects of albuterol enantiomers on ciliary beat frequency in ovine tracheal epithelial cells. J Appl Physiol (1985) 2002;92:2396–2402. doi: 10.1152/japplphysiol.00755.2001. [DOI] [PubMed] [Google Scholar]
- 5.Lieb T, Frei CW, Frohock JI, Bookman RJ, Salathe M. Prolonged increase in ciliary beat frequency after short-term purinergic stimulation in human airway epithelial cells. J Physiol. 2002;538:633–646. doi: 10.1113/jphysiol.2001.013222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmid A, Sutto Z, Nlend MC, Horvath G, Schmid N, Buck J, Levin LR, Conner GE, Fregien N, Salathe M. Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. J Gen Physiol. 2007;130:99–109. doi: 10.1085/jgp.200709784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nomura M, Vacquier VD. Proteins associated with soluble adenylyl cyclase in sea urchin sperm flagella. Cell Motil Cytoskeleton. 2006;63:582–590. doi: 10.1002/cm.20147. [DOI] [PubMed] [Google Scholar]
- 8.Lindemann CB, Goltz JS, Kanous KS. Regulation of activation state and flagellar wave form in epididymal rat sperm: evidence for the involvement of both Ca2+ and cAMP. Cell Motil Cytoskeleton. 1987;8:324–332. doi: 10.1002/cm.970080405. [DOI] [PubMed] [Google Scholar]
- 9.Monterisi S, Favia M, Guerra L, Cardone RA, Marzulli D, Reshkin SJ, Casavola V, Zaccolo M. CFTR regulation in human airway epithelial cells requires integrity of the actin cytoskeleton and compartmentalized cAMP and PKA activity. J Cell Sci. 2012;125:1106–1117. doi: 10.1242/jcs.089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nlend MC, Schmid A, Sutto Z, Ransford GA, Conner GE, Fregien N, Salathe M. Calcium-mediated, purinergic stimulation and polarized localization of calcium-sensitive adenylyl cyclase isoforms in human airway epithelia. FEBS Lett. 2007;581:3241–3246. doi: 10.1016/j.febslet.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng W, Wang Z, Zhang J, Reed BY, Pak CY, Moe OW. Cloning and characterization of the human soluble adenylyl cyclase. Am J Physiol Cell Physiol. 2005;288:C1305–C1316. doi: 10.1152/ajpcell.00584.2004. [DOI] [PubMed] [Google Scholar]
- 13.Jaiswal BS, Conti M. Identification and functional analysis of splice variants of the germ cell soluble adenylyl cyclase. J Biol Chem. 2001;276:31698–31708. doi: 10.1074/jbc.M011698200. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 15.Farrell J, Ramos L, Tresguerres M, Kamenetsky M, Levin LR, Buck J. Somatic ‘soluble’ adenylyl cyclase isoforms are unaffected in Sacy tm1Lex/Sacy tm1Lex ‘knockout’ mice. PLoS ONE. 2008;3:e3251. doi: 10.1371/journal.pone.0003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, van Duin M, Conti M, et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect Proc Natl Acad Sci USA 20041012993–2998.[Published erratum appears in Proc Natl Acad Sci USA 101:5180.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Martinez J, Milner TA, Buck J, Levin LR. Neuronal expression of soluble adenylyl cyclase in the mammalian brain. Brain Res. 2013;1518:1–8. doi: 10.1016/j.brainres.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You Y, Brody SL. Culture and differentiation of mouse tracheal epithelial cells. Methods Mol Biol. 2013;945:123–143. doi: 10.1007/978-1-62703-125-7_9. [DOI] [PubMed] [Google Scholar]
- 19.Horani A, Nath A, Wasserman MG, Huang T, Brody SL. Rho-associated protein kinase inhibition enhances airway epithelial basal-cell proliferation and lentivirus transduction. Am J Respir Cell Mol Biol. 2013;49:341–347. doi: 10.1165/rcmb.2013-0046TE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003;17:82–84. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- 21.Bitterman JL, Ramos-Espiritu L, Diaz A, Levin LR, Buck J. Pharmacological distinction between soluble and transmembrane adenylyl cyclases. J Pharmacol Exp Ther. 2013;347:589–598. doi: 10.1124/jpet.113.208496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuyumcu-Martinez NM, Cooper TA. Misregulation of alternative splicing causes pathogenesis in myotonic dystrophy. Prog Mol Subcell Biol. 2006;44:133–159. doi: 10.1007/978-3-540-34449-0_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lomovatskaya LA, Romanenko AS, Filinova NV, Salyaev RK. Detection of soluble adenylyl cyclase isoforms in plants. Dokl Biochem Biophys. 2008;420:124–126. doi: 10.1134/s1607672908030071. [DOI] [PubMed] [Google Scholar]
- 24.Steegborn C, Litvin TN, Levin LR, Buck J, Wu H. Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat Struct Mol Biol. 2005;12:32–37. doi: 10.1038/nsmb880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTPgammaS. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. (see comments) [DOI] [PubMed] [Google Scholar]
- 26.Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, Steegborn C. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol. 2006;362:623–639. doi: 10.1016/j.jmb.2006.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linder JU, Schultz JE. The class III adenylyl cyclases: multi-purpose signalling modules. Cell Signal. 2003;15:1081–1089. doi: 10.1016/s0898-6568(03)00130-x. [DOI] [PubMed] [Google Scholar]
- 28.Katsushika S, Chen L, Kawabe J, Nilakantan R, Halnon NJ, Homcy CJ, Ishikawa Y. Cloning and characterization of a sixth adenylyl cyclase isoform: types V and VI constitute a subgroup within the mammalian adenylyl cyclase family. Proc Natl Acad Sci USA. 1992;89:8774–8778. doi: 10.1073/pnas.89.18.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam BM, Moritz OL, Hurd LB, Papermaster DS. Identification of an outer segment targeting signal in the COOH terminus of rhodopsin using transgenic Xenopus laevis. J Cell Biol. 2000;151:1369–1380. doi: 10.1083/jcb.151.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Follit JA, Li L, Vucica Y, Pazour GJ. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J Cell Biol. 2010;188:21–28. doi: 10.1083/jcb.200910096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci. 2006;119:1383–1395. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- 32.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]