Abstract
Macrophage elastase (MMP12) is a key mediator of cigarette smoke (CS)-induced emphysema, yet its role in other smoking related pathologies remains unclear. The weight suppressing effects of smoking are a major hindrance to cessation efforts, and MMP12 is known to suppress the vascularization on which adipose tissue growth depends by catalyzing the formation of antiangiogenic peptides endostatin and angiostatin. The goal of this study was to determine the role of MMP12 in adipose tissue growth and smoking-related suppression of weight gain. Whole body weights and white adipose depots from wild-type and Mmp12-deficient mice were collected during early postnatal development and after chronic CS exposure. Adipose tissue specimens were analyzed for angiogenic and adipocytic markers and for content of the antiangiogenic peptides endostatin and angiostatin. Cultured 3T3-L1 adipocytes were treated with adipose tissue homogenate to examine its effects on vascular endothelial growth factor (VEGF) expression and secretion. MMP12 content and activity were increased in the adipose tissue of wild-type mice at 2 weeks of age, leading to elevated endostatin production, inhibition of VEGF secretion, and decreased adipose tissue vascularity. By 8 weeks of age, adipose MMP12 levels subsided, and the protein was no longer detectable. However, chronic CS exposure led to macrophage accumulation and restored adipose MMP12 activity, thereby suppressing adipose tissue mass and vascularity. Our results reveal a novel systemic role for MMP12 in postnatal adipose tissue expansion and smoking-associated weight loss by suppressing vascularity within the white adipose tissue depots.
Keywords: proteases, obesity, angiogenesis
Clinical Relevance
This study demonstrates a novel role for macrophage elastase (MMP12) in the inhibition of white adipose tissue vascularization. We show that during early postnatal growth, and again during cigarette smoke exposure, MMP12 generates endostatin from adipose tissue Collagen XVIII to suppress adipose tissue growth and vascularity.
Despite overwhelming evidence linking tobacco use to inflammatory and neoplastic diseases, cigarette smoking remains a tempting habit for many, particularly young women, due to its weight control capacity (1–3). Nicotine, the major active ingredient in tobacco smoke, is known to reduce appetite (4, 5), but decreased food intake fails to fully explain the weight loss associated with smoking (6, 7). Indeed, evidence of increased energy expenditure in humans after nicotine administration begins to fill this knowledge gap (8). However, the major impetus for smoking as a means of weight control may lie in its effects on energy storage, and these mechanisms remain unknown.
Many smokers develop chronic obstructive pulmonary disease (COPD), which is characterized by inflammation with release of serine and matrix metalloproteinases (MMPs) (9). One of these proteases, macrophage elastase (MMP12), is among the most highly up-regulated genes in response to smoking, and mice deficient in MMP12 are completely protected from the development of cigarette smoke (CS)-induced emphysema (10, 11). In the 15 years since we developed Mmp12 knockout mice (Mmp12−/−) for the study of COPD, we have noted that these mice are “fatter” than their wild-type (WT) littermates (unpublished data). MMPs are known to play important roles in adipose tissue development, and numerous MMPs are expressed by mature adipocytes and by the surrounding stromal-vascular cells (12, 13). Certain MMPs may promote adipose tissue growth; mice administered the broad-spectrum MMP inhibitor Galardin are mildly protected from obesity after high-fat feeding (14). Part of this effect is attributed to the abilities of Gelatinases A and B (MMP2 and MMP9, respectively) to promote angiogenesis, which is tightly coupled to adipogenesis (15–18). In contrast, we and others have shown that MMP12 inhibits angiogenesis in the tumor microenvironment via cleavage of plasmin(ogen) and type XVIII collagen to the antiangiogenic peptides angiostatin and endostatin, respectively (19–21). Taken together, these findings indicate that it is possible that a similar role for MMP12 in fat tissue would impede adipogenesis.
Cigarette smoking provokes a characteristic inflammatory state in human and mouse lungs with marked up-regulation of MMP12 in alveolar macrophages (10). Furthermore, smoking results in a systemic inflammatory state that may persist well beyond cessation (22). However, the association of systemic inflammation with weight suppression would appear to be at odds with our understanding of the metabolic syndrome whereby chronic macrophage inflammation induces weight gain and insulin intolerance (23–25). Therefore, smoking must yield a different brand of generalized inflammation, and systemic up-regulation of MMP12 by smoking, as occurs in the lungs, would help to explain this difference.
We proposed that MMP12 is prominent in white adipose tissue during early development, “putting the brakes on” adipose tissue growth and vascularization, and in its absence there would be weight gain. In adulthood, cigarette smoking would cause systemic inflammation with activation of macrophage MMP12 production. On entry in white adipose tissue, this would similarly inhibit adipose tissue expansion, leading to a relative reduction in body weight. We present data confirming this hypothesis and demonstrating that MMP12 generation of endostatin from type XVIII collagen is the basis for the decrease in adipose tissue growth and vascularization.
Materials and Methods
Mice
MMP12-deficient (Mmp12−/−) mice on a C57BL/6J background were generated as described (26). Age- and sex-matched WT C57BL/6J mice served as controls for all experiments. Mice were housed in ventilated Plexiglas cages (one to four animals per cage) within a pathogen-free barrier facility that maintained a 12-hour light/dark cycle. Mice had free access to autoclaved water and irradiated pellet food that derived 5% of calories from lipids. All mice were housed in accordance with guidelines from the American Association for Laboratory Animal Care and Research Protocols, and experiments were approved by the Animal Care and Use Committees of the University of Pittsburgh School of Medicine.
Isolation and Incubation of Adipocytes from White Adipose Tissue
Adipose tissue was isolated immediately after CO2 asphyxiation, placed in HEPES-buffered Dulbecco’s modified Eagle medium (DMEM) (Invitrogen Corp., Grand Island, NY) with 10 μg/ml fatty acid–poor BSA (Sigma-Aldrich, St. Louis, MO), and centrifuged at 1,000 × g for 10 minutes to pellet blood cells. Type 1 collagenase (1 μg/ml) (Sigma-Aldrich) was added to the tissue suspension and incubated at 37°C for 60 minutes. Samples were passed through a sterile, 250-μm nylon mesh, and the suspension was centrifuged at 300 × g for 10 minutes. Floating cells were collected as the adipocyte fraction, washed twice with DMEM, aliquoted into equal cell volumes (106 cells/ml), and incubated in DMEM plus 0.1% FBS with or without recombinant mouse endostatin (10 μg /ml) (Calbiochem, Billerica, MA) or recombinant mouse MMP12 (2 μg/ml) (R&D Systems Inc., Minneapolis, MN) for 24 hours. Culture medium was carefully collected below the floating adipocyte layer, with care taken not to disrupt the cells, and stored at –80°C.
3T3-L1 Cells Culture and Coincubation with White Adipose Tissue Homogenates
3T3-L1 preadipocytes were obtained from Zen-Bio, Inc. (Durham, NC). Cells were incubated for 2 days in PM-1-L1 (Zen-Bio), moved to DM-2-L1 (Zen-Bio), and incubated for 3 days. Differentiated adipocytes were maintained in AM-1-L1 (Zen-Bio). After differentiation, 3T3-L1 adipocytes were incubated in DMEM plus 0.1% FBS with or without 10 μg extracted protein from white adipose tissue homogenate with or without treatment of endostatin antibody (10 μg/ml) (Novus Biologicals, Littleton, CO), angiostatin antibody (10 μg/ml) (Novus Biologicals), and recombinant mouse MMP12 (2 μg /ml) (R&D Systems Inc.) for 24 hours. After incubation, the culture medium was collected. Samples were normalized for total protein content. Samples were stored at –80°C for ELISA or Western blot.
Statistical Analyses
Data were expressed as mean ± SEM. Differences between WT or MMP12−/− mice were compared with values from age-matched mice by Student’s t test. Comparisons within a single strain (WT or MMP12−/−) at different smoking statuses were made by Student’s t test. Differences in vascular endothelial growth factor (VEGF) secretion between greater than two samples were evaluated by one- or two-way ANOVA. Comparisons between time points were analyzed using repeated-measures ANOVA. Correlation coefficients (r) were determined using the Pearson product-moment method. A P value of < 0.05 was considered statistically significant.
Results
Mmp12−/− Mice Have Increased Body Weight Compared with WT Mice
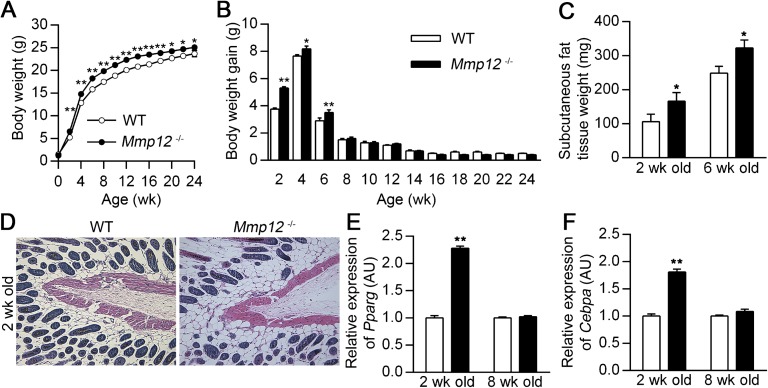
On the basis of gross observations that Mmp12−/− mice appeared larger than their WT littermates, we measured body weights on these mice at 2-week intervals from birth to 6 months of age. The weights of Mmp12−/− mice were significantly increased compared with WT mice beginning at 2 weeks and sustained through the first 24 weeks of life (Figure 1A). However, differences in weight gain were limited to the first 6 weeks of life (Figure 1B). All mice were fed a standard chow diet beginning 18 days after birth, and food intake was equivalent between the two groups (see Figure E1A in the online supplement). Litter numbers were similar between WT and Mmp12−/− mice (4.67 ± 0.6 versus 5.08 ± 0.56; P = nonsignificant [n = 6]), suggesting that access to food or breast milk did not account for the observed changes in body weight. Because the differences in weight gain were limited to the postnatal period, we evaluated the contribution of maternal breast milk. Mmp12−/− and WT pups were nursed by surrogate Swiss-Webster mothers so the quality of breast milk would be equivalent in the two groups. Even after adoptive nursing, postnatal weight gain in Mmp12−/− mice was increased compared with WT mice (Figures E1B and E1C).
Figure 1.
Mmp12−/− mice have increased adipose tissue. Body weight (A) and 2-week weight gain intervals (B) were recorded in wild-type (WT) and Mmp12−/− mice from 0 to 24 weeks of age (n = 10–25 mice/group). WT and Mmp12−/− mice were killed at 2 and 6 weeks of age, and subcutaneous adipose tissue was harvested. Analysis of WT and Mmp12−/− mice included weight of subcutaneous adipose tissue at 2 and 6 weeks of age (n = 5–8 mice/group) (C), representative histology by hematoxylin and eosin staining of subcutaneous tissue at 2 weeks of age (D), and Pparg (E) and Cebpa (F) mRNA expression measured by real-time RT-PCR (n = 4 mice/group). Data are mean ± SEM. *P < 0.05 and **P < 0.005 versus respective WT values.
Mmp12−/− Mice Have Increased Adipose Tissue Compared with WT Mice
Mmp12−/− displayed an expansion of adipose tissue on gross inspection compared with WT controls. Dissection and measurement of each compartment of adipose tissue confirmed an increase in adipose tissue weight in Mmp12−/− mice (Table 1). At 2 and 6 weeks of age, subcutaneous fat tissue weight was significantly increased in Mmp12−/− mice compared with WT mice (Figures 1C and 1D). Real-time PCR demonstrated that mRNA expression of Pparg and Cebpa, master regulators of adipogenesis (27), was increased in the subcutaneous fat tissue of Mmp12−/− mice at 2 weeks of age but not at 8 weeks of age (Figures 1E and 1F). Thus, adipose tissue expansion in white adipose tissue was increased in Mmp12−/− mice compared with WT mice and contributes to the observed body weight differences during early life.
Table 1.
Organ Weights in Wild-Type and Mmp12−/− Mice
| Genotype | Age (wk) | Subcutaneous Fat (mg) | Perigonadal Fat (mg) | Liver (mg) | Spleen (mg) | Kidney (mg) | Heart (mg) | Gastrocnemius Muscle (mg) |
|---|---|---|---|---|---|---|---|---|
| WT | 2 | 106.2 ± 21.9* | 5.6 ± 1.1 | 216.6 ± 11.2 | 31.2 ± 2.9 | 106.1 ± 9.4 | 56.5 ± 8.9 | 30.5 ± 3.5 |
| Mmp12−/− | 2 | 166.5 ± 25.3† | 8.4 ± 1.0† | 230.0 ± 17.6 | 30.8 ± 2.4 | 108.4 ± 7.3 | 60.7 ± 7.4 | 31.6 ± 2.7 |
| WT | 6 | 249.0 ± 20.3 | 101.8 ± 10.4 | 902.5 ± 21.2 | 66.0 ± 6.7 | 271.1 ± 12.4 | 103.8 ± 5.1 | 62.6 ± 6.5 |
| Mmp12−/− | 6 | 323.3 ± 22.8† | 148.0 ± 16.7† | 974.0 ± 76.9 | 70.8 ± 3.3 | 269.4 ± 14.3 | 113.7 ± 2.6 | 65.8 ± 7.4 |
Definition of abbreviation: WT, wild type.
Values are mean ± SEM.
P < 0.05 versus age-matched WT mice.
Organs were harvested from wild-type and Mmp12−/− mice and weighed at 2 and 6 wk of age.
Mmp12−/− Mice Have Increased Vascularity in White Adipose Tissue Compared with WT Mice
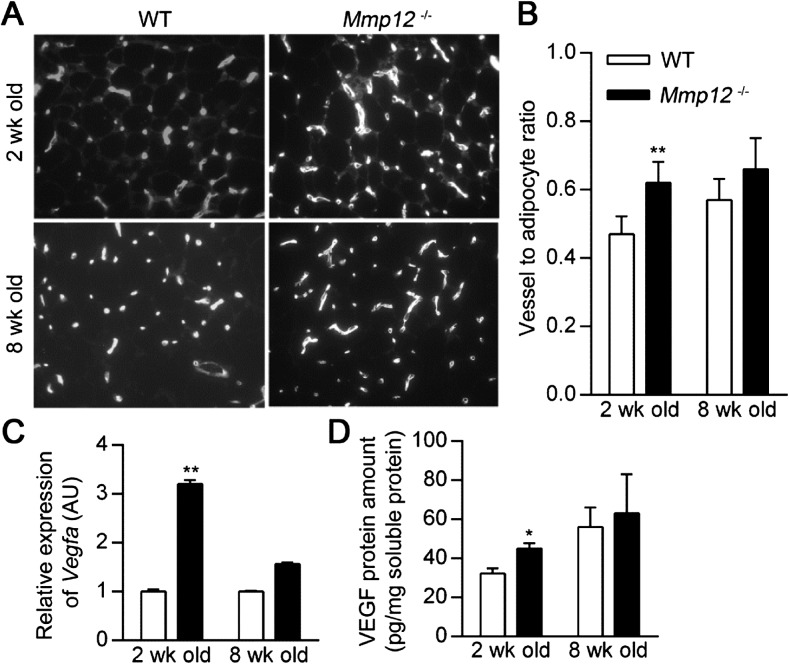
We have previously shown that MMP12 inhibits angiogenesis via the generation of angiostatic peptides from matrix components (19, 20). Because the vascular supply contributes to adipose tissue growth from prenatal life throughout adulthood (17), we examined whether MMP12 presence (WT) or absence (Mmp12−/−) influenced adipose tissue vascularity. Subcutaneous fat tissue vascularity was assessed by lectin staining at 2 and 8 weeks of age (Figure 2A). Vascularization was increased in Mmp12−/− mice compared with WT mice at 2 weeks of age but not at 8 weeks of age (Figure 2B). Likewise, Vegfa mRNA expression and VEGF protein levels in subcutaneous fat tissue were increased in Mmp12−/− mice compared with WT mice at 2 weeks but not at 8 weeks of age (Figures 2C–2D).
Figure 2.
Subcutaneous adipose tissue of WT mice has decreased vascularization. WT and Mmp12−/− mice were killed at 2 and 8 weeks of age, and the subcutaneous adipose tissue was harvested for analysis. (A) Representative blood vessel histology by lectin staining. (B) Vessel-to-adipocyte ratio was calculated by dividing the number of vessels by the number of adipocytes per measured surface area (n = 4–6 mice/group). (C) Relative expression of Vegfa mRNA was measured by real-time RT-PCR. (D) Vascular endothelial growth factor (VEGF) protein levels were measured by quantitative ELISA and normalized to total soluble protein concentration. Data are mean ± SEM. *P < 0.05 and **P < 0.005 versus respective WT values.
MMP12 Content and Activity Is Increased in White Adipose Tissue during Early Growth
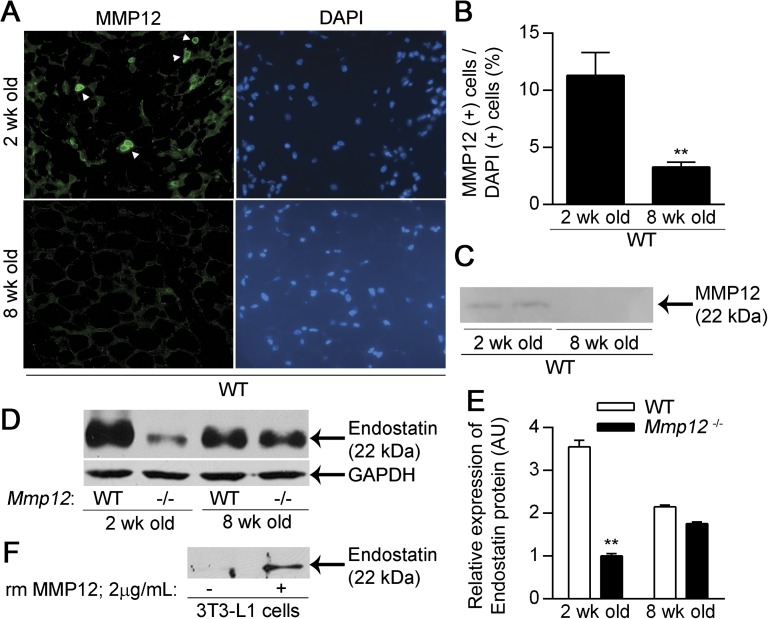
Consistent with a prior report (28), MMP12 expression and activity in the subcutaneous fat tissue of WT mice was not detectable at 8 weeks of age (Figures 3A–3C). However, MMP12 content and the percentage of MMP12-positive cells in subcutaneous fat tissue were increased at 2 weeks of age. We speculated that increased MMP12 activity in subcutaneous fat tissue could produce a relative angiostatic microenviroment via the generation of antiangiogenic peptides, as described for the tumor microenvironment (20). We found increased endostatin levels in subcutaneous fat tissue in WT mice compared with Mmp12−/− mice at 2 weeks of age but not at 8 weeks of age (Figures 3D–3E). MMP12 has been reported to cleave collagen XVIII into endostatin (21), and we confirmed that recombinant mouse MMP12 generates endostatin from collagen XVIII in 3T3-L1 cells (Figure 3F). Moreover, endostatin protein levels in the subcutaneous fat tissue of Mmp12−/− mice at 8 weeks of age are greater than those at 2 weeks of age. This is likely secondary to compensation by other MMPs (MMP3, -7, -9, or -13), which also can produce endostatin from collagen XVIII (21).
Figure 3.
MMP12 and endostatin are increased in the white adipose tissue of 2–week-old mice. WT mice were killed at 2 and 8 weeks of age, and the subcutaneous adipose tissue was analyzed for MMP12 expression (n = 4 mice/group). (A) Representative staining for MMP12. (B) The number of MMP12-expressing cells as a percentage of DAPI+ cells. (C) Representative Western blot for MMP12 protein expression in 60 μg of protein from WT and Mmp12−/− subcutaneous adipose. Endostatin levels were analyzed in the subcutaneous adipose tissue of 2- and 8-week-old WT and Mmp12−/− mice (n = 3 mice/group). (D) Representative Western blot for endostatin protein and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) endogenous control. (E) Endostatin levels were quantified by densitometry. (F) Representative Western blot demonstrating cleavage of Collagen XVIII to endostatin in 3T3-L1 adipocytes treated with recombinant murine (rm) MMP12. Data are mean ± SEM. **P < 0.005 versus 8-week-old mice from respective strain.
MMP12-Dependent Endostatin Production Modulates Adipose Tissue VEGF Secretion
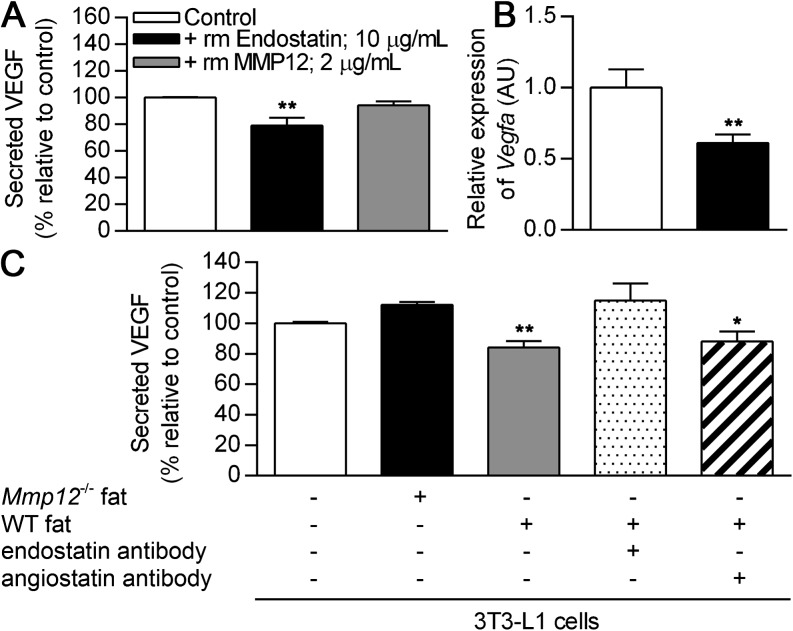
Endostatin has been shown to inhibit angiogenesis by decreasing VEGF transcription and hence endothelial cell proliferation in vascular cells (29, 30). Adipocytes are a major source of VEGF production in white adipose tissue (17). Therefore, it is plausible that the increased endostatin production in white adipose tissue of WT compared with Mmp12−/− mice could inhibit angiogenesis via decreased VEGF secretion. To exclude direct activity of MMP12 on adipocytes, we incubated isolated adipocytes with recombinant mouse MMP12 and observed no alteration in VEGF protein secretion (Figure 4A). In contrast, recombinant mouse endostatin significantly decreased Vegfa mRNA expression (Figure 4B) and decreased VEGF protein secretion (Figure 4A) from isolated adipocytes.
Figure 4.
Endostatin inhibits adipocyte secretion of VEGF and is responsible for decreased VEGF production in WT as compared with Mmp12−/− adipose tissue. Adipocytes were isolated from WT mice at 8 weeks of age and grown in culture. (A) VEGF secretion was quantified by ELISA in the presence of recombinant endostatin or MMP12 and expressed relative to untreated controls (n = 6 mice/group). (B) Vegfa mRNA expression was assessed by real-time RT-PCR. 3T3-L1 adipocytes were treated with subcutaneous adipose tissue homogenates from 2-week-old WT and Mmp12−/− mice (n = 3 mice/group). (C) VEGF secretion as quantified by ELISA in the presence or absence of function-blocking antibodies to endostatin or angiostatin (n = 3 mice/group). VEGF levels are expressed relative to untreated controls. Data are mean ± SEM. *P < 0.05 and **P < 0.005 versus respective untreated controls.
We applied a function blocking endostatin antibody to determine if endostatin production accounted for the inhibition of VEGF secretion in WT as opposed to Mmp12−/− mice. 3T3-L1 adipocytes were coincubated with subcutaneous fat tissue homogenates from 2-week-old WT and Mmp12−/− mice. Analysis of the culture supernatants demonstrated a decrease in VEGF induction by WT mouse fat homogenate. This decrease was abolished by the addition of endostatin antibody (Figure 4C). In contrast, the addition of an angiostatin blocking antibody had no abrogation of the inhibition by fat tissue supernatants. We were unable to demonstrate a role for anigiostatin on VEGF expression in white adipose tissue–like vascular cells, as previously reported (30). Thus, MMP12-dependent generation of endostatin is a key regulator of adipose tissue vascularity in mice.
MMP12 Is Required for CS-Induced Reduction in Adipose Tissue Content
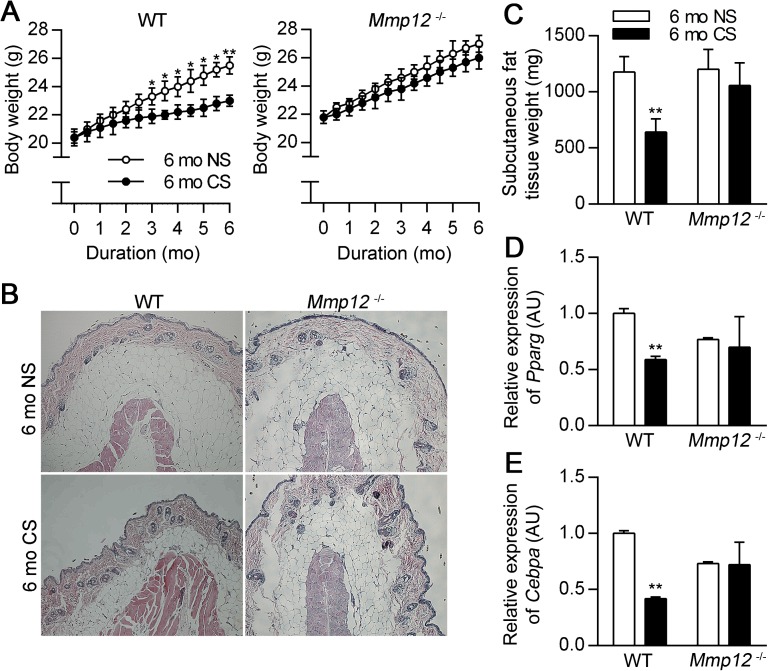
We have previously reported that CS-induced emphysema in mice is dependent on macrophage accumulation and production of MMP12 (11). Because cigarette smoking leads to “fat wasting” (31), we wondered whether a similar mechanism applied to limit adipose deposition as occurs in early postnatal life. To test this concept, we exposed Mmp12−/− and WT mice on a standard chow diet to the smoke of four cigarettes, 5 days per week, for 6 months. CS exposure induced emphysema in the lungs of WT but not Mmp12−/− mice (Figure E2), as has been previously reported (11). CS exposure also led to decreased body weight and perigonadal and subcutaneous fat tissue weight in WT mice, consistent with previous reports (7, 32), but not in Mmp12−/− mice (Figures 5A–5C; Figure E3A). Moreover, CS exposure decreased mRNA expression of Pparg and Cebpa in subcutaneous (Figures 5D–5E) and perigonadal fat tissue (Figures E3B–E3C) in WT mice but not in Mmp12−/− mice, suggesting that CS-induced weight loss is an MMP12 dependent process in mice.
Figure 5.
MMP12 is required for suppression of weight gain in response to cigarette smoke (CS) exposure. (A) Body weights were recorded biweekly for 6 months in WT and Mmp12−/− mice exposed to room air (NS) or CS (n = 6–11 mice/group). Mice were killed after 6 months of exposure, and subcutaneous and perigonadal adipose tissues were harvested. (B) Representative subcutaneous adipose tissue histology in WT mice in the absence and presence of CS exposure by hematoxylin and eosin staining. (C) Subcutaneous adipose tissue weights in WT and Mmp12−/− mice in the presence and absence of CS exposure (n = 5–7 mice/group). Relative expression of Pparg (D) and Cebpa mRNA (E) in subcutaneous adipose tissue as measured by real-time RT-PCR (n = 4 mice/group). Data are mean ± SEM. *P < 0.05 and **P < 0.005 versus respective NS controls.
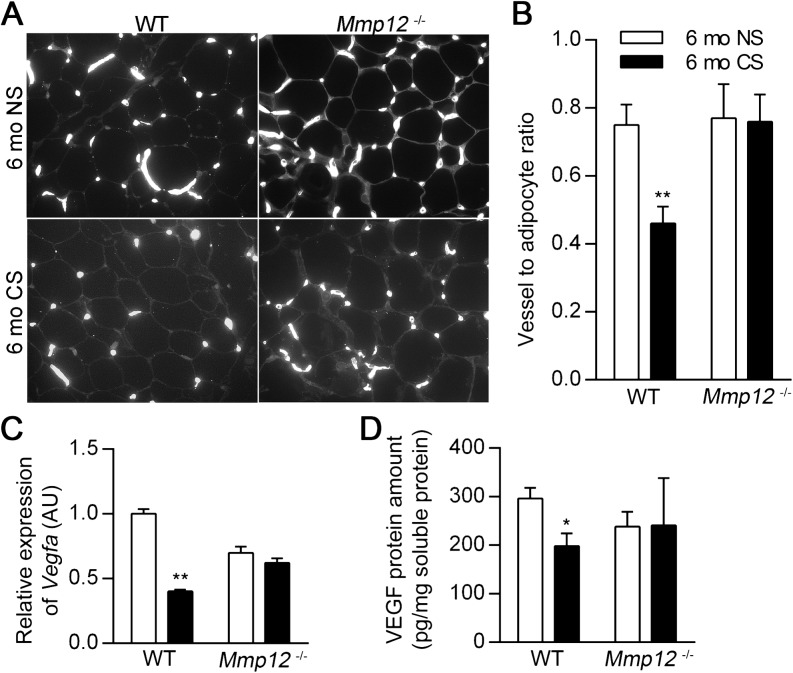
CS Exposure Decreases Adipose Tissue Growth and Vascularity
Chronic CS exposure (6 mo CS) decreased daily food intake during the first month, consistent with previous reports (7, 32), but did not decrease food intake thereafter when compared with nonsmoking (6 mo NS) controls (Figure E4). Similar to the findings in the neonatal period, the presence of MMP12 was associated with decreased vessel density after 6 months of CS exposure. Vascularization in subcutaneous (Figures 6A–6B) and perigonadal fat tissue (Figures E5A–E5B) was decreased in 6 mo CS WT mice compared with 6 mo NS WT mice, but there was no effect of smoke exposure on Mmp12−/− mice. VEGFA mRNA and protein levels in subcutaneous (Figures 6C–6D) and perigonadal fat (Figures E5C–E5D) were also decreased in 6 mo CS WT mice compared with 6 mo NS WT mice but were not decreased in 6 mo CS Mmp12−/− mice compared with 6 mo NS Mmp12−/− mice.
Figure 6.
WT mice have decreased adipose vasculature after 6 months of CS exposure. Subcutaneous adipose tissue was harvested from WT and Mmp12−/− mice after 6 months of exposure to NS or CS. Representative blood vessel histology by lectin staining (A) and vessel-to-adipocyte ratios in subcutaneous adipose tissue (B) (n = 5 mice/group). Vegfa mRNA (C) and VEGF protein (D) were quantified by real-time RT-PCR and ELISA, respectively, in subcutaneous adipose tissue (n = 4–7 mice/group). VEGF ELISA values were normalized to total soluble protein concentration. Data are mean ± SEM. *P < 0.05 and **P < 0.005 versus respective NS controls.
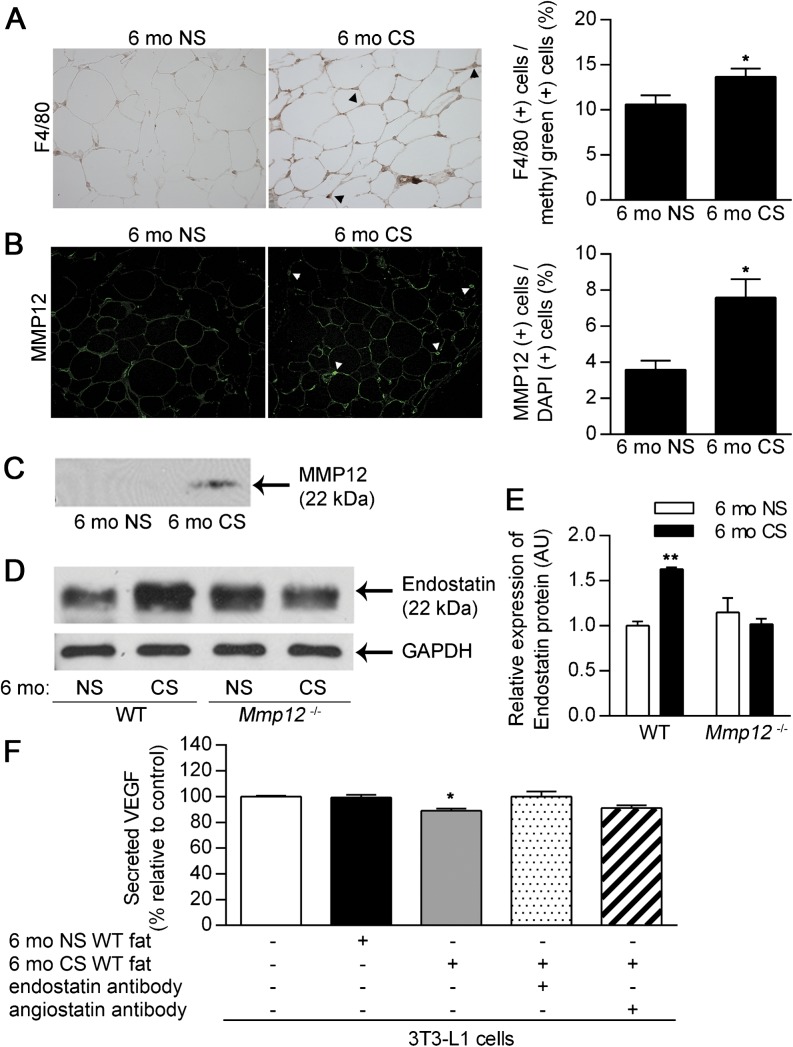
CS Exposure Increases Macrophage Accumulation, MMP12, and Endostatin Production in White Adipose Tissue
Because CS exposure decreased adipogenesis and vascularity in white adipose tissue in WT but not Mmp12−/− mice, we speculated that CS exposure would increase MMP12 activity in white adipose tissue as in the lung. CS exposure increased macrophage infiltration (F4/80 positive cell) in subcutaneous (Figure 7A) and perigonadal (Figure E6A) fat tissue in WT mice. We confirmed CS-induced increases in the F4/80-positive cell population in the stromal vascular fraction of perigonadal and subcutaneous fat tissue in WT mice by flow cytometry (Figure E7A). CS-induced macrophage infiltrates did not coexpress a high percentage of CD11c (Figures E7B and E7C), as reported in obesity models (33). As a result of increased macrophage infiltration, the expression and activity of MMP12 in subcutaneous (Figures 7B and 7C) and perigonadal (Figures E6B and E6C) fat tissue were increased in CS-exposed WT mice compared with NS controls. The CS-induced increase in MMP12 activity resulted in increased production of endostatin in subcutaneous (Figures 7D and 7E) and perigonadal (Figures E6D and E6E) fat tissue. Endostatin content in CS-exposed Mmp12−/− mice was comparable with NS control (Figures 7D and 7E). VEGF protein levels in the culture medium of 3T3-L1 adipocytes coincubated with subcutaneous (Figure 7F) and perigonadal (Figure E6F) fat tissue homogenates of 6 mo CS WT mice were significantly decreased compared with those of 6 mo NS WT mice. This decrease was abrogated by the addition of endostatin antibody (Figure 7F and Figure E6F). Thus, CS-induced MMP12 production in adipose tissue decreases vessel density via endostatin production and VEGF suppression.
Figure 7.
CS exposure enhances adipose tissue macrophage infiltration and MMP12 expression. Subcutaneous adipose was harvested from WT and Mmp12−/− mice after 6 months of exposure to NS or CS. Adipose tissue from WT mice was analyzed for macrophage infiltration and MMP12 expression (n = 5 mice/group). Representative staining for F4/80+ macrophages (A) and MMP12+ cells (B). The percentages of F4/80+ and MMP12+ cells were calculated relative to methyl green+ and DAPI+ cells, respectively. (C) Representative Western blot for MMP12 in WT mice (80 μg protein/well). Adipose tissue from WT and Mmp12−/− mice was assessed for endostatin protein in the absence or presence of CS exposure. (D) Representative Western blots for endostatin with densitometric quantification in subcutaneous adipose tissue (E). 3T3-L1 adipocytes were cocultured with subcutaneous adipose tissue homogenates from WT mice exposed to 6-months of NS or CS (n = 3 mice/group). (F) VEGF secretion was quantified by ELISA in the presence or absence of function-blocking antibodies to endostatin or angiostatin and expressed relative to untreated controls. Data are mean ± SEM. *P < 0.05 and **P < 0.005 versus respective NS or untreated controls.
Discussion
This study demonstrates that MMP12 limits weight gain during the period of peak mouse growth and development by limiting white adipose tissue expansion. MMP12, highly expressed in adipose tissue during postnatal development, cleaves type XVIII collagen, also abundant in adipose tissue, to endostatin, which in turn inhibits VEGF production and tissue vascularity. Quiescent in normal adult tissue, MMP12 is reactivated by CS and reinitiates this process, leading to inhibition of adipose tissue growth and vessel density in CS-exposed female mice.
Adipogenesis is highly dependent on the concurrent growth of a supportive vascular network during pre- and postnatal development (17, 34), and VEGF, principally from adipocytes, is the main mediator of angiogenesis in white adipose tissue (16, 17, 35). Type XVIII collagen is abundantly expressed in white adipose tissue, and its C-terminal cleavage product, endostatin, is a potent inhibitor of angiogenesis (29, 36). Subcutaneous administration of endostatin in mice is associated with markedly reduced body weight without affecting food intake (18), and our finding that endostatin inhibits adipocyte expression and secretion of VEGF begins to link reduced weight to stunted angiogenesis.
Macrophage elastase (MMP12) has been shown to block angiogenesis by cleaving plasmin(ogen) and type XVIII collagen to generate the bioactive peptides angiostatin and endostatin, respectively (19, 21). Mmp12 is up-regulated during in vitro adipogenesis (13), and this study elucidates a role in limiting adipocyte expansion by increasing endostatin levels and inhibiting adipose tissue vascularity. MMP12 can also activate MMP3, which can in turn produce endostatin from type XVIII collagen (21, 29). The body weights of MMP3-deficient mice are also increased compared with WT (12). Furthermore, it is possible that MMP12 deficiency alters additional metabolic parameters (e.g., basal metabolic rate and core body temperature), that were not addressed in the current study.
A recent study (37) found that deletion of MMP12 does not affect expansion of murine adipose tissue during early adulthood, in agreement with our finding that MMP12 activity is limited to the early postnatal period. However, long-term CS exposure restored MMP12 expression and adipogenesis inhibition, recapitulating the antiangiogenic milieu observed early in life. We demonstrate that chronic CS exposure leads to white adipose tissue infiltration by MMP12-expressing macrophages and decreased adipogenesis. We also observed no vascular alterations in the adipose tissue MMP12-deficient mice exposed to chronic CS, which may be a direct consequence of reduced Collagen XVIII to endostatin proteolysis or an indirect failure of MMP12-deficient macrophages to penetrate adipose depot basement membranes (26).
In the context of our findings, it is interesting to note that Lumeng and colleagues (33) identified a population of F4/80+ CD11c+ adipose tissue macrophages that were associated with obesity and insulin resistance (24). We observed increased numbers of F4/80+ CD11c− macrophages in the adipose of CS-exposed mice, which inhibited adipogenesis. Smoking has been shown to produce a unique activation state in alveolar macrophages that is characterized by elevated MMP12 (10), and it is possible that systemic changes due to smoking yield a similar phenotype in adipose tissue macrophages.
Cigarette smoking has long been associated with weight loss. Smoking as a means of weight control is particularly notable among young female patients (1–3), and our current study examined the role of MMP12 in adipogenesis in female C57BL/6J mice only. Our results address the lack of weight gain during smoke exposure versus after smoking cessation, although it is plausible that both effects contribute to the persistence of smoking as a weight control tool. Male C57BL/6J mice are less susceptible to smoking-induced pulmonary emphysema (data not shown), and we observed that they are also less sensitive to smoking-induced suppression of adipose tissue weight after 6 months of exposure (13 and 17% decreases in smoking versus nonsmoking perigonadal and subcutaneous fat, respectively). Hence, it is possible that gender differences in smoking-related weight suppression can help to explain the corresponding reasons for continued smoking.
In conclusion, MMP12 serves as an important modulator of adiposity during development and in conjunction with cigarette smoking. MMP12 cleaves type XVIII collagen in the white adipose tissue to generate endostatin, which decreases VEGF levels, inhibits adipose tissue vascularity, and blocks adipogenesis. These results link the pathogenesis of alveolar destruction and decreased fat mass in emphysema to a common protein, MMP12, while improving our understanding of the extrapulmonary manifestations of cigarette smoking.
Footnotes
This work was supported by National Institutes of Health, National Heart, Lung and Blood Institute grant 5P01 HL103455-02 (S.D.S.) and by the Flight Attendant Medical Research Institute (S.D.S.).
Author Contributions: T.T. designed and performed the research, analyzed the data, and wrote the manuscript. N.J.K., S.T., and A.S.L. performed the research, analyzed the data, and corrected the manuscript. A.M.H. and S.D.S. designed the research, analyzed the data, and corrected the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2014-0083OC on June 10, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fulkerson JA, French SA. Cigarette smoking for weight loss or control among adolescents: gender and racial/ethnic differences. J Adolesc Health. 2003;32:306–313. doi: 10.1016/s1054-139x(02)00566-9. [DOI] [PubMed] [Google Scholar]
- 2.Camp DE, Klesges RC, Relyea G. The relationship between body weight concerns and adolescent smoking. Health Psychol. 1993;12:24–32. doi: 10.1037//0278-6133.12.1.24. [DOI] [PubMed] [Google Scholar]
- 3.Akbartabartoori M, Lean ME, Hankey CR. Relationships between cigarette smoking, body size and body shape. Int J Obes (Lond) 2005;29:236–243. doi: 10.1038/sj.ijo.0802827. [DOI] [PubMed] [Google Scholar]
- 4.Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gundisch D, Diano S, De Biasi M, Horvath TL, Gao XB, et al. Nicotine decreases food intake through activation of pomc neurons. Science. 2011;332:1330–1332. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wager-Srdar SA, Levine AS, Morley JE, Hoidal JR, Niewoehner DE. Effects of cigarette smoke and nicotine on feeding and energy. Physiol Behav. 1984;32:389–395. doi: 10.1016/0031-9384(84)90252-x. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Hansen MJ, Jones JE, Vlahos R, Anderson GP, Morris MJ. Long-term cigarette smoke exposure increases uncoupling protein expression but reduces energy intake. Brain Res. 2008;1228:81–88. doi: 10.1016/j.brainres.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Hansen MJ, Jones JE, Vlahos R, Bozinovski S, Anderson GP, Morris MJ. Cigarette smoke exposure reprograms the hypothalamic neuropeptide y axis to promote weight loss. Am J Respir Crit Care Med. 2006;173:1248–1254. doi: 10.1164/rccm.200506-977OC. [DOI] [PubMed] [Google Scholar]
- 8.Perkins KA, Epstein LH, Stiller RL, Marks BL, Jacob RG. Acute effects of nicotine on resting metabolic rate in cigarette smokers. Am J Clin Nutr. 1989;50:545–550. doi: 10.1093/ajcn/50.3.545. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 10.Woodruff PG, Koth LL, Yang YH, Rodriguez MW, Favoreto S, Dolganov GM, Paquet AC, Erle DJ. A distinctive alveolar macrophage activation state induced by cigarette smoking. Am J Respir Crit Care Med. 2005;172:1383–1392. doi: 10.1164/rccm.200505-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 12.Maquoi E, Demeulemeester D, Voros G, Collen D, Lijnen HR. Enhanced nutritionally induced adipose tissue development in mice with stromelysin-1 gene inactivation. Thromb Haemost. 2003;89:696–704. [PubMed] [Google Scholar]
- 13.Maquoi E, Munaut C, Colige A, Collen D, Lijnen HR. Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes. 2002;51:1093–1101. doi: 10.2337/diabetes.51.4.1093. [DOI] [PubMed] [Google Scholar]
- 14.Lijnen HR, Maquoi E, Hansen LB, Van Hoef B, Frederix L, Collen D. Matrix metalloproteinase inhibition impairs adipose tissue development in mice. Arterioscler Thromb Vasc Biol. 2002;22:374–379. doi: 10.1161/hq0302.104522. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–115. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 18.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD. Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol. 1998;161:6845–6852. [PubMed] [Google Scholar]
- 20.Houghton AM, Grisolano JL, Baumann ML, Kobayashi DK, Hautamaki RD, Nehring LC, Cornelius LA, Shapiro SD. Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res. 2006;66:6149–6155. doi: 10.1158/0008-5472.CAN-04-0297. [DOI] [PubMed] [Google Scholar]
- 21.Ferreras M, Felbor U, Lenhard T, Olsen BR, Delaisse J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486:247–251. doi: 10.1016/s0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- 22.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131:1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166–E174. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci USA. 1996;93:3942–3946. doi: 10.1073/pnas.93.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chavey C, Mari B, Monthouel MN, Bonnafous S, Anglard P, Van Obberghen E, Tartare-Deckert S. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem. 2003;278:11888–11896. doi: 10.1074/jbc.M209196200. [DOI] [PubMed] [Google Scholar]
- 29.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 30.Hajitou A, Grignet C, Devy L, Berndt S, Blacher S, Deroanne CF, Bajou K, Fong T, Chiang Y, Foidart JM, et al. The antitumoral effect of endostatin and angiostatin is associated with a down-regulation of vascular endothelial growth factor expression in tumor cells. FASEB J. 2002;16:1802–1804. doi: 10.1096/fj.02-0109fje. [DOI] [PubMed] [Google Scholar]
- 31.Engelen MP, Schols AM, Lamers RJ, Wouters EF. Different patterns of chronic tissue wasting among patients with chronic obstructive pulmonary disease. Clin Nutr. 1999;18:275–280. doi: 10.1016/s0261-5614(98)80024-1. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Hansen MJ, Jones JE, Vlahos R, Anderson GP, Morris MJ. Detrimental metabolic effects of combining long-term cigarette smoke exposure and high-fat diet in mice. Am J Physiol Endocrinol Metab. 2007;293:E1564–E1571. doi: 10.1152/ajpendo.00442.2007. [DOI] [PubMed] [Google Scholar]
- 33.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997;4:211–232. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- 35.Lijnen HR. Angiogenesis and obesity. Cardiovasc Res. 2008;78:286–293. doi: 10.1093/cvr/cvm007. [DOI] [PubMed] [Google Scholar]
- 36.Inoue-Murayama M, Sugimoto Y, Niimi Y, Aso H. Type xviii collagen is newly transcribed during bovine adipogenesis. Differentiation. 2000;65:281–285. doi: 10.1046/j.1432-0436.2000.6550281.x. [DOI] [PubMed] [Google Scholar]
- 37.Bauters D, Van Hul M, Lijnen HR. Macrophage elastase (mmp-12) in expanding murine adipose tissue. Biochim Biophys Acta. 2013;1830:2954–2959. doi: 10.1016/j.bbagen.2012.12.024. [DOI] [PubMed] [Google Scholar]