Abstract
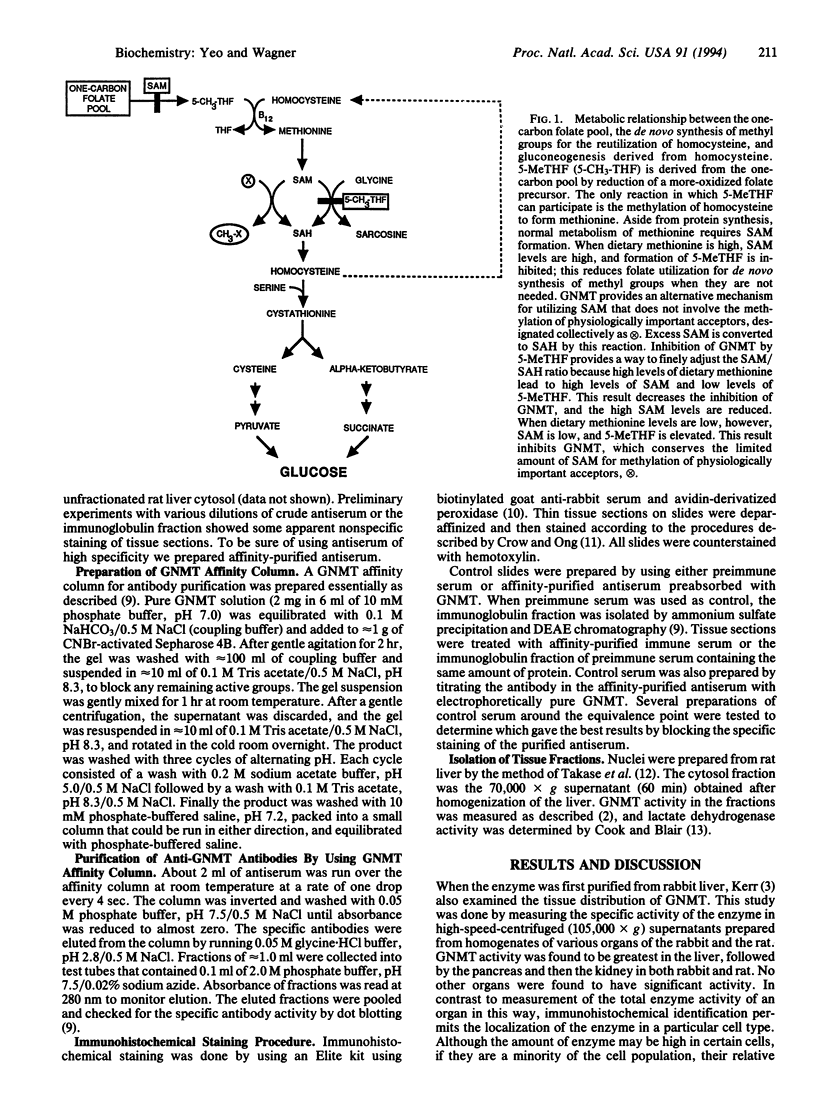
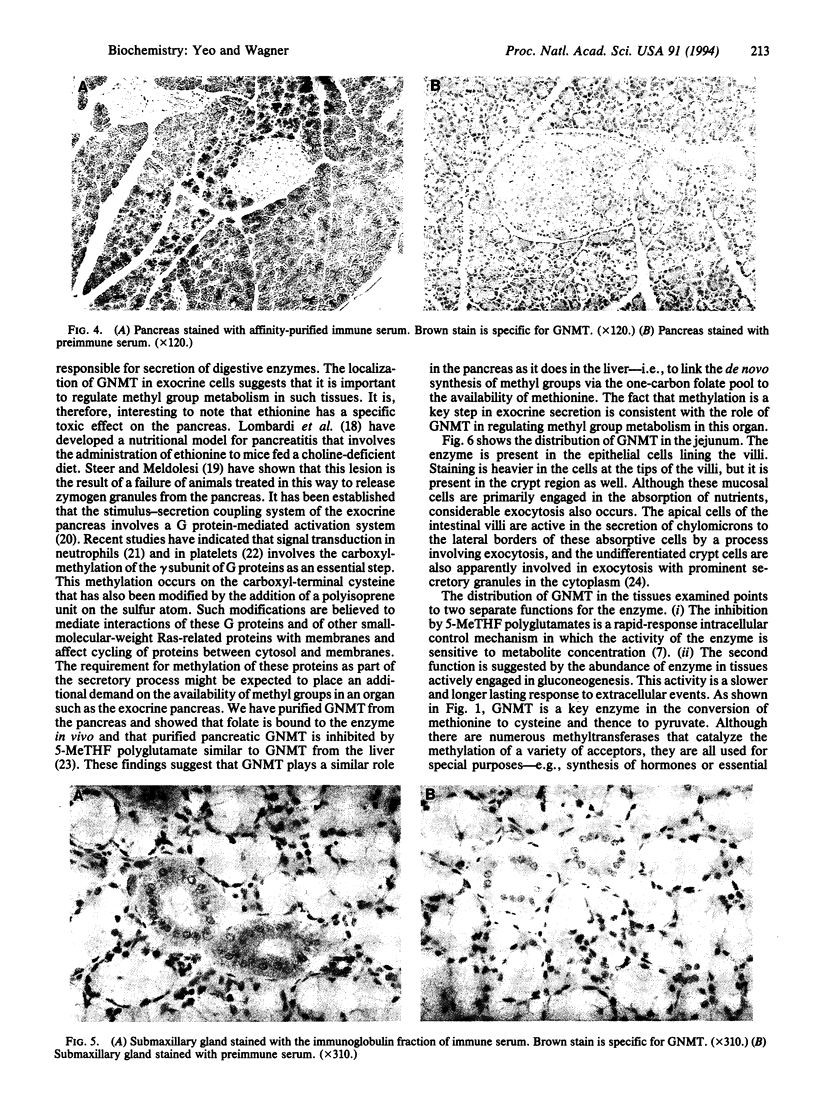
Glycine N-methyltransferase (GNMT; S-adenosyl-L-methionine:glycine N-methyltransferase, EC 2.1.1.20) is a major protein in rat liver that binds 5-methyltetrahydrofolate polyglutamate in vivo. This enzyme is believed to function in the regulation of the availability of S-adenosylmethionine, the primary donor of methyl groups in the body. The distribution of GNMT in a variety of rat tissues was examined immunohistochemically. In liver, GNMT was most abundant in the periportal region, whereas in kidney it was seen primarily in the proximal convoluted tubules. In pancreas, GNMT was abundant, principally in the exocrine tissue. GNMT was present in the striated duct cells of the submaxillary gland. In the jejunum, GNMT was found in the epithelial cells of the villi. Close examination of the liver indicated GNMT in the nucleus; this site was confirmed by purification of the nuclei and measurement of enzyme activity. The location of GNMT in the liver and kidney suggests that this enzyme plays a role in gluconeogenesis, while its presence in the exocrine cells suggests it may also be a factor in secretion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook R. J., Blair J. A. The distribution and chemical nature of radioactive folates in rat liver cells and rat liver mitochondria. Biochem J. 1979 Mar 15;178(3):651–659. doi: 10.1042/bj1780651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. J., Wagner C. Glycine N-methyltransferase is a folate binding protein of rat liver cytosol. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3631–3634. doi: 10.1073/pnas.81.12.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow J. A., Ong D. E. Cell-specific immunohistochemical localization of a cellular retinol-binding protein (type two) in the small intestine of rat. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4707–4711. doi: 10.1073/pnas.82.14.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guder W. G., Schmidt U. The localization of gluconeogenesis in rat nephron. Determination of phosphoenolpyruvate carboxykinase in microdissected tubules. Hoppe Seylers Z Physiol Chem. 1974 Mar;355(3):273–278. doi: 10.1515/bchm2.1974.355.1.273. [DOI] [PubMed] [Google Scholar]
- Heady J. E., Kerr S. J. Purification and characterization of glycine N-methyltransferase. J Biol Chem. 1973 Jan 10;248(1):69–72. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Huzoor-Akbar, Wang W., Kornhauser R., Volker C., Stock J. B. Protein prenylcysteine analog inhibits agonist-receptor-mediated signal transduction in human platelets. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):868–872. doi: 10.1073/pnas.90.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman S. Regulation of the activity of hepatic phenylalanine hydroxylase. Adv Enzyme Regul. 1986;25:37–64. doi: 10.1016/0065-2571(86)90007-5. [DOI] [PubMed] [Google Scholar]
- Kerr S. J. Competing methyltransferase systems. J Biol Chem. 1972 Jul 10;247(13):4248–4252. [PubMed] [Google Scholar]
- Lombardi B., Estes L. W., Longnecker D. S. Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by DL-ethionine fed with a choline-deficient diet. Am J Pathol. 1975 Jun;79(3):465–480. [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Evidence suggesting that a novel guanine nucleotide regulatory protein couples receptors to phospholipase C in exocrine pancreas. Biochem J. 1986 Jun 1;236(2):337–343. doi: 10.1042/bj2360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd S. H., Ebert M. H., Scriver C. R. Labile methyl group balances in the human: the role of sarcosine. Metabolism. 1980 Aug;29(8):707–720. doi: 10.1016/0026-0495(80)90192-4. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Fujioka M. Purification and properties of glycine N-methyltransferase from rat liver. J Biol Chem. 1982 Apr 10;257(7):3447–3452. [PubMed] [Google Scholar]
- Ogawa H., Gomi T., Horii T., Ogawa H., Fujioka M. Molecular cloning of cDNA for rat glycine methyltransferase. Biochem Biophys Res Commun. 1984 Oct 15;124(1):44–50. doi: 10.1016/0006-291x(84)90913-6. [DOI] [PubMed] [Google Scholar]
- Philips M. R., Pillinger M. H., Staud R., Volker C., Rosenfeld M. G., Weissmann G., Stock J. B. Carboxyl methylation of Ras-related proteins during signal transduction in neutrophils. Science. 1993 Feb 12;259(5097):977–980. doi: 10.1126/science.8438158. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Meldolesi J. The cell biology of experimental pancreatitis. N Engl J Med. 1987 Jan 15;316(3):144–150. doi: 10.1056/NEJM198701153160306. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Wagner C. Purification and characterization of a folate binding protein from rat liver cytosol. Arch Biochem Biophys. 1980 Jan;199(1):236–248. doi: 10.1016/0003-9861(80)90277-5. [DOI] [PubMed] [Google Scholar]
- Takase S., Ong D. E., Chytil F. Cellular retinol-binding protein allows specific interaction of retinol with the nucleus in vitro. Proc Natl Acad Sci U S A. 1979 May;76(5):2204–2208. doi: 10.1073/pnas.76.5.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C., Briggs W. T., Cook R. J. Inhibition of glycine N-methyltransferase activity by folate derivatives: implications for regulation of methyl group metabolism. Biochem Biophys Res Commun. 1985 Mar 29;127(3):746–752. doi: 10.1016/s0006-291x(85)80006-1. [DOI] [PubMed] [Google Scholar]
- Wagner C., Decha-Umphai W., Corbin J. Phosphorylation modulates the activity of glycine N-methyltransferase, a folate binding protein. In vitro phosphorylation is inhibited by the natural folate ligand. J Biol Chem. 1989 Jun 5;264(16):9638–9642. [PubMed] [Google Scholar]
- Wittwer A. J., Wagner C. Identification of the folate-binding proteins of rat liver mitochondria as dimethylglycine dehydrogenase and sarcosine dehydrogenase. Flavoprotein nature and enzymatic properties of the purified proteins. J Biol Chem. 1981 Apr 25;256(8):4109–4115. [PubMed] [Google Scholar]
- Wittwer A. J., Wagner C. Identification of the folate-binding proteins of rat liver mitochondria as dimethylglycine dehydrogenase and sarcosine dehydrogenase. Purification and folate-binding characteristics. J Biol Chem. 1981 Apr 25;256(8):4102–4108. [PubMed] [Google Scholar]
- Xue G. P., Snoswell A. M. Disturbance of methyl group metabolism in alloxan-diabetic sheep. Biochem Int. 1985 Jun;10(6):897–905. [PubMed] [Google Scholar]
- Yeo E. J., Wagner C. Purification and properties of pancreatic glycine N-methyltransferase. J Biol Chem. 1992 Dec 5;267(34):24669–24674. [PubMed] [Google Scholar]








