Significance
Atmospheric secondary organic aerosol has substantial impacts on climate, air quality, and human health. However, the formation mechanisms of secondary organic aerosol remain uncertain, especially on how anthropogenic pollutants (from human activities) control aerosol formation from biogenic volatile organic compounds (emitted by vegetation) and the magnitude of anthropogenic influences. Although possible mechanisms have been proposed based on laboratories studies, a coherent understanding of anthropogenic−biogenic interactions in ambient environments has not emerged. Here, we provide direct observational evidence that secondary organic aerosol formed from biogenic isoprene and monoterpenes is greatly mediated by anthropogenic SO2 and NOx emissions based on integrated ambient measurements and laboratory studies.
Keywords: fine particulate matter, biogenic secondary organic aerosol, anthropogenic emissions, sulfate, organic nitrates
Abstract
Secondary organic aerosol (SOA) constitutes a substantial fraction of fine particulate matter and has important impacts on climate and human health. The extent to which human activities alter SOA formation from biogenic emissions in the atmosphere is largely undetermined. Here, we present direct observational evidence on the magnitude of anthropogenic influence on biogenic SOA formation based on comprehensive ambient measurements in the southeastern United States (US). Multiple high-time-resolution mass spectrometry organic aerosol measurements were made during different seasons at various locations, including urban and rural sites in the greater Atlanta area and Centreville in rural Alabama. Our results provide a quantitative understanding of the roles of anthropogenic SO2 and NOx in ambient SOA formation. We show that isoprene-derived SOA is directly mediated by the abundance of sulfate, instead of the particle water content and/or particle acidity as suggested by prior laboratory studies. Anthropogenic NOx is shown to enhance nighttime SOA formation via nitrate radical oxidation of monoterpenes, resulting in the formation of condensable organic nitrates. Together, anthropogenic sulfate and NOx can mediate 43–70% of total measured organic aerosol (29–49% of submicron particulate matter, PM1) in the southeastern US during summer. These measurements imply that future reduction in SO2 and NOx emissions can considerably reduce the SOA burden in the southeastern US. Updating current modeling frameworks with these observational constraints will also lead to more accurate treatment of aerosol formation for regions with substantial anthropogenic−biogenic interactions and consequently improve air quality and climate simulations.
Organic aerosol (OA) is an important atmospheric component that influences climate, air quality, and human health (1). A large fraction of OA is secondary organic aerosol (SOA), which is formed through oxidation of volatile organic compounds (VOCs) emitted from human activities (anthropogenic) and vegetation (biogenic). In particular, biogenic VOCs (BVOCs), such as isoprene (C5H8) and monoterpenes (C10H16), are key precursors for global SOA formation owing to their larger emissions and higher reactivity with atmospheric oxidants compared with anthropogenic VOCs (1). However, the extent to which anthropogenic pollutants mediate the formation of SOA from biogenic VOCs (referred to as biogenic SOA) in the ambient environments is poorly understood and highly uncertain. For example, while radiocarbon analysis repeatedly indicated that more than half of the carbon in SOA is of modern (biogenic) origin in the southeastern United States (SE US) (2, 3), aircraft measurements in the same region showed that SOA correlates with anthropogenic tracers, such as CO (3).
One possible explanation to reconcile the seemingly contradictory results from radiocarbon studies and ambient measurements is that the majority of SOA is produced from naturally emitted BVOCs, but its formation processes also involve pollutants originated from anthropogenic emissions (3, 4). Laboratory studies have recently revealed that biogenic SOA formation can be largely affected by anthropogenic pollutants such as NOx and SO2 (1, 5). According to 2011 US national emission inventory (www.epa.gov/ttn/chief/net/2011inventory.html), 90% of NOx and 97% of SO2 are anthropogenically emitted. NOx can alter SOA formation by influencing peroxy radical chemistry in BVOCs oxidation mechanisms (5). The reaction of NO2 with O3 forms nitrate radicals, which can oxidize BVOCs to form condensable products that often have high SOA yields (6, 7). The effect of SO2 was investigated but often explained in the context of particle acidity in laboratory studies (8). Despite intense laboratory investigations, only a few proposed mechanisms are consistent with ambient observations (9), and the reasons for the observed enhancement of biogenic SOA formation in certain polluted environments remain unclear (10, 11). For instance, while some laboratory studies found that particle acidity can enhance isoprene SOA formation (8, 12), only weak correlations have been observed in the atmosphere between tracers of isoprene SOA and particle acidity (13–16). Thus, a coherent understanding of the enhancement of biogenic SOA in polluted environments has not emerged, and these proposed mechanisms from laboratory studies have not been quantitatively established in ambient environments.
Here, we provide direct observational evidence and quantification of anthropogenically enhanced biogenic SOA formation in the Southern Oxidant and Aerosol Study (SOAS; SI Appendix, Southern Oxidant and Aerosol Study) field campaign in June and July 2013. In addition, we also conducted ambient measurements from May 2012 to February 2013 at multiple rural and urban sites in the greater Atlanta area as part of the Southeastern Center of Air Pollution and Epidemiology study (SCAPE, EPA Clean Air Center; SI Appendix). The SE US is ideal for studying anthropogenic−biogenic interactions due to high natural emissions and the proximity to anthropogenic pollution sources. Here, we investigate the sources of OA using factor analysis of high-time-resolution mass spectrometry data coupled with a suite of comprehensive and collocated measurements (SI Appendix, Instrumentation). We have also performed complementary laboratory studies to examine possible chemical mechanisms to interpret results from ambient measurements. From these integrated ambient and laboratory studies, we show that anthropogenic SO2 and NOx emissions substantially mediate SOA formation from BVOCs such as isoprene and monoterpenes in the SE US.
OA Source Apportionment
We obtain quantitative, real-time measurements of five nonrefractory submicron particulate matter (PM1) components (organics, sulfate, nitrate, ammonium, and chloride) with High Resolution Time-of-Flight Aerosol Mass Spectrometer (HR-ToF-AMS) from June 1 to July 15, 2013 (SI Appendix, Fig. S2) at the SouthEastern Aerosol Research and Characterization (SEARCH) network site near Centreville in rural Alabama, which served as the SOAS ground site. The measured campaign average of nonrefractory PM1 mass is 7.5 ± 5.3 μg⋅m−3 (average ± 1 SD), which is similar to the campaign-average PM2.5 mass of 7.8 ± 4.6 μg⋅m−3 (average ± 1 SD), considering the detection differences in particle size range and particle composition (refractory species are not detected by HR-ToF-AMS) between the two methods. We find that organics are the dominant components in PM1, with a mass fraction of 67%, followed by sulfate at 26%.
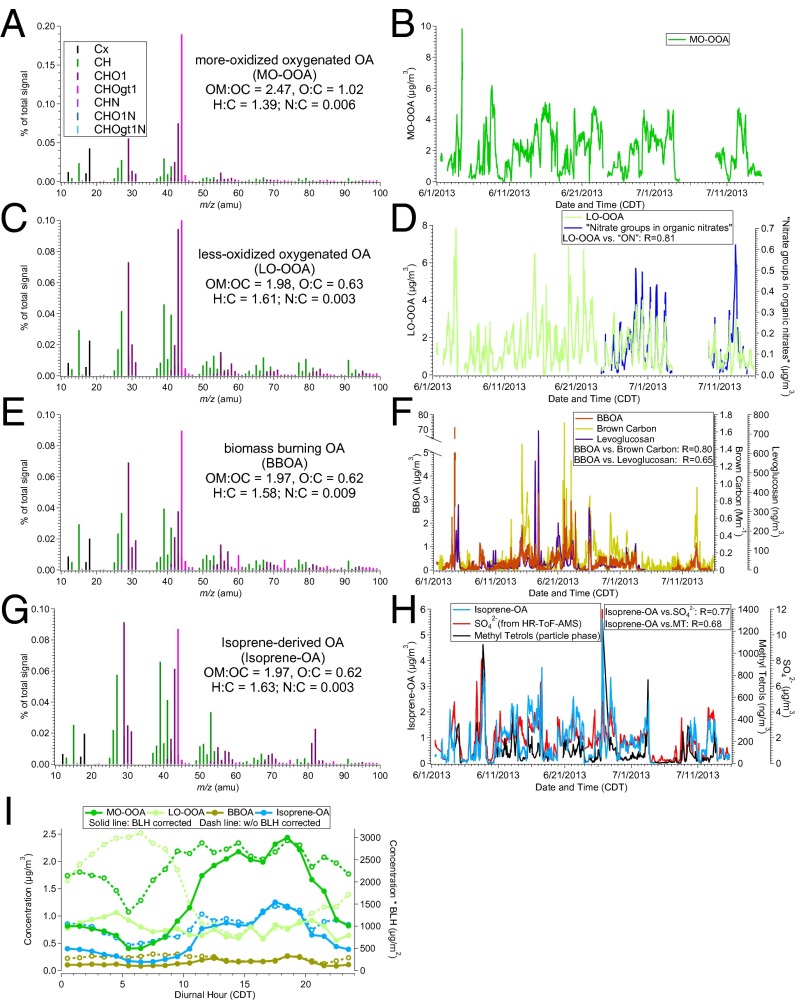
To determine the sources of OA measured at Centreville during SOAS, we perform multivariate factor analysis with positive matrix factorization (PMF) and identify four subtypes of OA (SI Appendix, Positive Matrix Factorization). Fig. 1 shows the unique mass spectrum and time series of each of these factors. The first two factors are oxygenated OA (OOA) with high but differing atomic oxygen-to-carbon (O:C) ratio. We refer to the OOA factor with higher O:C ratio as more-oxidized OOA (MO-OOA, O:C = 1.02) and the one with lower O:C ratio as less-oxidized OOA (LO-OOA, O:C = 0.63). MO-OOA and LO-OOA represent 39% and 33% of OA, respectively. The mass spectrum of the third factor is characterized by ions at m/z 60 (C2H4O2+) and 73 (C3H5O2+), which are known to be produced by levoglucosan, a tracer for biomass burning (17) and indeed correlates with the third factor (R = 0.65, Fig. 1F) in Centreville. In addition, the third factor shows good correlation with brown carbon (R = 0.8; Fig. 1F) (SI Appendix, Particle into Liquid Sampler), which appears to be significant in biomass combustion emissions (18). Thus, we identify this factor as biomass burning OA (BBOA), which represents 10% of OA.
Fig. 1.
(A, C, E, G) Normalized high-resolution aerosol mass spectra (colored by the ion type) and elemental ratios of the PMF factors. (B, D, F, H) Time series of the PMF factors and tracer compounds, along with their correlation coefficient. (I) Diurnal trends of PMF factors with (solid line) and without (dash line) multiplying by boundary layer height (BLH).
A fourth factor, characterized by tracer ions at m/z 53 (C4H5+) and m/z 82 (C5H6O+) in its mass spectrum, contributes 18% of OA. This same factor has been observed in several recent field studies (15, 19, 20) and has been linked to isoprene, given its mass spectral similarity to laboratory-generated isoprene SOA via the reactive uptake of epoxydiols (IEPOX), an important oxidation product of isoprene when organic peroxy radicals mainly react with hydroperoxy radicals (21). Previous ambient measurements (15) showed that this factor correlated with 24-h integrated filter-based 2-methylerythritol and 2-methylthreitol (collectively referred to as methyltetrols), which are known isoprene SOA tracers likely formed from IEPOX uptake (8, 22). In Centreville, we continuously measured particle-phase methyltetrols with a semivolatile thermal desorption aerosol gas chromatograph (SV-TAG) and found that the fourth factor indeed correlates with methyltetrols (R = 0.68, Fig. 1H), which provides further evidence that this factor is related to isoprene. Additionally, isoprene is the most abundant BVOC (highest mixing ratio) during daytime in Centreville and exhibits a similar diurnal trend as the fourth OA factor (SI Appendix, Fig. S4). PMF analysis of our six SCAPE datasets (SI Appendix, Southeastern Center for Air Pollution and Epidemiology, EPA Clean Air Center) also revealed a factor of similar mass spectral features (i.e., prominent signals at C4H5+ and C5H6O+) only in the warmer months, from May to September (Fig. 2) when isoprene emissions are strongest and methyltetrols concentrations are highest (23, 24). Based on all of this evidence, we name the fourth factor, which is likely related to isoprene SOA formed via reactive uptake of IEPOX, as isoprene-derived organic aerosol (Isoprene-OA).
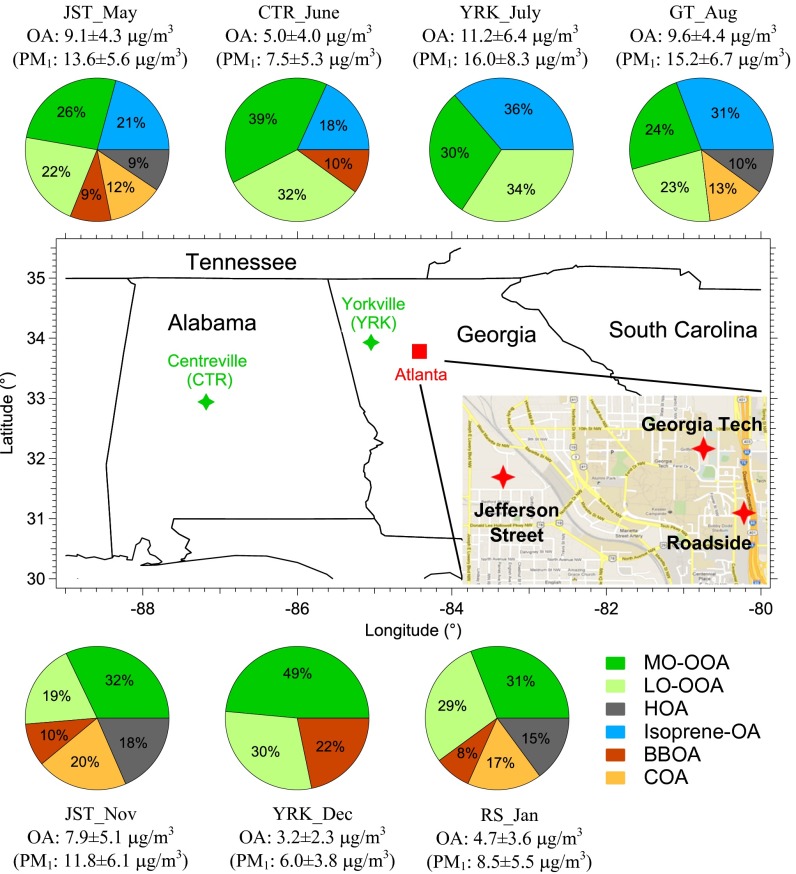
Fig. 2.
Geographical locations and organic aerosol characterization of SOAS and SCAPE field campaigns in the southeastern US. Inset shows a detailed map of Atlanta (adapted from Google Maps). Abbreviations correspond to Centerville (CTR), Yorkville (YRK), Jefferson Street (JST), Georgia Institute of Technology (GT), and Roadside (RS). Details about sampling period at each site are listed in SI Appendix, Table S1. Measurement sites are classified based on their locations as urban (red star) and rural (green star). The pie charts report the source apportionment of organic aerosol. The mass concentrations ± 1 SD of organics and PM1 as measured by HR-ToF-AMS are also reported. The identified OA subtypes are MO-OOA (more-oxidized oxygenated OA), LO-OOA (less-oxidized oxygenated OA), Isoprene-OA (isoprene-derived OA), BBOA (biomass burning OA), HOA (hydrocarbon-like OA), and COA (cooking OA). Isoprene-OA is only identified in the warmer months (from May to September), and LO-OOA is identified at various rural and urban sites throughout the year. While the first four factors are discussed in OA Source Apportionment, the identification of HOA and COA is discussed in SI Appendix, Positive Matrix Factorization. Isoprene-OA and LO-OOA account for 43–70% of total measured OA in summer time.
In this work, we aim at quantifying the extent of anthropogenically mediated biogenic SOA in the SE US. We focus on the discussion of Isoprene-OA and LO-OOA, as these two OA subtypes could originate from biogenic isoprene and monoterpenes, respectively, and be greatly mediated by anthropogenic emissions. We note that MO-OOA accounts for 24–49% of measured organic aerosol in the SE US (Fig. 2), although the source of this OA subtype is currently unclear and warrants future investigations. As MO-OOA has the highest O:C ratio among all OA factors, it likely represents highly aged organic aerosol from multiple origins (25).
Effects of Sulfate on Isoprene-OA
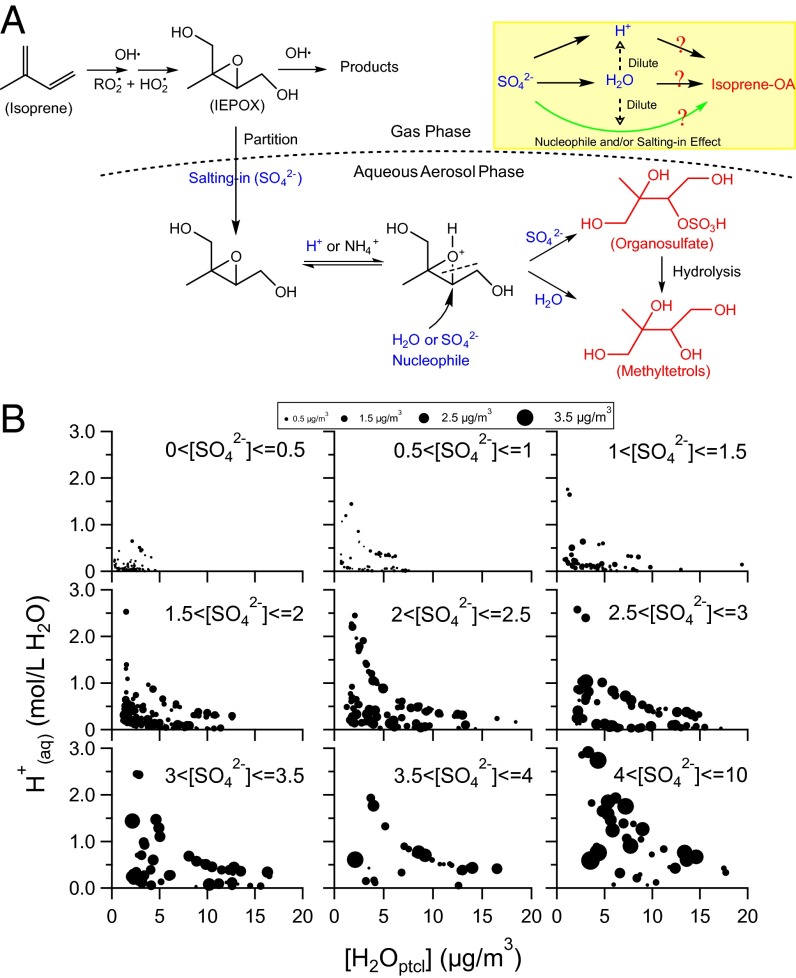
A striking feature of the Centreville aerosol is a strong association (R = 0.77) between Isoprene-OA and sulfate (SO42-). This feature appears to be common throughout the SE US based on our SCAPE datasets in the greater Atlanta area (range of R between Isoprene-OA and sulfate is 0.73–0.88). For instance, in Centreville, a spike in Isoprene-OA and known isoprene oxidation products methyltetrols was observed from about noon to 1800 hours on June 26 when the site was influenced by a sulfate-rich plume (Fig. 1H). Similarly, in Yorkville (SEARCH site in Georgia), when a dramatic decrease in sulfate concentration occurred on July 6, 2012, among the three OA factors, only the Isoprene-OA showed a corresponding decrease (SI Appendix, Fig. S5). These observations, and other observed similar events highlight the importance of sulfate in isoprene SOA formation in the SE US. However, how exactly and to what extent sulfate mediates isoprene SOA formation in the ambient environments remains elusive. Recent laboratory studies proposed that isoprene SOA formation from IEPOX requires particle water (H2Optcl) for IEPOX uptake, proton donors (e.g., H+ or NH4+) for catalyzing IEPOX ring opening, and nucleophiles (e.g., H2O, SO42−, NO3−) to facilitate further particle-phase reactions (8, 26). A simplified mechanism of this process is shown in Fig. 3A. The highly convoluted interactions among particle water, particle acidity, and sulfate present a challenge in elucidating the roles of each of these parameters in isoprene SOA formation (Fig. 3A). For example, the direct effect of sulfate on SOA formation (possibly through nucleophilic addition) may be misinterpreted as the effect of particle water and acidity because they are typically driven by sulfate (27).
Fig. 3.
(A) A simplified mechanism of isoprene SOA formation via reactive uptake of IEPOX based on refs. 8 and 26. Only one IEPOX isomer is shown. Compounds colored blue are the parameters we investigate in this study. Compounds colored red are two representative species for isoprene SOA. Inset shows a schematic explaining the relationship between particle water (H2Optcl), particle acidity (H+), sulfate (SO42−), and Isoprene-OA. The direct role of SO42− on isoprene OA formation is colored green. The indirect role of SO42− through H2Optcl and/or H+ is colored black. (B) H+(aq) (moles per liter H2O) as a function of [H2Optcl] (micrograms per cubic meter of air). All data points are grouped into nine subplots based on a 0.5 μg⋅m−3 increment in [SO42−], and the size of data points represents [Isoprene-OA]. In some cases, a range of H+(aq) is observed for the same [H2Optcl], which is likely due to difference in gas-phase [NH3] (SI Appendix, Fig. S6).
Another critical challenge to elucidate the effects of each parameter is the determination of particle water content ([H2Optcl]; [ ] denotes micrograms per cubic meter of air) and particle acidity (H+(aq); (aq) denotes moles per liter of H2O). For Centreville and all SCAPE datasets, we comprehensively calculate [H2Optcl] by including the contribution from organics based on measured organic hygroscopicity (28) and the contribution from inorganics based on the thermodynamic model ISORROPIA II (27). Detailed calculations of [H2Optcl] can be found in Guo et al. (29). The calculated [H2Optcl] agrees with our indirect measurements of particle water content (29). Further, we calculate particle pH based on [H2Optcl] and ISORROPIA II output [H+] (micrograms per cubic meter of air). The ISORROPIA equilibrium calculations accurately predict the measured gas-phase ammonia concentrations, providing a strong validation for our particle acidity calculation (29). Our results show that aerosol throughout the SE US is very acidic (pH ranging between 0 and 2) and contains high particle water contents (average [H2Optcl] ranging between 5.1 and 8.4 μg⋅m−3) in the summertime (29).
In the SE US, H+ is a more efficient proton donor than NH4+ since NH4+ is an effective catalyst only for pH > 4 (26). Bisulfate (HSO4−) could also act as a proton donor, which may provide electrostatic stabilization of partially formed intermediate (30). Although the efficiency of bisulfate in catalyzing IEPOX ring opening is uncertain, it is expected to be lower than H+ under the low-pH condition in the SE US. Regarding nucleophiles, SO42− is the most important because of its high particle concentration and stronger nucleophilic strength (31) compared with other species (NO3− and H2O). Together, these results indicate that H2Optcl, H+, and SO42− are the most important parameters affecting isoprene SOA formation from IEPOX uptake in the SE US.
We perform multivariate linear regression analysis on Centreville data to gain quantitative insights into the effects of particle water ([H2Optcl]), particle acidity (H+(aq)), and sulfate ([SO42−]) (SI Appendix, Multivariate Linear Regression) on isoprene SOA formation (i.e., Isoprene-OA factor). Importantly, we find that SO42− has a statistically significant (P < 0.0001) positive linear relationship with Isoprene-OA factor with a regression coefficient of 0.42 (SI Appendix, Table S2). These results suggest that a 1 μg⋅m−3 increase in SO42− will (on average) increase Isoprene-OA by an estimated 0.42 μg⋅m−3, when holding the other covariates constant. In contrast, the estimated effects of H2Optcl and H+ are not statistically significant, suggesting that particle water and acidity are not the limiting parameters in isoprene SOA formation.
To visualize this important finding, nine scatter plots of H+(aq) vs. [H2Optcl] are generated by sorting all data points into nine bins based on a 0.5 μg⋅m−3 increment in [SO42−] and representing [Isoprene-OA] by the size of data points (Fig. 3B). These plots are useful because they offer a clear view of the effect of one parameter on Isoprene-OA while holding the other two covariates constant. We note that in each subplot (similar [SO42−]), the data point size ([Isoprene-OA]) appears to be independent of H+(aq) and [H2Optcl], indicating that varying H+(aq) or [H2Optcl] under similar [SO42−] causes little change in [Isoprene-OA]. In contrast, the data point size ([Isoprene-OA]) tends to increase appreciably with increasing [SO42−], indicating that sulfate could greatly mediate isoprene OA formation directly through its abundance. We perform the same analysis on our SCAPE datasets where Isoprene-OA factor is identified (SI Appendix, Fig. S6) and arrive at the same conclusion that Isoprene-OA formation over broad regions of the SE US is directly controlled by the abundance of sulfate.
One possible explanation for the remarkable control of isoprene SOA by sulfate might be the concerted nucleophilic addition to the IEPOX ring. Both experimental and computational studies (31, 32) have revealed that this step is the rate-determining step in OA formation from IEPOX. Thus, an increase in [SO42−] may effectively facilitate the ring-opening reaction of IEPOX and subsequent OA formation, especially the formation of organosulfates (8), which have been detected previously in the SE US (33) and could hydrolyze to form methyltetrols (34) (Fig. 3A). To a less extent than sulfate, water could also react with IEPOX by acting as a nucleophile, which forms methyltetrols (8). Sulfate may also affect Isoprene-OA formation through salting-in effect. Salting-in refers to the effect that increasing salt concentration in aqueous solution would increase the solubility of polar organic compounds. For example, Kampf et al. (35) found that the effective Henry’s law coefficient of glyoxal increases exponentially with SO42−(aq) until glyoxal uptake is kinetically limited. Considering that IEPOX is also highly water soluble as glyoxal, sulfate may also cause salting-in effect on IEPOX. Once IEPOX is in the particle phase, further reactions such as ring opening and subsequent nucleophilic attack by sulfate prevent the reversible partitioning of IEPOX back to the gas phase. However, no systematic study about the salting-in effect of IEPOX uptake is currently available, and this warrants further study.
Our finding that particle acidity does not influence isoprene SOA formation in the SE US is striking and contrasts with several previous laboratory and modeling studies, which suggested the importance of particle acidity in isoprene SOA formation (8, 36). The weak correlation between IEPOX-derived SOA tracers and particle acidity has been observed in prior field measurements, but the explanations are rather inconclusive (13, 14). For example, Lin et al. (14) attributed the weak correlation as SOA not being formed locally and concluded that the particle acidity could change as the aerosol is advected to the sampling site from other regions. However, isoprene OA measured in Centreville, or the SE US in general, is expected to be formed locally (SI Appendix, Backtrajectory Analysis and Fig. S10) owing to regionally abundant isoprene emissions in summertime (36) and the short lifetime of isoprene (∼1.4 h at 25 °C assuming [OH] ≈ 2*106 molecules per cubic centimeter of air), consistent with our observations of similar processes at different sites. Here, we hypothesize that the weak influence of particle acidity on isoprene OA is a result of consistently high particle acidity in the SE US (29). A recent chamber study (26) showed that the reactive partitioning coefficient of IEPOX increases by only 1.5 times as H+(aq) increases by many orders of magnitude (in the pH range relevant to our study). Our ambient measurements show that the particle pH throughout the SE US is very low (ranging between 0 and 2; the average value is 0.94 ± 0.59 for Centreville) (29), which means that isoprene OA formation is insensitive to H+ in the SE US. Moreover, as the isoprene OA formation is limited by nucleophiles instead of catalyst activity (31, 32), increasing particle acidity (within pH range 0–2) may not facilitate the isoprene OA formation rate, and hence the association between H+(aq) and [Isoprene-OA] is not significant. Therefore, although our analysis does not discount the important role of particle acidity in isoprene OA formation via IEPOX uptake, it suggests that particle acidity is not the limiting parameter given the acidic nature of aerosol in the SE US.
The weak influence of particle water on isoprene OA formation, which is surprising, could be a result of the competition between particle water abundance and dilution of ions at high relative humidity typically found in these regions. Increasing particle water content would provide more medium for gas-phase water-soluble species to dissolve and potentially increase SOA (37); increasing water, however, reduces SO42−(aq) (moles per liter of H2O) by diluting the particle concentration of sulfate. This dilution could not only reduce the reaction rate due to lower nucleophile concentration but also suppress IEPOX uptake due to weakening ionic strength and salting-in effect. Thus, increasing water could potentially decrease SOA. In Centreville, [H2Optcl] reached a daily minimum at about 1600 local time when SO42−(aq) is highest and isoprene is near its maximum (SI Appendix, Figs. S4 and S7), indicating that the two opposing effects of particle water are competing with each other when IEPOX is abundant.
As particle water content correlates with sulfate, we perform additional multivariate linear regression by considering water uptake by organics only (Org-H2O), which is not related to sulfate and contributes about 36% of total particle water (29), to deconvolute the interaction between sulfate and particle water on isoprene OA formation. In contrast to total particle water, which is not significantly associated with Isoprene-OA, Org-H2O shows a significantly positive relationship with Isoprene-OA (P = 0.002, SI Appendix, Table S2). Even though significant, Org-H2O still does not have a dominant effect on isoprene OA formation as its β-coefficient is 80 times smaller than that of sulfate. Further, the contrasting regression results between total particle water vs. Org-H2O indicate that increasing [H2Optcl] under low particle water levels (i.e., Org-H2O) would enhance Isoprene-OA formation. However, under high particle water levels (i.e., total particle water), which is typical in the SE US, particle water is not a limiting parameter for Isoprene-OA formation.
Effects of NOx on LO-OOA
Similar to the spatial uniformity of the association between Isoprene-OA and sulfate during summer, LO-OOA factor shows consistently similar diurnal patterns (SI Appendix, Fig. S8) in Centreville and SCAPE datasets at various rural and urban sites, but in this case, LO-OOA factor is observed throughout the year (Fig. 2). To determine whether LO-OOA is locally produced or from long-range transport, we focus on Centreville, where auxiliary data are available. Measurements in Centreville are split into four subsets based on 72-h back trajectories of air mass geographical origins relative to the location of measurement site: northwest, northeast, southwest, and southeast (SI Appendix, Backtrajectory Analysis and Figs. S9 and S10). The diurnal patterns of LO-OOA are similar regardless of the origin of the air masses. Combined with similar diurnal patterns at multiple sites, this suggests that the source of LO-OOA is local. As seen in Fig. 1I, the LO-OOA concentration shows a diurnal maximum at night and a minimum at around 1700 hours. From 1700 hours to sunrise, the LO-OOA concentration increases by nearly four times. This increase still exists after the LO-OOA concentration is adjusted by the boundary layer height, indicating that the nighttime increase in LO-OOA is likely caused by nighttime aerosol production instead of the nocturnal boundary layer becoming shallow.
Since the NO3• radical (a product of NO2 and ozone reaction) is a well-known nocturnal oxidant, we hypothesize that NO3• oxidation of BVOCs contributes to the nighttime increase in LO-OOA. Laboratory studies have revealed that organic nitrates make up a substantial fraction of the SOA from BVOC+NO3• (6, 7). To examine the role of organic nitrates in LO-OOA formation, we calculate the mass concentration of the nitrate functional groups in organic compounds (NO3,org−) from the difference between HR-ToF-AMS measurements (NO3− from both organic and inorganic species) and PILS-IC measurements (NO3− from inorganic nitrate only) (SI Appendix, Organic Nitrate Estimation). We find a good correlation (R = 0.81) between LO-OOA and NO3,org−, which supports that LO-OOA is most likely related to nighttime NO3• chemistry. Further, we calculate that organic nitrates contribute 40–60% of LO-OOA in early morning based on the concentration of nitrate functional groups and the assumption that the average molecular weight of organic nitrates ranges from 200 g⋅mol−1 to 300 g⋅mol−1 (9) (SI Appendix, Fig. S11).
To quantitatively constrain the contribution of NO3• chemistry to LO-OOA, we estimate aerosol formation from isoprene, α-pinene, and β-pinene, which are the most abundant biogenic SOA precursors measured in Centreville, via various oxidation pathways. We estimate [NO3•] to be about 7.6 × 10−2 parts per trillion (SI Appendix, [NO3•] Estimation) with a corresponding lifetime of 8 s. We further calculate that NO3• chemistry at night accounts for 17%, 20%, and 38% of the reacted isoprene, α-pinene, and β-pinene, respectively (SI Appendix, Table S4). The contribution of each BVOC and reaction pathway to total nighttime SOA formation would depend on their respective SOA yields at mass loadings relevant to Centreville (i.e., ∼8 μg⋅m−3), which are available in the literature (SI Appendix, Table S5), except for β-pinene+NO3•. To this end, we performed comprehensive laboratory chamber studies to investigate SOA formation from β-pinene+NO3• under conditions relevant to Centreville and SE US (SI Appendix, Laboratory Chamber Experiments). The SOA yield is found to be 50%, which is ∼17 times higher than β-pinene SOA yields from ozonolysis and photooxidation for a mass loading of ∼8 μg⋅m−3. This implies that β-pinene could still form aerosol in the absence of NO3•, although the amount of aerosol formed would be substantially smaller. Based on our β-pinene SOA yields, and yields for other BVOCs via various oxidation pathways from prior chamber studies, we calculate that 0.7 μg⋅m−3 of SOA would be produced, which agrees within a factor of three with the measured nighttime LO-OOA production (1.7 μg⋅m−3 from 1700 hours to sunrise).
According to model estimation, 64% of total nighttime OA production arises from the NO3• oxidation pathway (SI Appendix, Table S5 and Fig. S12), which is consistent with the estimated contribution of organic nitrates to LO-OOA. For the amount of OA produced from NO3• oxidation pathway, 80% originates from monoterpenes, which is much greater than the contribution from isoprene (20%) as suggested by our model. Taken together, monoterpenes+NO3• chemistry accounts for 50% of total nighttime OA production. The large contribution is likely due to the large abundance of monoterpenes at night, which exhibit the same diurnal pattern as LO-OOA (SI Appendix, Fig. S4), as well as the high SOA yield from β-pinene+NO3• as revealed by our chamber studies. Additionally, the presence of LO-OOA throughout the year in the greater Atlanta area (Fig. 2) is in agreement with the fact that monoterpene emissions exist in all seasons in the SE US (24). Therefore, we conclude from our integrated ambient observations and laboratory studies that nighttime monoterpenes+NO3• chemistry contributes substantially to LO-OOA.
Our results highlight the important role of BVOC composition in evaluating their contribution to ambient OA via NO3• oxidation. A recent study (9) observed that high [BVOC] can suppress SOA formation from NO3• oxidation in Bakersfield (CA), where NO3• activity is dominated by limonene. The authors attributed the suppression to high concentrations of limonene depleting [NO3•], thus inhibiting the further oxidation of their first-generation products and subsequent aerosol formation. Nevertheless, our study shows that BVOCs+NO3• can still be an important pathway for OA production in the SE US. In contrast to limonene, BVOCs like β-pinene, whose first-generation oxidation products are condensable (6), could lead to substantial nighttime aerosol production.
Implications
We provide direct evidence from ambient measurements to show that anthropogenic pollution can greatly mediate SOA formation from biogenic VOCs under current conditions in the SE US. Being strongly influenced by the NO3• radical and sulfate, LO-OOA (mainly from monoterpenes oxidation by NO3•) and Isoprene-OA (isoprene SOA formed via reactive uptake of IEPOX in the presence of hydrated sulfate) account for 19–34% and 18–36% (May−September only) of OA, respectively, in Centreville and the greater Atlanta area (Fig. 2). In the SE US, the majority of sulfate (photochemical reactions of SO2) and NO3• radical (a product of NO2 and ozone reaction) is of anthropogenic origin (38). Using measurement at Centreville (SEARCH site) from 2006 to 2010, we find that the correlation between organic carbon (OC) and sulfate (hourly average data) is substantially better in summer (June−August) than winter (December−February) (SI Appendix, Fig. S13). As isoprene emission is higher in warmer months, our proposed interaction between sulfate and Isoprene-OA provides a possible explanation for the seasonal variation in the correlation between OC and sulfate, although we cannot rule out other possibilities. In addition, over the past 15 years, the OC at rural SEARCH sites in the SE US declined by about 38% as calculated from the trends shown in Hidy et al. (39). During the same period, the emission of SO2 and NOx has also decreased by about 65% and 52%, respectively (39), suggesting that our proposed mechanism about anthropogenic emissions mediating biogenic SOA formation contributes in a potentially significant way to the decrease in OC. As SO2 and NOx emissions continue to fall, other biogenic SOA formation pathways (e.g., isoprene SOA formation in the absence of sulfate and monoterpenes SOA from ozonolysis and photooxidation) may become more important, although these pathways have relatively lower SOA yields compared with the mechanisms discussed in this study (8). The decreasing SO2 and NOx emissions may not only reduce the biogenic SOA burden but also have impacts on climate and health. For example, while SOA from IEPOX uptake in the presence of sulfate (i.e., Isoprene-OA factor) is found to have the highest hygroscopicity (tendency to absorb water vapor) of all OA components (28), biogenic SOA formed under lower sulfate and NOx environments could have substantially different properties than those formed in polluted environments and warrants further studies.
Although isoprene OA formation via IEPOX uptake has been reported in several field campaigns, our study performs detailed analyses of particle water, particle acidity, and sulfate, and then provides comprehensive assessment to deconvolute their individual effects on isoprene OA formation in the atmosphere (28, 29). Our observation in Centreville and the greater Atlanta area shows that it is sulfate, instead of particle water and acidity, that controls isoprene OA formation in the SE US during summer, although the exact mechanisms of this direct sulfate effect need further investigation. The influence of these parameters can vary regionally and globally. Therefore, SOA models need to carefully consider the fate of IEPOX and the complexity of isoprene OA formation under various atmospheric conditions. Moreover, our results reveal that the direct effect of sulfate may complicate the role of particle water in the partitioning of water-soluble organics. Finally, these findings emphasize the importance of careful calculations of both particle water content and particle acidity when investigating these SOA formation processes.
Supplementary Material
Acknowledgments
The authors would like to thank Charles L. Blanchard for helpful discussions. Georgia Tech SOAS researchers were supported by National Science Foundation (NSF) Grant 1242258, US Environmental Protection Agency (EPA) STAR Grants RD-83540301 (early career) and R835410, and National Oceanic and Atmospheric Administration Climate Program Office Award NA10OAR4310102. SCAPE Clean Air Center is supported through US EPA Grant R834799. G.I.-V. was supported by an NSF Graduate Research Fellowship (Grant DGE 1106400), and SV-TAG data collection was funded by NSF Grant 1250569. S.-H.L. is supported by NSF (AGS-1241498). National Center for Atmospheric Research (NCAR) is sponsored by NSF. We acknowledge high-performance computing support from Yellowstone (ark:/85065/d7wd3xhc) provided by NCAR's Computational and Information Systems Laboratory, sponsored by NSF. Operation of the SEARCH network and analysis of its data collection are sponsored by the Southern Co. and Electric Power Research Institute. A.B. acknowledges the research project Mediterranean Aerosol, Cloud Activation and Volatility Experiments implemented within the framework of the Action “Supporting Postdoctoral Researchers” of the Operational Program “Education and Lifelong Learning,” and is cofinanced by the European Social Fund and the Greek State.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417609112/-/DCSupplemental.
References
- 1.Hallquist M, et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos Chem Phys. 2009;9(14):5155–5236. [Google Scholar]
- 2.Schichtel BA, et al. Fossil and contemporary fine particulate carbon fractions at 12 rural and urban sites in the United States. J Geophys Res. 2008;113(D2):D02311. [Google Scholar]
- 3.Weber RJ, et al. A study of secondary organic aerosol formation in the anthropogenic-influenced southeastern United States. J Geophys Res. 2007;112(D13):D13302. [Google Scholar]
- 4.Spracklen DV, et al. Aerosol mass spectrometer constraint on the global secondary organic aerosol budget. Atmos Chem Phys. 2011;11(23):12109–12136. [Google Scholar]
- 5.Kroll JH, Seinfeld JH. Chemistry of secondary organic aerosol: Formation and evolution of low-volatility organics in the atmosphere. Atmos Environ. 2008;42(16):3593–3624. [Google Scholar]
- 6.Ng NL, et al. Secondary organic aerosol (SOA) formation from reaction of isoprene with nitrate radicals (NO3) Atmos Chem Phys. 2008;8(14):4117–4140. [Google Scholar]
- 7.Fry JL, et al. Organic nitrate and secondary organic aerosol yield from NO3 oxidation of beta-pinene evaluated using a gas-phase kinetics/aerosol partitioning model. Atmos Chem Phys. 2009;9(4):1431–1449. [Google Scholar]
- 8.Surratt JD, et al. Reactive intermediates revealed in secondary organic aerosol formation from isoprene. Proc Natl Acad Sci USA. 2010;107(15):6640–6645. doi: 10.1073/pnas.0911114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rollins AW, et al. Evidence for NOx control over nighttime SOA formation. Science. 2012;337(6099):1210–1212. doi: 10.1126/science.1221520. [DOI] [PubMed] [Google Scholar]
- 10.Shilling JE, et al. Enhanced SOA formation from mixed anthropogenic and biogenic emissions during the CARES campaign. Atmos Chem Phys. 2013;13(4):2091–2113. [Google Scholar]
- 11.Goldstein AH, Koven CD, Heald CL, Fung IY. Biogenic carbon and anthropogenic pollutants combine to form a cooling haze over the southeastern United States. Proc Natl Acad Sci USA. 2009;106(22):8835–8840. doi: 10.1073/pnas.0904128106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaston CJ, et al. Reactive uptake of an isoprene-derived epoxydiol to submicron aerosol particles. Environ Sci Technol. 2014;48(19):11178–11186. doi: 10.1021/es5034266. [DOI] [PubMed] [Google Scholar]
- 13.Worton DR, et al. Observational insights into aerosol formation from isoprene. Environ Sci Technol. 2013;47(20):11403–11413. doi: 10.1021/es4011064. [DOI] [PubMed] [Google Scholar]
- 14.Lin YH, Knipping EM, Edgerton ES, Shaw SL, Surratt JD. Investigating the influences of SO2 and NH3 levels on isoprene-derived secondary organic aerosol formation using conditional sampling approaches. Atmos Chem Phys. 2013;13(16):8457–8470. [Google Scholar]
- 15.Budisulistiorini SH, et al. Real-time continuous characterization of secondary organic aerosol derived from isoprene epoxydiols in downtown Atlanta, Georgia, using the Aerodyne Aerosol Chemical Speciation Monitor. Environ Sci Technol. 2013;47(11):5686–5694. doi: 10.1021/es400023n. [DOI] [PubMed] [Google Scholar]
- 16.Tanner RL, Olszyna KJ, Edgerton ES, Knipping E, Shaw SL. Searching for evidence of acid-catalyzed enhancement of secondary organic aerosol formation using ambient aerosol data. Atmos Environ. 2009;43(21):3440–3444. [Google Scholar]
- 17.Schneider J, et al. Mass spectrometric analysis and aerodynamic properties of various types of combustion-related aerosol particles. Int J Mass Spectrom. 2006;258(1-3):37–49. [Google Scholar]
- 18.Hecobian A, et al. Water-Soluble Organic Aerosol material and the light-absorption characteristics of aqueous extracts measured over the Southeastern United States. Atmos Chem Phys. 2010;10(13):5965–5977. [Google Scholar]
- 19.Robinson NH, et al. Evidence for a significant proportion of Secondary Organic Aerosol from isoprene above a maritime tropical forest. Atmos Chem Phys. 2011;11(3):1039–1050. [Google Scholar]
- 20.Slowik JG, et al. Photochemical processing of organic aerosol at nearby continental sites: Contrast between urban plumes and regional aerosol. Atmos Chem Phys. 2011;11(6):2991–3006. [Google Scholar]
- 21.Paulot F, et al. Unexpected epoxide formation in the gas-phase photooxidation of isoprene. Science. 2009;325(5941):730–733. doi: 10.1126/science.1172910. [DOI] [PubMed] [Google Scholar]
- 22.Claeys M, et al. Formation of secondary organic aerosols through photooxidation of isoprene. Science. 2004;303(5661):1173–1176. doi: 10.1126/science.1092805. [DOI] [PubMed] [Google Scholar]
- 23.Guenther A, et al. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature) Atmos Chem Phys. 2006;6(11):3181–3210. [Google Scholar]
- 24.Ding X, et al. Spatial and seasonal trends in biogenic secondary organic aerosol tracers and water-soluble organic carbon in the southeastern United States. Environ Sci Technol. 2008;42(14):5171–5176. doi: 10.1021/es7032636. [DOI] [PubMed] [Google Scholar]
- 25.Ng NL, et al. Organic aerosol components observed in Northern Hemispheric datasets from Aerosol Mass Spectrometry. Atmos Chem Phys. 2010;10(10):4625–4641. [Google Scholar]
- 26.Nguyen TB, et al. Organic aerosol formation from the reactive uptake of isoprene epoxydiols (IEPOX) onto non-acidified inorganic seeds. Atmos Chem Phys. 2014;14(7):3497–3510. [Google Scholar]
- 27.Fountoukis C, Nenes A. ISORROPIA II: A computationally efficient thermodynamic equilibrium model for K+−Ca2+−Mg2+−NH4+−Na+−SO42–NO3−–Cl−–H2O aerosols. Atmos Chem Phys. 2007;7(17):4639–4659. [Google Scholar]
- 28.Cerully K, et al. On the link between hygroscopicity, volatility, and oxidation state of ambient and water-soluble aerosol in the southeastern United States. Atmos Chem Phys Discuss. 2014 14(22):30835–30877. [Google Scholar]
- 29.Guo H, et al. Particle water and pH in the southeastern United States. Atmos Chem Phys Discuss. 2014;14(19):27143–27193. [Google Scholar]
- 30.Whalen DL. Buffer catalysis in epoxide hydrolyses. J Am Chem Soc. 1973;95(10):3432–3434. [Google Scholar]
- 31.Piletic IR, Edney EO, Bartolotti LJ. A computational study of acid catalyzed aerosol reactions of atmospherically relevant epoxides. Phys Chem Chem Phys. 2013;15(41):18065–18076. doi: 10.1039/c3cp52851k. [DOI] [PubMed] [Google Scholar]
- 32.Eddingsaas NC, VanderVelde DG, Wennberg PO. Kinetics and products of the acid-catalyzed ring-opening of atmospherically relevant butyl epoxy alcohols. J Phys Chem A. 2010;114(31):8106–8113. doi: 10.1021/jp103907c. [DOI] [PubMed] [Google Scholar]
- 33.Surratt JD, et al. Organosulfate formation in biogenic secondary organic aerosol. J Phys Chem A. 2008;112(36):8345–8378. doi: 10.1021/jp802310p. [DOI] [PubMed] [Google Scholar]
- 34.Hu KS, Darer AI, Elrod MJ. Thermodynamics and kinetics of the hydrolysis of atmospherically relevant organonitrates and organosulfates. Atmos Chem Phys. 2011;11(16):8307–8320. [Google Scholar]
- 35.Kampf CJ, et al. Effective Henry’s law partitioning and the salting constant of glyoxal in aerosols containing sulfate. Environ Sci Technol. 2013;47(9):4236–4244. doi: 10.1021/es400083d. [DOI] [PubMed] [Google Scholar]
- 36.Pye HOT, et al. Epoxide pathways improve model predictions of isoprene markers and reveal key role of acidity in aerosol formation. Environ Sci Technol. 2013;47(19):11056–11064. doi: 10.1021/es402106h. [DOI] [PubMed] [Google Scholar]
- 37.Carlton AG, Turpin BJ. Particle partitioning potential of organic compounds is highest in the Eastern US and driven by anthropogenic water. Atmos Chem Phys. 2013;13(20):10203–10214. [Google Scholar]
- 38.Carlton AG, Pinder RW, Bhave PV, Pouliot GA. To what extent can biogenic SOA be controlled? Environ Sci Technol. 2010;44(9):3376–3380. doi: 10.1021/es903506b. [DOI] [PubMed] [Google Scholar]
- 39.Hidy GM, et al. Chemical climatology of the southeastern United States, 1999−2013. Atmos Chem Phys. 2014;14(21):11893–11914. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.