Locomotion, described as “the combination of forward progression carrying the animal from one location to the next, and stopping/scanning involving investigation of particular locations” (1), is an innate behavior heavily used as a defining phenotypic trait in animal studies related to motor disorders, including Parkinson’s disease. Hence, it is important to design and analyze behavioral experiments in ways that allow the distinction between motor vs. cognitive contributions to movement patterns. In a recent study on the effect of selectively reducing glutamate transmission in a subtype 2 Vesicular glutamate transporter (Vglut2)-expressing subpopulation of the subthalamic nucleus [Vglut2f/f;Pitx2-Cre conditional knockout (cKO) mice], Schweizer et al. use an elegant multidisciplinary methodology that combines behavioral, anatomical, electrophysiological, and molecular experiments (2). Notwithstanding the power of their molecular approach, we have strong reservations about Schweizer et al.’s interpretation of the behavioral assessment. The authors measure spontaneous locomotion over 60 min in an “open field setting” on 2 consecutive days; they find increased total activity in cKO mice and conclude that the selective genetic manipulation is sufficient to cause hyperlocomotion while largely sparing cognitive and affective behavior. Here we argue that their data do not unequivocally establish the former and only incompletely examine the latter.
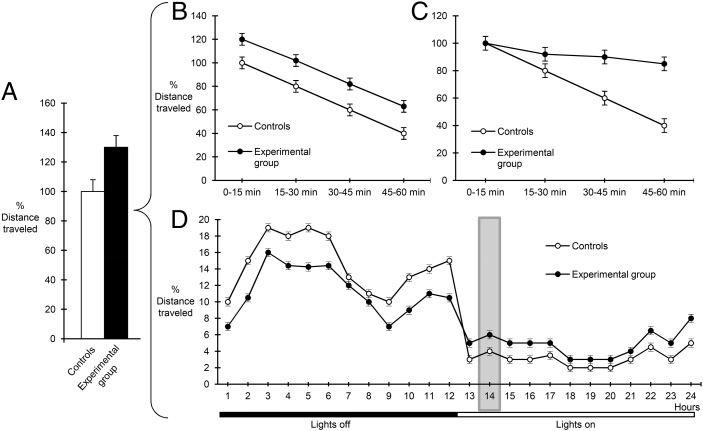
To establish a purely motor deficit, it is essential to control the degree of environmental novelty to exclude differences in the process of habituation. The experimental design in Schweizer et al. (2) is ambiguously described and the environment appears to be neither completely novel nor fully familiar. In addition, activity is measured over a 1-h period (Fig. 1A), which precludes the differentiation between true hyperactivity, where experimental animals cover greater distances throughout the observation period (Fig. 1B), and a habituation deficit, where animals fail to show the expected activity reduction with time (Fig. 1C). The significant increase in vertical locomotion (rearings), considered a reliable indicator of environmental novelty (3) and observed in cKO animals (only or particularly) on the second day, would be consistent with the interpretation of deficient habituation. Hence, a more detailed analysis of horizontal/vertical activity in 5- to 15-min periods would be required to establish a genuine hyperlocomotion phenotype.
Fig. 1.
Graphic illustration of different movement patterns that can lead to increases in apparent animal locomotion. (A) Measurement of the total distance covered in a 60-min period in a locomotor activity task shows a 30% increase in apparent locomotion in experimental (black bar) vs. control animals (white bar). Such an increase in total movement in a novel environment can reflect either genuine hyperactivity (B), with greater distances equally spread over the entire 1-h observation period, or a deficit in habituation (C), where the two groups of animals show similar initial mobility (first 15-min period) but fail to adapt to the novel environment with time (subsequent three 15-min periods). (D) In a familiar home-cage environment, a 30% increase in locomotion observed in the experimental animals during a 1-h period in the active phase (time point 14, shaded area), as reported in Schweizer et al. (2), could reflect alterations in locomotor circadian rhythm and not the animals’ overall activity levels (identical total values in the two groups over the 24 h).
Even if the testing environment were more closely approximated as “familiar,” the justification of hyperlocomotion would require examining the circadian rhythm of motor activity. As illustrated in Fig. 1D, a snapshot of measured activity during a 1-h period of the cycle can lead to the misleading impression of hyperactivity (Fig. 1A), as opposed to an altered circadian pattern. Indeed, given the involvement of the subthalamic nucleus in sleep patterns (4), this interpretation cannot be excluded on the basis of currently reported data.
A final note of caution concerns the purported lack of cognitive side-effects, which are not sufficiently explored. Additionally, the significantly increased number of incomplete trials of cKO mice in the radial maze should not be considered as “minor exceptions” in normal cognitive behavior (2). The subthalamic nucleus receives inputs from frontal areas (in rodents, prelimbic-medial orbital areas) involved in executive control (5), and incomplete visits suggest that cKO mice cannot sustain an efficient pattern of self-correction. Further investigation would be necessary to differentiate automatic from controlled processing in cKO mice.
Acknowledgments
This work was supported by FP7-Regpot Infrastructure Grant TRANSMED; Onassis and Propondis Foundations fellowships; and funding from the Foundation for Education and European Culture (to E.K.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Drai D, Kafkafi N, Benjamini Y, Elmer G, Golani I. Rats and mice share common ethologically relevant parameters of exploratory behavior. Behav Brain Res. 2001;125(1-2):133–140. doi: 10.1016/s0166-4328(01)00290-x. [DOI] [PubMed] [Google Scholar]
- 2.Schweizer N, et al. Limiting glutamate transmission in a Vglut2-expressing subpopulation of the subthalamic nucleus is sufficient to cause hyperlocomotion. Proc Natl Acad Sci USA. 2014;111(21):7837–7842. doi: 10.1073/pnas.1323499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lever C, Burton S, O’Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci. 2006;17(1-2):111–133. doi: 10.1515/revneuro.2006.17.1-2.111. [DOI] [PubMed] [Google Scholar]
- 4.Vetrivelan R, Qiu MH, Chang C, Lu J. Role of basal ganglia in sleep-wake regulation: Neural circuitry and clinical significance. Front Neuroanat. 2010;4:145. doi: 10.3389/fnana.2010.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jahanshahi M. Effects of deep brain stimulation of the subthalamic nucleus on inhibitory and executive control over prepotent responses in Parkinson’s disease. Front Syst Neurosci. 2013;7:118. doi: 10.3389/fnsys.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]