Significance
Obesity and associated metabolic disorders (e.g., cardiovascular disease and type 2 diabetes) are major public health concerns. These disorders result, in part, from hormonal dysregulation, particularly of glucocorticoids (GCs; central regulators of metabolism and adipogenesis). The specific mechanisms by which GCs modulate these processes remain largely unknown, but GCs increase production of endocannabinoids—potent central and peripheral regulators of appetite, energy balance, and metabolism. Our results show that sustained exposure to GCs produces obesity and metabolic syndrome through a peripheral endocannabinoid mechanism. These data further our understanding of the role of endocannabinoid signaling to promote not only diet-induced, but also, hormonal-mediated obesity and support the argument that peripheral blockade of endocannabinoid signaling could be a potential treatment for obese conditions.
Keywords: corticosterone, 2-AG, anandamide, obesity, liver
Abstract
Glucocorticoids are known to promote the development of metabolic syndrome through the modulation of both feeding pathways and metabolic processes; however, the precise mechanisms of these effects are not well-understood. Recent evidence shows that glucocorticoids possess the ability to increase endocannabinoid signaling, which is known to regulate appetite, energy balance, and metabolic processes through both central and peripheral pathways. The aim of this study was to determine the role of endocannabinoid signaling in glucocorticoid-mediated obesity and metabolic syndrome. Using a mouse model of excess corticosterone exposure, we found that the ability of glucocorticoids to increase adiposity, weight gain, hormonal dysregulation, hepatic steatosis, and dyslipidemia was reduced or reversed in mice lacking the cannabinoid CB1 receptor as well as mice treated with the global CB1 receptor antagonist AM251. Similarly, a neutral, peripherally restricted CB1 receptor antagonist (AM6545) was able to attenuate the metabolic phenotype caused by chronic corticosterone, suggesting a peripheral mechanism for these effects. Biochemical analyses showed that chronic excess glucocorticoid exposure produced a significant increase in hepatic and circulating levels of the endocannabinoid anandamide, whereas no effect was observed in the hypothalamus. To test the role of the liver, specific and exclusive deletion of hepatic CB1 receptor resulted in a rescue of the dyslipidemic effects of glucocorticoid exposure, while not affecting the obesity phenotype or the elevations in insulin and leptin. Together, these data indicate that glucocorticoids recruit peripheral endocannabinoid signaling to promote metabolic dysregulation, with hepatic endocannabinoid signaling being especially important for changes in lipid metabolism.
Obesity and associated cardiometabolic diseases, such as type 2 diabetes, represent major contributors to morbidity and mortality (1, 2). Persistent exposure to environmental and psychological stress and the concomitant increase in circulating glucocorticoids (GCs; the primary stress hormones) are believed to be contributing factors to the epidemic of obesity and metabolic syndrome (3–6). Furthermore, patients receiving exogenous GCs subsequent to an organ transplant or for the treatment of inflammatory-related illnesses show several symptoms of the metabolic syndrome (7). Despite this relationship, the mechanisms by which GCs produce these changes in weight regulation and metabolism remain unclear.
It has been clearly shown that GCs can mobilize the endocannabinoid (eCB) system, which is essential for many of the effects of GCs, including negative feedback regulation of the hypothalamic–pituitary–adrenal axis, suppression of sexual behavior, and alterations in memory consolidation (8–11). eCBs are also potent regulators of feeding and metabolism, with effects that parallel those of GCs, such as increased feeding, reduced energy expenditure, fat accumulation within the liver, dyslipidemia, and development of adiposity and obesity (12–16). These observations led us to hypothesize that long-term exposure to elevations in GCs results in a hyperactive eCB system, which contributes to metabolic syndrome and obesity. Using a combination of genetic and pharmacological tools to ablate the eCB system, we show that eCB signaling through the CB1 receptor (CB1R) within the periphery contributes to the development of obesity and metabolic syndrome in a mouse model of excess GC exposure. Moreover, these effects seem independent of central feeding mechanisms. In this study, we use our group’s recently characterized noninvasive approach to deliver a high dose of corticosterone (CORT; 100 μg/mL) in the drinking water to mice, resulting in the rapid development of a metabolic syndrome phenotype (14). This model mirrors clinical symptoms observed in chronic CORT treatment (such as after organ transplantation or for the treatment of inflammatory disease). Our findings substantiate the role of the eCB system in obesity and metabolic syndrome and extend these findings to show an important role of eCB signaling in hormonally mediated obesity. This pattern of results supports the development of peripherally restricted CB1R antagonists as possible therapeutics for these conditions.
Results
Metabolic Assessment of Mice Treated with CORT.
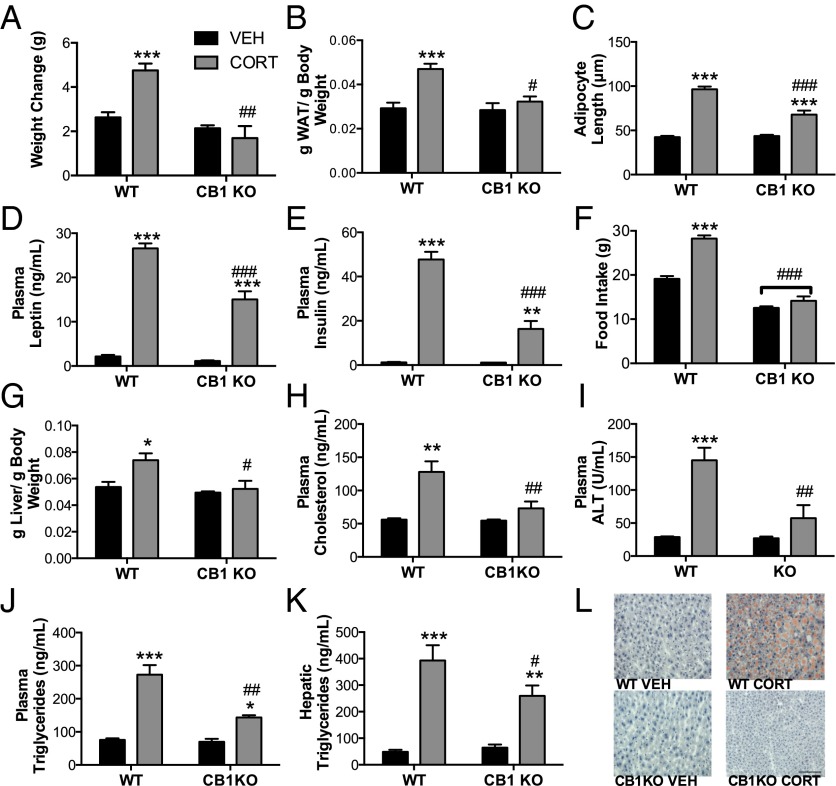
Consistent with previous data from our group (14, 17), mice exposed to CORT rapidly developed a metabolic syndrome phenotype characterized by significant increases in body weight, adiposity, hyperlipidemia, hyperleptinemia, hyperglycemia, hyperinsulinemia, hyperphagia, hepatic steatosis, and an array of other metabolic measures (Fig. 1 and Fig. S1). As long-term GC exposure has been shown to stimulate hunger and increase the rewarding aspects of food intake (18–21), we conducted a pair-feeding study to further explore if hyperphagia contributed to the obesity phenotype shown herein. CORT-treated animals became comparably obese, even when the amount of food was restricted to that consumed by untreated mice (Fig. S2); thus, the ability of CORT to induce obesity does not require hyperphagia and is likely the result of changes in metabolism. To probe this possibility, we determined the respiratory quotient (RQ) in CORT-treated mice as a measure of fuel-partitioning patterns. Mice exposed to chronic CORT exhibit a range from 0.91 to 0.97 compared with from 0.77 to 0.95 in vehicle-treated mice, suggesting a preference for carbohydrate oxidation and an inability to fluctuate between carbohydrate oxidation and fatty acid oxidation (Fig. S3).
Fig. 1.
CB1R signaling is required for GC-mediated metabolic abnormalities. Graphs show that CORT-treated CB1R−/− mice have reduced (A) weight, (B) adiposity, and (C) adipocyte size. Similarly, (D) plasma insulin and (E) plasma leptin as measured by ELISA show a blunted CORT-induced increase compared with WT. (F) The CORT-induced increase in food consumption is reduced in CB1R−/− mice as shown in week 4 of food intake; however, pair-feeding studies show that this hyperphagia does not mediate the development of obesity (Fig. S2). CB1R−/− mice are also protected against the CORT-induced increase in (G) liver weight, (H) plasma cholesterol, (I) alanine aminotransferase (ALT), (J) triglycerides, and (K) hepatic triglycerides. (L) CB1R−/− mice are also protected against the development of hepatic steatosis as noted by the decreased accumulation of lipid droplets in the liver as measured by Oil Red O staining as well as decreased macrovesicular steatosis as measured by H&E staining (Fig. S1B). Data are expressed as means ± SEMs (n = 4–5 per group). Asterisks indicate the significant effects of CORT treatment relative to vehicle treatment in mice. Pound signs indicate statistically significant differences between CORT-treated WT and CB1R−/− mice. VEH, vehicle. *P < 0.05; **P < 0.01; ***P < 0.001; #P < 0.05; ##P < 0.01; ###P < 0.001. (Scale bar, 100 µm.)
Genetic and Pharmacological Evidence That CORT-Induced Metabolic Dysregulation Is CB1R-Dependent.
To elucidate the role of CB1R signaling in metabolic dysregulation, global CB1R KO (CB1R−/−) mice and their WT littermates were exposed to the drinking water CORT model for 28 d. Genetic ablation of the CB1R significantly attenuated or completely abolished the effects of chronic CORT on the metabolic measures (Fig. 1 and Fig. S1). Circulating CORT levels and the degree of splenic atrophy induced by CORT were comparable in WT and CB1R−/− mice, supporting the notion that differences in the CORT effect in WT and CB1R−/− mice were not caused by differences in exposure to CORT (Fig. S4). CORT-treated CB1R−/− mice had an RQ range of 0.87–0.96 resulting from greater fatty acid oxidation compared with WT CORT mice (RQ range = 0.91–0.97) (Fig. S3). Together, these data support the hypothesis that CB1R-mediated signaling is required for metabolic dysregulation induced by chronic GC exposure.
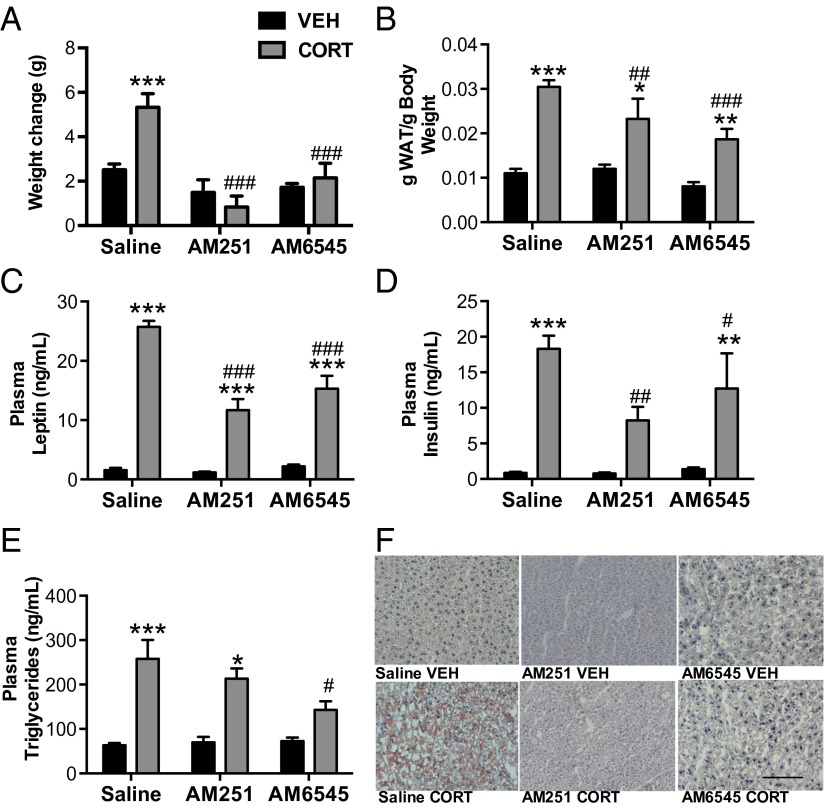
To further establish the role of the eCB system in GC-induced metabolic syndrome, WT mice were treated with daily injections of the global CB1R antagonist AM251 (2 mg/kg) throughout the 28-d CORT treatment. Consistent with the effects seen in CB1R−/− mice, AM251 treatment significantly attenuated CORT-induced increases in body weight, adiposity, circulating leptin, and insulin (Fig. 2). AM251 treatment also resulted in a reduction of CORT-stimulated fat accumulation in the liver (Fig. 2F), confirming the hypothesis that CB1R signaling is necessary for the development of CORT-induced obesity and metabolic alterations. These data are consistent with findings that CB1R signaling is elevated in obese individuals and functionally contributes to the development of metabolic syndrome (13).
Fig. 2.
Blockade of the CB1R modulates the effect of chronic CORT. Concurrent treatment of CORT in the water for 28 d with either the global CB1R antagonist AM251 or the peripheral specific CB1R antagonist AM6545 results in decreased (A) weight, (B) adiposity, (C) circulating plasma leptin, and (D) insulin compared with WT controls. (E) Triglycerides were significantly decreased in AM6545-treated mice but remained unaltered in AM251-treated mice. (F) However, both AM251 and AM6545 treatments prevent development of hepatic steatosis as indicated by Oil Red O staining. Data are expressed as means ± SEMs (n = 4–9 per group). Asterisks indicate the significant effects of CORT treatment relative to vehicle treatment in mice. Pound signs indicate the effect of AM251 or AM6545 compared with saline in CORT-treated mice. VEH, vehicle. *P < 0.05; **P < 0.01; ***P < 0.001; #P < 0.05; ##P < 0.01; ###P < 0.001. (Scale bar, 100 µm.)
Alterations in the Central and Peripheral eCB Systems in CORT-Treated Mice.
Metabolic function in several organ systems is modulated by eCB signaling (13). Within the brain, hypothalamic eCB signaling stimulates increased energy intake, whereas in the liver and white adipose tissue (WAT), eCB signaling contributes to various metabolic components of obesity (13, 22). To localize eCB effects in tissues important for metabolic regulation, the eCBs 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (AEA) as well as the related fatty acid ethanolamides N-oleoylethanolamine (OEA) and N-palmitoylethanolamine (PEA) were quantified by MS in the hypothalamus, blood, WAT, and liver (Table 1). CORT treatment did not alter the content of AEA or PEA in the hypothalamus but decreased the amount of OEA and 2-AG, supporting the argument against a central site of action for enhanced eCB driving the CORT-mediated metabolic phenotype through an increase in feeding (23).
Table 1.
The effects of chronic CORT administration on eCB levels
| Tissue | AEA | 2-AG | PEA | OEA |
| Hypothalamus tissue (pmol/g) | ||||
| Vehicle | 6.74 ± 0.39 | 4.46 × 104 ± 4.9 | 222.56 ± 19.93 | 109.98 ± 6.49 |
| CORT | 6.05 ± 0.64 | 3.17 × 104 ± 2.5* | 214.62 ± 4.87 | 90.04 ± 2.9* |
| Blood serum (pmol/mL) | ||||
| Vehicle | 5.82 ± 0.920 | 8.55 ± 1.26 | 22.74 ± 2.67 | 8.23 ± 0.43 |
| CORT | 12.129 ± 2.737* | 9.72 ± 2.14 | 39.05 ± 6.38* | 10.17 ± 0.82* |
| WAT tissue (pmol/g) | ||||
| Vehicle | 9.68 ± 0.97 | 2,008 ± 634 | 788.9 ± 116.3 | 301.2 ± 30.18 |
| CORT | 5.93 ± 0.39† | 800.05 ± 95.99 | 590.0 ± 148.3 | 216.5 ± 40.57 |
| Liver tissue(pmol/g) | ||||
| Vehicle | 4.51 ± 0.86 | 840 ± 70 | 389.76 ± 29.56 | 70.19 ± 8.45 |
| CORT | 8.23 ± 1.10† | 270 ± 20‡ | 503.72 ± 37.12* | 32.85 ± 3.01† |
Data are expressed as means ± SEMs. For eCB measures, n = 4–5 per group for hypothalamus, and n = 8–10 per group for blood, WAT, and liver.
Significant difference at P < 0.05.
Significant difference at P < 0.01.
Significant difference at P < 0.001.
The liver and WAT are the primary organ systems identified as being responsible for the regulation of metabolism by eCB signaling (22, 24). CORT exposure nearly doubled hepatic AEA compared with vehicle-treated controls. Liver PEA content was similarly increased, but both OEA and 2-AG contents were significantly reduced by chronic CORT exposure. Although the decline in 2-AG content was somewhat unexpected, elevation in AEA content (without a concomitant increase in 2-AG) is consistent with results after DIO (25). CORT exposure significantly decreased AEA in the WAT and tended to decrease 2-AG (nearly reaching statistical significance), an effect mirroring animal models of diet-induced obesity (DIO) (26). In humans, circulating concentrations of these blood lipids positively relate to body mass index and obesity (16, 27), motivating us to examine circulating levels of eCBs for comparison. Chronic CORT treatment doubled plasma concentrations of AEA and increased concentrations of PEA and OEA as well, whereas plasma 2-AG concentrations were unaffected by CORT.
To further characterize the eCB system in these peripheral compartments, we examined the effects of CORT on gene expression of components of the eCB system within the liver and WAT. Specifically, we quantified mRNA for N-acyl-phosphatidylethanolamine–specific phospholipase D and fatty acid amide hydrolase (putative synthetic and catabolic enzymes, respectively) for AEA in liver and WAT (Table 2). Hepatic levels of N-acyl-phosphatidylethanolamine–specific phospholipase D mRNA were significantly increased, whereas fatty acid amide hydrolase mRNA levels were significantly reduced, consistent with increased liver contents of AEA and PEA. Additionally, mRNA for the CB1R was increased within the liver after CORT treatment. There were no effects of CORT on the synthetic or catabolic enzymes responsible for 2-AG, diacylglycerol lipase-α, and monoacylglycerol lipase. Given the significant drop in 2-AG content, the lack of change in these catabolic enzymes is surprising but could be explained by alterations in enzymatic activity rather than expression levels. In WAT, the only significant effect of chronic CORT exposure was decreased expression of CB1R mRNA. In whole, our data suggest that CORT exposure significantly increases hepatic AEA/CB1R signaling, which was observed in DIO (22, 25), leading to the hypothesis that liver CB1R signaling is necessary for chronic CORT to produce symptoms of metabolic disorder.
Table 2.
The effects of chronic CORT administration on mRNA of eCB parameters
| Tissue | CB1R | FAAH | NAPE-PLD | MAGL | DAGL |
| WAT fold change | |||||
| Vehicle | 1.84 ± 0.35 | 1.12 ± 0.28 | 1.10 ± 2.4 | 1.11 ± 0.36 | 1.11 ± 0.26 |
| CORT | 0.48 ± 0.16* | 0.33 ± 0.14* | 0.92 ± 0.18 | 0.66 ± 0.24 | 0.86 ± 0.30 |
| Liver fold change | |||||
| Vehicle | 1.01 ± 0.11 | 1.07 ± 0.09 | 0.73 ± 0.19 | 1.02 ± 0.05 | 1.46 ± 0.67 |
| CORT | 1.32 ± 0.03* | 0.41 ± 0.016† | 3.35 ± 0.37‡ | 1.41 ± 0.30 | 1.16 ± 0.39 |
Data are expressed as means ± SEMs (n = 3–4 per group). DAGL, diacylglycerol lipase; FAAH, fatty acid amide hydrolase; MAGL, monoacylglycerol lipase; NAPE-PLD, N-acyl-phosphatidylethanolamine–specific phospholipase D.
Significant difference at P < 0.05.
Significant difference at P < 0.01.
Significant difference at P < 0.001.
Peripheral CB1R Antagonism Prevents Metabolic Dysregulation.
Our data showing elevation in both circulating and hepatic AEA levels implicate a role for eCB signaling in peripheral compartments. To dissociate the central and peripheral effects of CB1R signaling, we used the peripherally restricted, neutral (i.e., does not exhibit inverse agonist activity) CB1R antagonist AM6545 (10 mg/kg) at a dose shown to exclusively occupy peripheral CB1Rs (with no brain penetrance) and prevent the metabolic effects of DIO (28). Concurrent treatment with AM6545 and CORT resulted in blockade of CORT-induced weight gain, a significant decrease in CORT-induced adiposity, and blunting of elevations in circulating levels of leptin, insulin, and triglycerides (Fig. 2). AM6545 also prevented the development of hepatic steatosis relative to vehicle-treated animals exposed to CORT.
Liver-Specific Deletion of CB1R Prevents CORT-Mediated Dyslipidemia but Not Obesity.
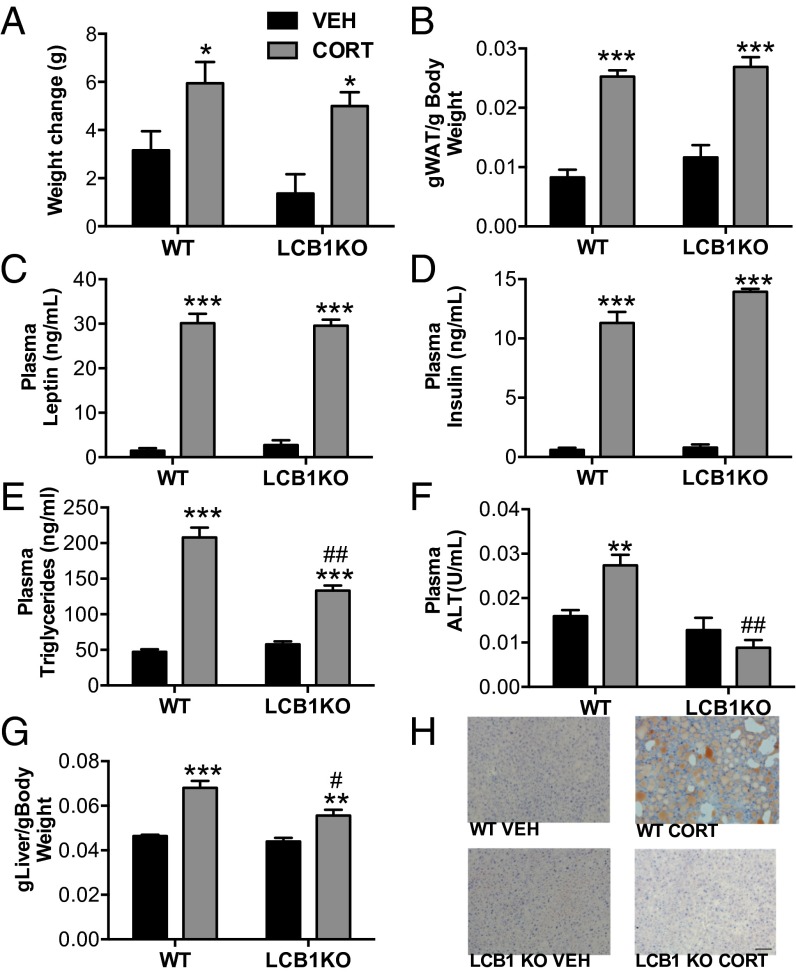
Based on the results of our physiological, histological, and biochemical data, localizing the peripheral organ responsible for mediating the effects of eCB signaling on CORT-induced metabolic effects was important. Hepatocyte-specific CB1R−/− (LCB1R−/−) mice are resistant to DIO-induced lipid dysregulation (22, 29, 30) and provide a powerful tool to explore involvement of liver eCB signaling. LCB1R−/− mice revealed a unique role of liver eCB signaling in the metabolic effects of chronic CORT. The effects of chronic CORT on body weight, leptin, and insulin were not different between WT and LCB1R−/− mice. However, the LCB1R−/− mice exhibited significantly reduced effects of CORT on all measures of dyslipidemia, including plasma triglyceride and alanine aminotransferase (ALT) concentrations, elevated liver weight, and hepatic steatosis (Fig. 3). The pattern of effects seen in the LCB1R−/− mice is largely consistent with the protection against the hepatic effects of DIO afforded by loss of CB1R in hepatocytes (22). We conclude that enhanced hepatic AEA/CB1R signaling mediates the dyslipidemic component of GC-induced metabolic syndrome: nonhepatic CB1R signaling processes elsewhere in the periphery, such as adipose tissue, muscle, kidney, or pancreas (all of which have been shown to play some role in the metabolic effects of eCB signaling), mediate the effects of CORT on adiposity, hyperleptinemia, and hyperinsulinemia.
Fig. 3.
Liver-specific CB1R−/− reveals a unique role of hepatic signaling in CORT-treated mice. LCB1R−/− does not prevent the CORT-induced (A) weight gain, (B) increased adiposity, or increased (C) plasma leptin and (D) insulin. In contrast, LCB1R−/− does attenuate CORT-induced increases in (E) plasma triglycerides, (F) plasma alanine aminotransferase (ALT), and (G) hepatic triglycerides. (H) Furthermore, liver-specific KO of CB1R prevents development of hepatic steatosis as indicated by Oil Red O staining. Data are expressed as means ± SEMs (n = 6–8 per group). Asterisks indicate the significant effects of CORT treatment relative to vehicle treatment in mice. Pound signs indicate statistically significant differences between CORT-treated WT and LCB1R−/− mice. VEH, vehicle. *P < 0.05; **P < 0.01; ***P < 0.001; #P < 0.05; ##P < 0.01. (Scale bar, 100 µm.)
Discussion
Weight gain accompanies chronic stress exposure in many humans, and obesity is often associated with changes in the regulation of GC (3, 31–33). Although some (32, 34) but not all (35) studies indicate that adrenalectomy can prevent weight gain in rodent models of obesity, few animal models recapitulate the effects of hypercortisolemia on metabolic function. Our model reliably produces rapid and significant changes in body weight, feeding, and metabolic function in response to excess GC treatment, allowing for the investigation of the mechanisms by which GCs modulate these processes. This study shows that the CB1R is necessary for the development of most of the metabolic effects of excessive GC hormone exposure. Our data indicate a significant role for hepatic CB1R signaling in some of these effects, showing an important role of hepatocytes in the dyslipidemic effects of long-term hypercortisolemia. This study builds on the established obesogenic function of eCB signaling in response to dietary fat consumption by extending eCB involvement to hormonal mediators of metabolic dysregulation.
From our data, we also postulate an interaction between GCs and eCBs in the effects of DIO. Given that some studies have shown that DIO is dependent on both GC levels (32) and eCB signaling (36), our findings lead us to posit that GCs may be the mediating factor in mobilizing eCB signaling after high-fat feeding. Given our results and the findings of others (37), it seems likely that these two signaling systems function cooperatively to promote the development of metabolic syndrome. This data is consistent with data from other physiological systems, whereby many effects of GCs are mediated by increased eCB signaling (8). The mechanisms by which GCs increase eCB signaling are not well-understood, and different mechanisms seem to act in different tissues. Within the brain, evidence for both nongenomic and genomic (38–41) means for GC-induced eCB signaling exists. Our data suggest that, at least within the liver, these effects are likely mediated by genomic mechanisms, because sustained exposure to CORT resulted in changes in gene expression of many components of the eCB system that mapped onto functional changes in AEA levels and CB1R signaling. More so, these data are consistent with reports in humans that circulating cortisol levels positively correlate with circulating levels of both AEA and PEA (42, 43). The specific mechanism of these interactions requires additional study, but our data support the overarching hypothesis that cross-talk between GC and eCB signaling is an important relationship for many physiological processes, including metabolic function.
The ability of eCB signaling to regulate metabolic function is clearly a complex process that involves many sites of action (12, 13). There is substantial evidence that eCB signaling within the brain, particularly within the hypothalamus (as well as the nucleus accumbens and olfactory bulb) (23, 44–49), promotes feeding behavior and its regulation by satiety state. Similarly, there is experimental evidence for both central and peripheral sites of action by which eCB signaling can modulate metabolic processes (12, 13, 50, 51), particularly through the regulation of sympathetic tone in adipose tissue. Peripheral eCB signaling has been suggested to account for various aspects of metabolic dysregulation in numerous tissue depots. Hepatic eCB signaling has been shown to promote de novo hepatic lipogenesis, hepatic steatosis, and global dysregulation of lipid metabolism (22, 29, 30, 52), whereas preliminary evidence suggests that CB1R signaling within adipose tissue is important for the development of obesity and insulin resistance (53). Consistent with these findings, our data indicate that CB1R within the liver drives the dyslipemic component of excess GC exposure, whereas blockade of all CB1R in the periphery attenuates all aspects of the metabolic dysregulation. Based on the data from the adipose-specific CB1R−/− mice (53), we predict that this tissue depot contributes significantly to many of the remaining metabolic effects of GCs that are not attributable to CB1R within the liver. The exception here may be the residual leptin signaling produced by CORT treatment that was not reliably reversed by any form of CB1R blockade or inactivation. Because there is evidence that CORT can directly drive leptin production in adipocytes (14, 54–57), the hyperleptinemic effects of GCs seem to be independent of CB1R activity and are likely mediated by direct genomic actions of GCs. Future research will be required to examine this possibility.
Although their efficacy was promising in reducing the morbid effects of obesity, global CB1R antagonists have been abandoned for the treatment of metabolic disorders because of centrally mediated psychiatric side effects (28). However, data from DIO models and our GC model indicate that eCB signaling seems to primarily mediate metabolic function through peripheral mechanisms. As such, peripherally restricted CB1R antagonists may prove to be a valuable tool in the treatment of metabolic conditions without adverse psychiatric side effects, particularly those that are neutral antagonists and lack inverse agonist activity. Our data indicate the importance of peripheral eCB signaling in GC-mediated metabolic dysregulation and support the clinical investigation of peripherally restricted CB1R in the treatment of both diet-induced and hormonally mediated forms of obesity.
Materials and Methods
Animals and Protocols.
The CB1R−/− global and liver-specific mice used in this study were originally generated and backcrossed to a C57/Bl6J background (22). LCB1R−/− mice were generated by crossing mice homozygous for the CB1-floxed allele (CB1f/f), which were on a predominantly C57BL/6N background (seven to eight crossings), with mice expressing the bacterial Cre recombinase driven by the mouse albumin promoter (triglyceride[Alb-cre]′21 Mgn) that had been backcrossed seven times to a C57BL/6J background [Jackson Laboratory (58)] to obtain CB1f/f × CB1f/fAlbCre breeding pairs. All animal procedures were undertaken with approval of The Rockefeller University’s Institutional Animal Care and Use Committee.
Pharmacological Manipulations.
The general procedure for CORT-treated mice was conducted as previously described (14). Chronic treatment with AM251 [Tocris: DMSO; Tween 80: 0.9% saline 2:1:97 (vol/vol)] or AM6545 [DMSO; Tween 80: 0.9% saline 2:1:97 (vol/vol)] commenced concurrently with CORT in drinking water. One hour before lights off, mice were injected i.p. with vehicle [DMSO; Tween 80: 0.9% saline 2:1:97 (vol/vol)], AM251 at 2 mg/kg, or AM6545 at 10 mg/kg.
eCB Extraction and Analysis.
Brain regions and liver samples were subjected to a lipid extraction process as described previously (59), and blood was analyzed as described elsewhere (60). eCB analysis for adipose tissue (61) is described in SI Materials and Methods. Oil Red O and H&E histology, pair feeding, quantitative RT-PCR (see Table S1 for a list of primers used), indirect calorimetery and derived metabolic measures (28), measurements of hepatic triglycerides, and measurements of very LDL triglyceride production are described in SI Materials and Methods.
Statistical Analyses.
For all statistical analyses, we used Prism 5 (GraphPad Software, Inc.). Independent t test, one- or two-way ANOVA, or repeated measures ANOVAs were undertaken where appropriate. Our a priori hypothesis is that the CB1R−/− mice will be resistant to the effects of CORT administration, and therefore, regardless of significance of the interactions, Bonferroni posttests were used to examine differences in all variables among treatment conditions. In all cases, results were considered significant at P < 0.05. All statistical tests and results from these experiments can be seen in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Sarah Bhagat for her technical assistance at the early stage of this study. This research was supported, in part, by an operating grant from the Hope for Depression Research Foundation and an unrestricted operating grant from Johnson and Johnson Pharmaceuticals (to B.S.M.). NIH Grants DK 020541 Einstein DRTC Animal Physiology Core (to G.J.S.), DA9158 (to A.M.M.), DA23142 (to A.M.M.), and DA026996 (to C.J.H.); the Research and Education Component of the Advancing a Healthier Wisconsin Endowment to the Medical College of Wisconsin; and intramural funds from the National Institute on Alcohol Abuse and Alcoholism, NIH (G.K.) also supported this research. N.P.B. was a recipient of a predoctoral Ford Foundation Fellowship. M.N.H. is the recipient of a Tier II Canada Research Chair, and this research was supported by an operating grant from the Natural Sciences and Engineering Research Council of Canada.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421420112/-/DCSupplemental.
References
- 1.Rosenzweig JL, et al. Endocrine Society Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(10):3671–3689. doi: 10.1210/jc.2008-0222. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. American Heart Association National Heart, Lung, and Blood Institute Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 3.Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21(3):159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2-3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wardle J, Chida Y, Gibson EL, Whitaker KL, Steptoe A. Stress and adiposity: A meta-analysis of longitudinal studies. Obesity (Silver Spring) 2011;19(4):771–778. doi: 10.1038/oby.2010.241. [DOI] [PubMed] [Google Scholar]
- 6.Patterson ZR, Abizaid A. Stress induced obesity: Lessons from rodent models of stress. Front Neurosci. 2013;7(2013):130. doi: 10.3389/fnins.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trikudanathan S, McMahon GT. Optimum management of glucocorticoid-treated patients. Nat Clin Pract Endocrinol Metab. 2008;4(5):262–271. doi: 10.1038/ncpendmet0791. [DOI] [PubMed] [Google Scholar]
- 8.Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(5):791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campolongo P, et al. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci USA. 2009;106(12):4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coddington E, Lewis C, Rose JD, Moore FL. Endocannabinoids mediate the effects of acute stress and corticosterone on sex behavior. Endocrinology. 2007;148(2):493–500. doi: 10.1210/en.2006-0740. [DOI] [PubMed] [Google Scholar]
- 11.Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151(10):4811–4819. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engeli S, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54(10):2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17(4):475–490. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Karatsoreos IN, et al. Endocrine and physiological changes in response to chronic corticosterone: A potential model of the metabolic syndrome in mouse. Endocrinology. 2010;151(5):2117–2127. doi: 10.1210/en.2009-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser R, et al. Cortisol effects on body mass, blood pressure, and cholesterol in the general population. Hypertension. 1999;33(6):1364–1368. doi: 10.1161/01.hyp.33.6.1364. [DOI] [PubMed] [Google Scholar]
- 16.Björntorp P, Rosmond R. Neuroendocrine abnormalities in visceral obesity. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S80–S85. doi: 10.1038/sj.ijo.0801285. [DOI] [PubMed] [Google Scholar]
- 17.Cassano AE, et al. Anatomic, hematologic, and biochemical features of C57BL/6NCrl mice maintained on chronic oral corticosterone. Comp Med. 2012;62(5):348–360. [PMC free article] [PubMed] [Google Scholar]
- 18.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology. 2004;145(8):3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 19.Dallman MF, et al. Minireview: Glucocorticoids—food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145(6):2633–2638. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- 20.De Vriendt T, Moreno LA, De Henauw S. Chronic stress and obesity in adolescents: Scientific evidence and methodological issues for epidemiological research. Nutr Metab Cardiovasc Dis. 2009;19(7):511–519. doi: 10.1016/j.numecd.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Santana P, et al. Aldosterone and dexamethasone both stimulate energy acquisition whereas only the glucocorticoid alters energy storage. Endocrinology. 1995;136(5):2214–2222. doi: 10.1210/endo.136.5.7720670. [DOI] [PubMed] [Google Scholar]
- 22.Osei-Hyiaman D, et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118(9):3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136(4):550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagotto U, et al. How many sites of action for endocannabinoids to control energy metabolism? Int J Obes (Lond) 2006;30(Suppl 1):S39–S43. doi: 10.1038/sj.ijo.0803277. [DOI] [PubMed] [Google Scholar]
- 25.Osei-Hyiaman D, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115(5):1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matias I, et al. Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: Effect of high fat diets. Mol Cell Endocrinol. 2008;286(1-2 Suppl 1):S66–S78. doi: 10.1016/j.mce.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Côté M, et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 2007;31(4):692–699. doi: 10.1038/sj.ijo.0803539. [DOI] [PubMed] [Google Scholar]
- 28.Tam J, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120(8):2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cinar R, et al. Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology. 2014;59(1):143–153. doi: 10.1002/hep.26606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology. 2012;142(5):1218.e1–1228.e1. doi: 10.1053/j.gastro.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29(11):2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- 32.Rothwell NJ, Stock MJ, York DA. Effects of adrenalectomy on energy balance, diet-induced thermogenesis and brown adipose tissue in adult cafeteria-fed rats. Comp Biochem Physiol A. 1984;78(3):565–569. doi: 10.1016/0300-9629(84)90597-8. [DOI] [PubMed] [Google Scholar]
- 33.Mantha L, Palacios E, Deshaies Y. Modulation of triglyceride metabolism by glucocorticoids in diet-induced obesity. Am J Physiol. 1999;277(2 Pt 2):R455–R464. doi: 10.1152/ajpregu.1999.277.2.R455. [DOI] [PubMed] [Google Scholar]
- 34.Makimura H, et al. Adrenalectomy reverses obese phenotype and restores hypothalamic melanocortin tone in leptin-deficient ob/ob mice. Diabetes. 2000;49(11):1917–1923. doi: 10.2337/diabetes.49.11.1917. [DOI] [PubMed] [Google Scholar]
- 35.Makimura H, Mizuno TM, Beasley J, Silverstein JH, Mobbs CV. Adrenalectomy stimulates hypothalamic proopiomelanocortin expression but does not correct diet-induced obesity. BMC Physiol. 2003;3:4. doi: 10.1186/1472-6793-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. J Obes Relat Metab Disord. 2004;28(4):640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- 37.Scerif M, et al. CB1 receptor mediates the effects of glucocorticoids on AMPK activity in the hypothalamus. J Endocrinol. 2013;219(1):79–88. doi: 10.1530/JOE-13-0192. [DOI] [PubMed] [Google Scholar]
- 38.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. J Neurosci. 2003;23(12):4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Shen RY, Haj-Dahmane S. Endocannabinoids mediate the glucocorticoid-induced inhibition of excitatory synaptic transmission to dorsal raphe serotonin neurons. J Physiol. 2012;590(Pt 22):5795–5808. doi: 10.1113/jphysiol.2012.238659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M, et al. Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol. 2012;26(1):56–70. doi: 10.1177/0269881111409606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill MN, et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci. 2011;31(29):10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dlugos A, Childs E, Stuhr KL, Hillard CJ, de Wit H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology. 2012;37(11):2416–2427. doi: 10.1038/npp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill MN, et al. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology. 2013;38(12):2952–2961. doi: 10.1016/j.psyneuen.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Marzo V, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410(6830):822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 45.Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134(6):1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verty AN, McGregor IS, Mallet PE. Paraventricular hypothalamic CB(1) cannabinoid receptors are involved in the feeding stimulatory effects of Delta(9)-tetrahydrocannabinol. Neuropharmacology. 2005;49(8):1101–1109. doi: 10.1016/j.neuropharm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 47.Higuchi S, et al. The cannabinoid 1-receptor silent antagonist O-2050 attenuates preference for high-fat diet and activated astrocytes in mice. J Pharmacol Sci. 2010;112(3):369–372. doi: 10.1254/jphs.09326sc. [DOI] [PubMed] [Google Scholar]
- 48.Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: Anandamide in nucleus accumbens shell enhances 'liking' of a sweet reward. Neuropsychopharmacology. 2007;32(11):2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- 49.Soria-Gómez E, et al. The endocannabinoid system controls food intake via olfactory processes. Nat Neurosci. 2014;17(3):407–415. doi: 10.1038/nn.3647. [DOI] [PubMed] [Google Scholar]
- 50.Quarta C, et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010;11(4):273–285. doi: 10.1016/j.cmet.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 51.Buettner C, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14(6):667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jourdan T, et al. Antagonism of peripheral hepatic cannabinoid receptor-1 improves liver lipid metabolism in mice: Evidence from cultured explants. Hepatology. 2012;55(3):790–799. doi: 10.1002/hep.24733. [DOI] [PubMed] [Google Scholar]
- 53.Mancini GQC, Srivastava RK, Klaus S, Pagotto U, Lutz B. Proceedings of the 20th Annual Symposium on the Cannabinoids. International Cannabinoid Research Society; Research Triangle Park, NC: 2010. Adipocyte-specific CB1 conditional knock-out mice: New insights in the study of obesity and metabolic syndrome; p. 17. [Google Scholar]
- 54.Larsson H, Ahrén B. Short-term dexamethasone treatment increases plasma leptin independently of changes in insulin sensitivity in healthy women. J Clin Endocrinol Metab. 1996;81(12):4428–4432. doi: 10.1210/jcem.81.12.8954054. [DOI] [PubMed] [Google Scholar]
- 55.Miell JP, Englaro P, Blum WF. Dexamethasone induces an acute and sustained rise in circulating leptin levels in normal human subjects. Horm Metab Res. 1996;28(12):704–707. doi: 10.1055/s-2007-979882. [DOI] [PubMed] [Google Scholar]
- 56.Russell CD, et al. Leptin expression in adipose tissue from obese humans: Depot-specific regulation by insulin and dexamethasone. Am J Physiol. 1998;275(3 Pt 1):E507–E515. doi: 10.1152/ajpendo.1998.275.3.E507. [DOI] [PubMed] [Google Scholar]
- 57.Kolaczynski JW, Goldstein BJ, Considine RV. Dexamethasone, OB gene, and leptin in humans; effect of exogenous hyperinsulinemia. J Clin Endocrinol Metab. 1997;82(11):3895–3897. doi: 10.1210/jcem.82.11.4341. [DOI] [PubMed] [Google Scholar]
- 58.Postic C, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274(1):305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 59.Patel S, et al. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005;46(2):342–349. doi: 10.1194/jlr.M400377-JLR200. [DOI] [PubMed] [Google Scholar]
- 60.Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: A preliminary report. Pharmacopsychiatry. 2008;41(2):48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams J, et al. Quantitative method for the profiling of the endocannabinoid metabolome by LC-atmospheric pressure chemical ionization-MS. Anal Chem. 2007;79(15):5582–5593. doi: 10.1021/ac0624086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.