Significance
Due to its bonding environments, carbon can make up numerous compounds with many other elements. However, the abundance and dynamics of carbon in the deep Earth remains uncertain due to its complex behavior during the primary accretion and differentiation of the Earth in its early history. The naturally occurring stable isotopes of carbon serve as a useful tracer to study the carbon cycle, both on the surface and in the deep Earth. Here, a new model is presented for understanding a first-order carbon cycle within the Earth, including the sources of the building blocks and core formation processes. Low carbon isotope compositions of carbonaceous materials from ancient rocks have to be used with a caution as biosignatures.
Keywords: carbon, isotope, deep Earth, diamond, carbide
Abstract
The carbon budget and dynamics of the Earth’s interior, including the core, are currently very poorly understood. Diamond-bearing, mantle-derived rocks show a very well defined peak at δ13C ≈ −5 ± 3‰ with a very broad distribution to lower values (∼−40‰). The processes that have produced the wide δ13C distributions to the observed low δ13C values in the deep Earth have been extensively debated, but few viable models have been proposed. Here, we present a model for understanding carbon isotope distributions within the deep Earth, involving Fe−C phases (Fe carbides and C dissolved in Fe−Ni metal). Our theoretical calculations show that Fe and Si carbides can be significantly depleted in 13C relative to other C-bearing materials even at mantle temperatures. Thus, the redox freezing and melting cycles of lithosphere via subduction upwelling in the deep Earth that involve the Fe−C phases can readily produce diamond with the observed low δ13C values. The sharp contrast in the δ13C distributions of peridotitic and eclogitic diamonds may reflect differences in their carbon cycles, controlled by the evolution of geodynamical processes around 2.5–3 Ga. Our model also predicts that the core contains C with low δ13C values and that an average δ13C value of the bulk Earth could be much lower than ∼−5‰, consistent with those of chondrites and other planetary body. The heterogeneous and depleted δ13C values of the deep Earth have implications, not only for its accretion−differentiation history but also for carbon isotope biosignatures for early life on the Earth.
Major Earth-forming processes (accretion, magma ocean formation, Moon-forming giant impact, and core segregation) might have caused significant loss of the primordial volatiles, including carbon to space and the core. Our current estimate of a carbon budget in the mantle varies more than an order of magnitude (1−40 ×1023 g of C), due to uncertainties in carbon concentrations and proportions of different mantle components (1, 2). Carbon in shallow oceanic mantle generally exists as dissolved CO2 in melts or C−O−H fluids due to low carbon solubility in silicates (3). With increasing depth, various carbonates become the major C-bearing solid phases. Graphite and then diamond become main hosts for C at deeper depths. Recent experimental studies suggest that Earth's mantle as shallow as 150–250 km may be saturated with iron and the Fe–Ni metals (4). In the presence of Fe-rich metals, diamond can be converted to iron carbides (Fe3C and Fe7C3) and dissolved C in Fe−Ni metals (5–7). The occurrence of cohenite, (Fe, Ni)3C, has been well documented from iron meteorites, but only recently, several investigators reported rare terrestrial occurrences of Fe carbides (Fe3C, Fe2C, and Fe23C6) from kimberlite (8), from minerals of subcratonic lithosphere or possible lower-mantle origins (9, 10) and others (11). Thus, Fe carbides and Fe−Ni metals are increasingly recognized as potential C-bearing phases in the lower mantle and core. The occurrence of other carbides (SiC, WC, and Cr3C2) has also been verified. These carbide phases are formed under very reducing conditions at depths and possibly during adiabatic decompression such as eruption (12). Carbon also appears to substitute for oxygen in silicates under deep-Earth conditions (13).
Extensive investigations of various C-bearing phases from mantle-derived volcanic rocks and xenoliths (bulk carbons, C−O−H inclusions, carbonatite, and graphite−diamond) in the past decades (14–18) established a strong, primary mode of δ13C values at −5 ± 3‰. A blurred secondary mode of δ13C values also occurs at −10‰ to −20‰ in diamonds and other forms of carbon with the lowest observed δ13C values of −42‰ (17, 18). Several models have been put forward to explain these low δ13C values. Firstly, mantle carbon could have inherited isotopic heterogeneity of original Earth-building materials despite Earth’s early, violent history. Chondrites and other silicate planetary objects have varying δ13C values of the total carbon (organic matter, graphite, carbide) from −24‰ to +5‰ (2, 19). Secondly, the subduction of lithosphere with marine organic sediments has been widely advocated for the depleted δ13C values of eclogitic and other types of diamond. However, the lack of a carbon component representing the δ13C value of sedimentary carbonates (∼0‰) has been noticed (14, 20), which is far more abundant than organic matter in pelagic marine sediments (21). Nitrogen contents and δ15N values of eclogitic diamonds are also inconsistent with the hypothesis of the recycled organic matter (22). Thirdly, Rayleigh-type isotope fractionation processes at mantle conditions (>1,000 °C) have been proposed to explain the low δ13C values, including diamond deposition from C−O−H fluids, CO2 degassing from carbonatite melts (16, 23, 24). However, only Rayleigh-type fractionation processes to near completion can produce very negative δ13C values, because of small (≤3‰) carbon isotope fractionation among C−O−H fluids, CaCO3, and diamond under mantle conditions.
Very low δ13C values (−18‰ to −35‰) have been reported for SiC from several localities (25). Recently, Mikhail et al. (8) reported that diamond and coexisting iron carbide from Jagersfontein kimberlite, South Africa, have low δ13C values: −12.7‰ to −17.3‰ (diamond) and −18.6‰ to −25.2‰ (cohenite). An increasing number of the occurrences of carbides from the upper and lower mantles, associated with diamond with low δ13C values, points to a genetic relationship between these diamonds with low δ13C values and carbides. Thus, various carbides, particularly the Fe−C phases (dissolved C in Fe−Ni metals and Fe carbides), may play an important role in the deep carbon cycle, not only in terms of a carbon reservoir, but also in carbon isotope distributions in the deep Earth.
Results and Discussion
Here, we have calculated the reduced partition function ratios (β-factors) for carbon isotopes (13C/12C) of two key carbides, cementite (Fe3C) and moissanite (SiC) (Materials and Methods). Our calculations of the 13C β-factors of cementite and silicon carbide can be expressed as
| [1] |
| [2] |
where x = 106/T2. Then, an equilibrium fractionation factor between two substances A and B (αA-B) can be expressed by
| [3] |
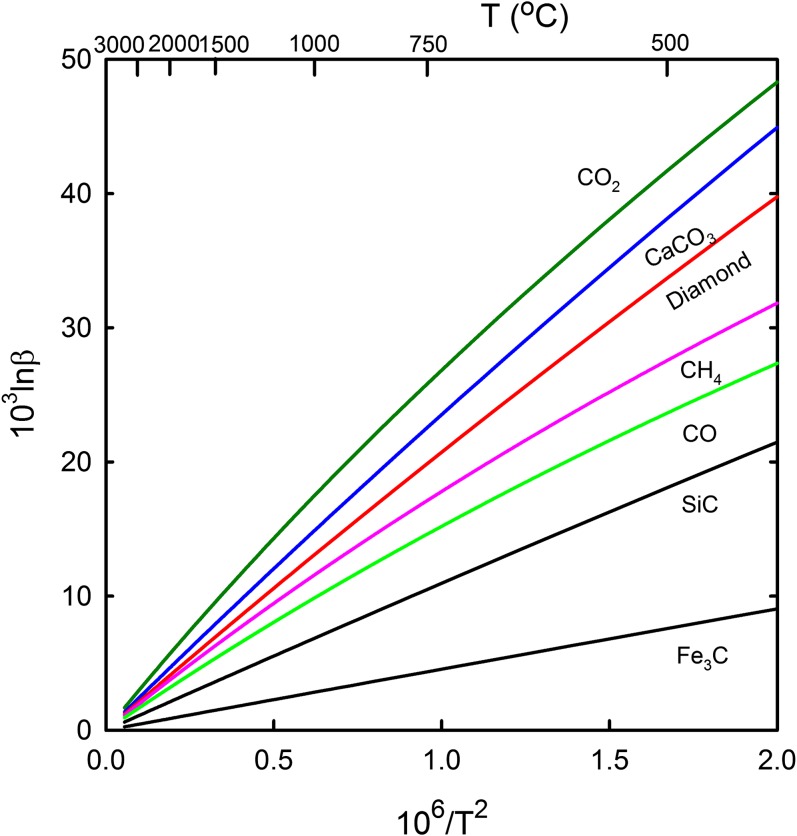
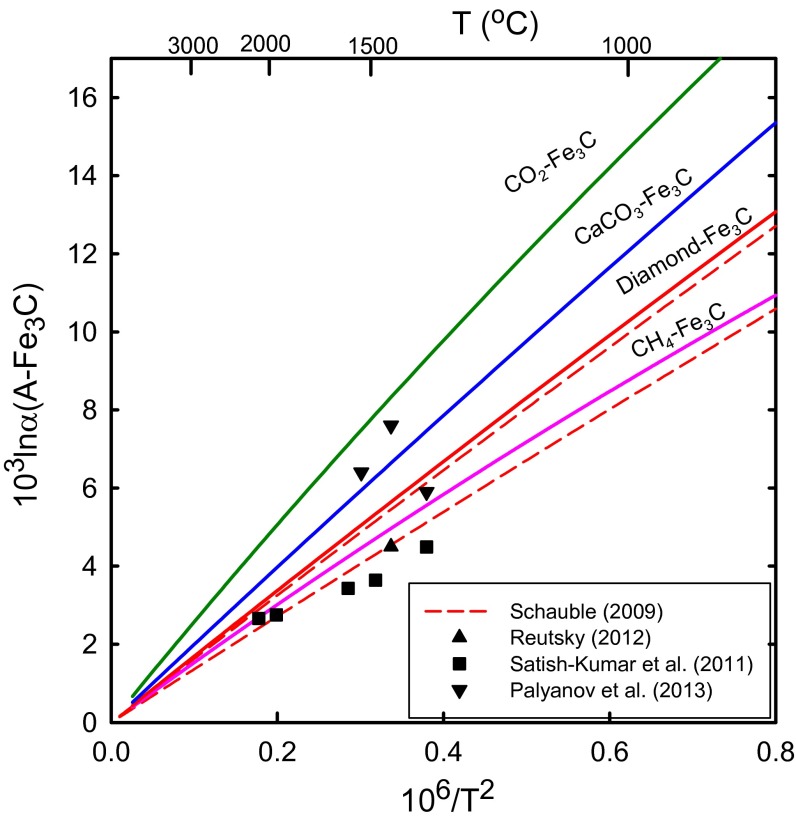
The 13C β-factors for the two carbides are smaller than those for any other carbon-bearing compounds ever reported in the literature, including methane and CO (Fig. 1). The effect of pressure on 13C/12C fractionation between Fe3C and other mantle phases are not significant at mantle temperatures (26) (SI Text). Thus, δ13C values of Fe3C are lower than any other C-bearing mantle phases (diamond, CO2, CH4, and CaCO3) by 10–14‰ and 3–5‰ at 1,000 °C and 2,000 °C, respectively (Fig. 2). The δ13C values of SiC are also lower by 6–11‰ and 2–4‰ at 1,000 °C and 2,000 °C, respectively. The effect of polymorphic transitions of CaCO3 (and CO2) with increasing pressure is probably insignificant (27, 28), although not quantified yet. Our calculations for 13C β-factors of Fe3C are very close to density functional theory calculations for Fe3C and η-Fe2C (29) (Fig. 2). Recent experimental results of 13C/12C isotope fractionation factors between graphite/diamond and Fe−C melts/Fe carbides at 1200–2100 °C and 2–15 GPa vary from 2.7‰ to 7.6‰ (30–32) (Fig. 2). It should be noticed that the attainment of isotope equilibrium has not been demonstrated in these experimental studies. Our calculations generally agree with the experimental data, especially at the high temperature (≥2,000 °C). It is likely that Fe7C3, a probable main phase in the lower mantle and the inner core, has β-factors similar to Fe3C. Collectively, the theoretical and experimental studies clearly demonstrate significant depletion of 13C in various carbides (Fe carbides and SiC) and C dissolved in Fe−Ni metals at mantle temperatures.
Fig. 1.
Comparison of the 13C β-factors for carbides (SiC and Fe3C) and other carbon-bearing compounds at high temperatures. Data sources: SiC, Fe3C, CaCO3 (this study), CO (60), CO2 and diamond (61), and CH4 (62).
Fig. 2.
Calculated equilibrium 13C/12C fractionation between mantle carbon phases (A) and Fe3C, compared with experimental data. Two dashed red lines are density functional theory calculations for diamond−Fe3C (above) and diamond−η−Fe2C (below) fractionation (29−32).
Cohenite in iron meteorites is depleted in 13C by 11.5–12.5‰ relative to coexisting graphite (33–35). Carbon dissolved in taenite (Fe−Ni alloy) from several types of meteorites is also depleted in 13C relative to graphite (34, 36). Assuming 13C β-factors of cohenite are very similar to those of cementite, the graphite and cohenite equilibrated at 850–900 °C. Recently, Mikhail et al. (8) reported that the difference in the δ13C values between the diamond and coexisting iron carbide from Jagersfontein kimberlite, South Africa, averaged 7.2 ± 1.3‰. This isotope fractionation corresponds to a formation temperature of 1,250 °C ± 150 °C, which is within a typical range for the formation of subcontinental lithospheric diamonds (1,150 °C ± 100 °C). The δ13C values of terrestrial SiC have also been reported to range from −18‰ to −35‰, which are much lower than coexisting diamond or CaCO3 (37, 38). Thus, the observed low δ13C values of carbide minerals are consistent with their low 13C β-factors relative to other C-bearing mantle minerals even at mantle temperatures.
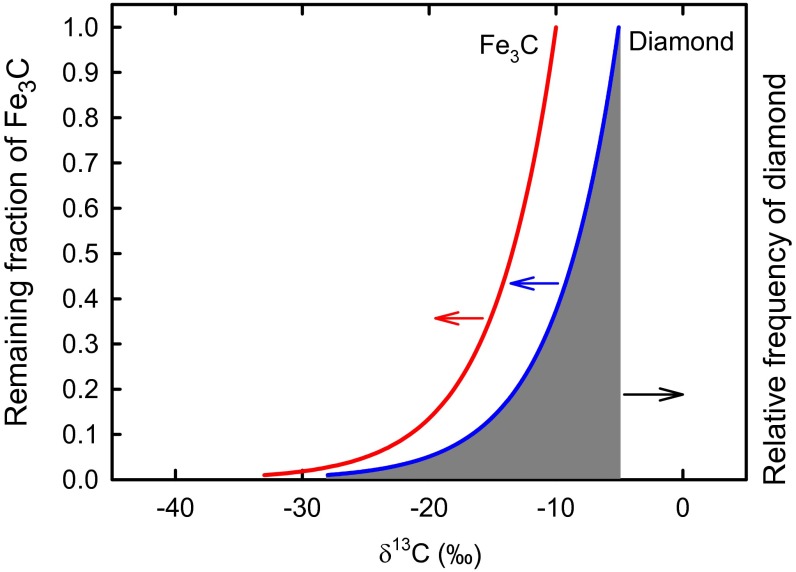
With increasing depth within the mantle, CO2 and carbonates become unstable with respect to diamond at 120–200 km. In greater depths, the fO2 further decreases to that of iron-wustite, and eventually (Fe, Ni) metal becomes stable at about 250 km and below (“redox freezing”) (39, 40). With typical concentrations of C in the oceanic mantle (20−300 ppm), all diamond is converted first to dissolved C in the Fe−Ni metal phase, and then to a discrete iron carbide phase (Fe3C and Fe7C3) after carbon saturation, followed by diamond. A recent experimental study between Mg−Ca carbonate and metallic Fe at 1,000–1,650 °C and 6.5–7.5 GPa (30) demonstrated the formation of diamond and Fe3C together with metastable graphite along the steep fO2 gradient. Using the results of our theoretical calculations of 13C β-factors, we now can build a first-order model for δ13C distributions among these C-bearing mantle minerals (carbonate, diamond, and Fe3C) during mantle−slab interactions within the deep Earth. When carbonate-rich lithosphere is subducted into the metal-saturated zone, mantle domains with the Fe−C phase form (collective term used here for C dissolved in Fe−Ni metal and various Fe carbides) with excess carbon as diamond. Because δ13C values of Fe3C are lower by ∼5–6‰ than diamond at ∼250 km (∼1,400–1,600 °C for modern and Archean mantles), lithosphere with a typical δ13C of −5‰ that undergoes deep subduction would contain Fe3C and diamond with δ13C of −11‰ to −5‰ and −5‰ to +1‰, respectively, depending on Fe3C/diamond ratios. The subducted lithosphere may further penetrate deep into the transition zone and even into the upper part of the lower mantle. When the domains of subducted lithosphere with Fe−C phases (and diamond) become entrained into mantle upwelling, first reoxidation of the Fe−C phase to diamond, then diamond to carbonatite melts occurs (“redox melting”). The oxidation of the Fe−C to diamond/carbonate could occur below the metal saturation (250 km) to the 660-km discontinuity, corresponding to ∼1,400−1,600 °C (modern mantle) or to ∼1,600−1,900 °C (Archean mantle). Thus, equilibrium δ13C fractionation between diamond and Fe3C ranges from ∼3.5‰ to ∼6‰. This oxidation process could occur by Rayleigh-type open-system fractionation, because C diffuses very rapidly in Fe−C phases, but very slowly in diamond (D ≈ 10−23 m2/s to 10−25 m2/s in the transition zone) (41), limiting the isotope exchange with the Fe−C phase. Thus, it is very likely that during oxidation of Fe−C to diamond, their δ13C values can become progressively lower from their starting values, reaching δ13C < −20‰ at low remaining fractions of carbide (f < 0.1) (Fig. 3). Further oxidation of the diamonds to carbonatite could slightly lower the δ13C value of remaining diamond (<∼1‰ at 1,400–1,500 °C). Thus, within the framework of the redox freezing and melting cycle, the Fe−C phases and diamond with δ13C values much lower than −5‰ can be readily produced (Fig. 3). It has been well documented that the bulk of diamond are metasomatic in origin, deposited from C-bearing fluids. It is very likely that the carbon cycle during the redox freezing and melting discussed above (CO2/carbonates to diamond to Fe−C phases and reverse) is mediated by small amounts of C−O−H fluids [CO2, CH4 and possibly other hydrocarbons (42)] or carbonate melts. However, the δ13C distribution of diamond and Fe carbides discussed above and in Fig. 3 remain essentially the same.
Fig. 3.
Modeling of δ13C distribution during a Rayleigh-type redox melting of Fe3C to diamond: red and blue lines (δ13C of Fe3C and diamond, respectively, as a function of the remaining fraction of Fe3C) and gray area (δ13C relative frequency of diamond produced by the oxidation of Fe3C).
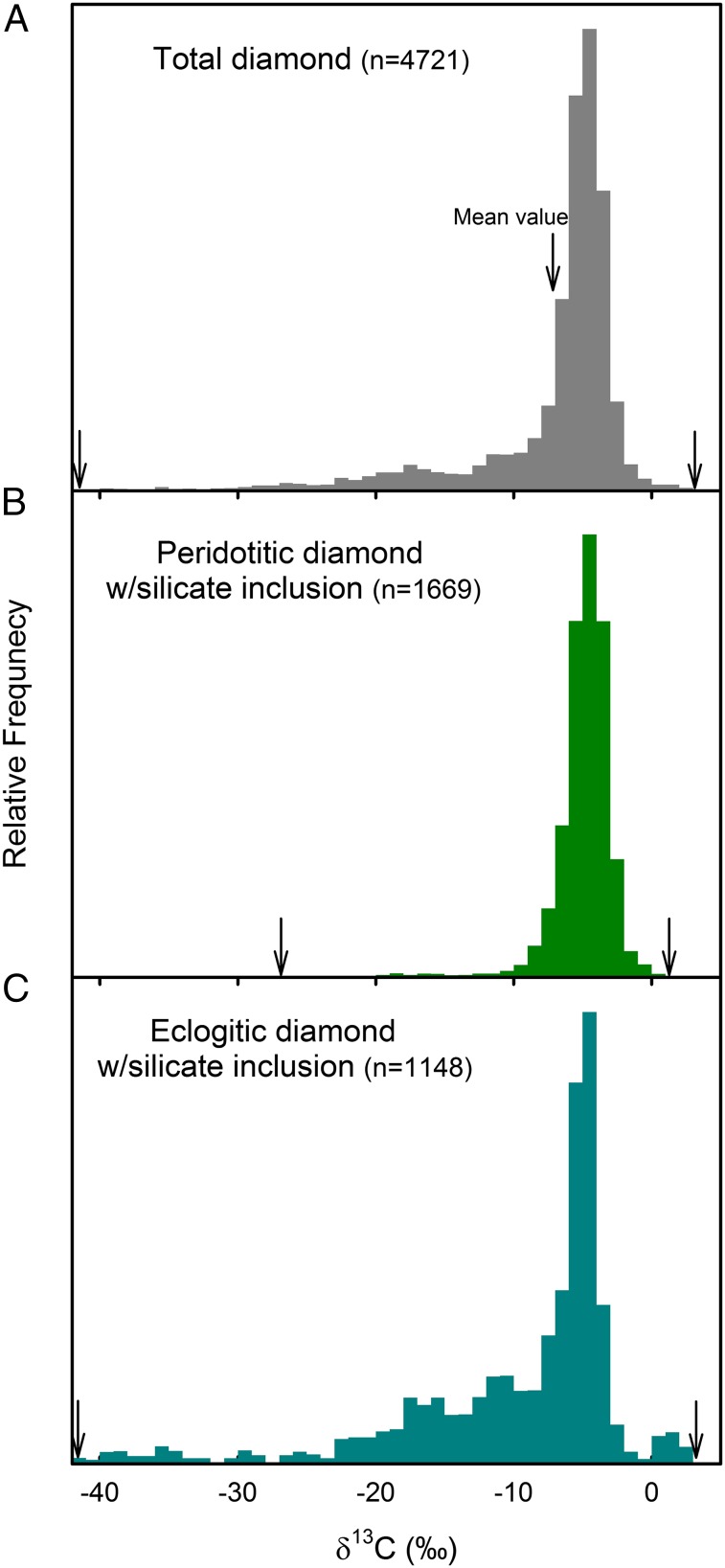
A comparison of results from our modeling for δ13C relative distribution of diamonds (gray area, Fig. 3) with the compiled δ13C distribution of natural diamonds (Fig. 4A) reveals similar semiexponential tailings to lower values, but a much stronger peak for the natural diamonds at δ13C = −5 ± 3‰ than our modeling. It is also well recognized that the δ13C distribution of eclogitic diamonds show a very pronounced tailing pattern to lower values than peridotitic diamonds (Fig. 4 B and C) despite the fact that the both types of diamonds are formed in the keel of subcontinental lithospheric mantle (SCLM) at similar temperature–pressure conditions (1,150 °C ± 100 °C and ∼5 GPa) (43). The appearance of eclogite may reflect the change in geodynamical processes from shallow subduction and delamination or “intraplate” lithospheric extension/mantle upwelling before ∼3 Ga to modern, large-scale plate tectonics with deep subduction of oceanic crust (44, 45). The Archaean oceanic crust was probably thicker and hotter than present-day oceanic crust, owing to higher heat flow and higher degrees of melting at midocean ridges. Thus, shallow subduction with flatter angles and recycling of oceanic crust were dominant. The period from 3 Ga to the end of the Archean (2.5 Ga) is increasingly viewed as a time of the onset of subduction-driven plate tectonics, the Wilson cycle. The oceanic crust became steadily thinner, and the whole crust could be subducted deep by the late Archaean. Subcretion of oceanic lithosphere to the base of continents resulted in deep SCLM with the core of old (>2.5–3 Ga) rheologically stiffer peridotitic nuclei and surrounding younger (<2.5–3 Ga) terranes of eclogitic compositions. In fact, compilations of the ages of diamond deposits (46, 47) show a general pattern that the majority of peridotitic diamonds formed in the Paleo to Neoarchean (2.7–3.5 Ga), while eclogitic diamonds have much younger ages (1−2.9 Ga).
Fig. 4.
The δ13C relative distributions of natural diamonds: (A) worldwide, (B) peridotitic with silicate inclusion, and (C) eclogitic with silicate inclusion. The arrows indicate the highest and lowest δ13C values of each group. A mean δ13C value of the worldwide diamond is −7.2‰. The data are from ref. 18.
Within this scenario of the tectonic evolution, the source of carbon for peridotitic diamonds before 2.5–3 Ga was largely restricted to the upperpart of the mantle (≤200 km), where major C-bearing materials included CO2, carbonates, and diamond/graphite. After the onset of large-sale plate tectonics and deep subduction of oceanic crusts after 2.5–3 Ga, mantle rocks of eclogitic compositions started to form in the lower upper mantle and below (>250 km). The carbon cycle to this depth includes the Fe−C phases via the redox freezing and melting processes as discussed above. During the upwelling and subcretion of eclogitic domains, the Fe−C phases are either oxidized to diamond or mobilized as CH4-bearing fluids, which are trapped and then oxidized to diamonds under SCLM keels. Thus, when subducted deep (>250 km), some of eclogitic diamond are produced via the net oxidation from the Fe−C phases to diamond, which induces large isotope fractionation as discussed above (Fig. 3). Peridotite components of lithosphere might have experienced the same redox freezing/melting, involving the Fe−C phases. In fact, peridotitic diamonds also show a wide δ13C distribution to lower values (−26.4‰ to +0.2‰), although much less pronounced relative to eclogitic diamonds (Fig. 4) (18). This sharp contrast in their δ13C distributions could be due to the change in their carbon cycles that was controlled by the evolution of global geodynamical processes: carbon from the upperpart of the mantle for old (>2.5–3 Ga) peridotite diamonds vs. carbon from deeply subducted lithosphere for young (<2.5–3 Ga) eclogitic diamonds. Diamonds from the transition zone and the lower mantle also show wide δ13C distributions to lower values (−24‰ to +5‰), which is generally consistent with the new model presented here that involves the deep carbon cycle via the redox freezing and melting. Porous, polycrystalline diamond (carbonado) and diamonds in ophiolites also have very low δ13C distributions: −32‰ to −5‰ (carbonado) and −28‰ to −18‰ (ophiolite). Their origins are still in debate, but they are undoubtedly formed under (local?) very reducing conditions as metal (Fe, Fe−Ni, Ni−Mn−Co) and carbides (SiC and Fe−C) occur along with the diamond. Diamond formation via these carbide phases is a likely process for producing the observed low δ13C values.
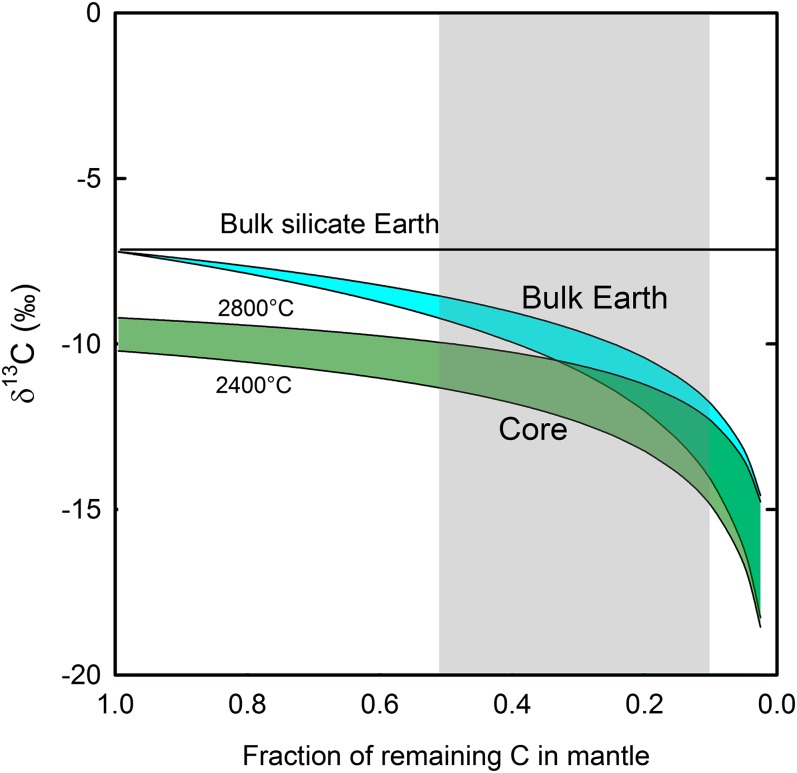
The proto-Earth likely experienced protracted (30–40 My) reequilibration between Fe metal and silicates during planetary accretion−growth, resulting in the core−mantle segregation. Carbon in the “magma ocean” was partitioned into iron droplets, which were then sequestered into the core via metal diapirs (48). An estimated total C content in the mantle varies widely (∼1–40 × 1023 g of C) (1, 2). Assuming that δ13C values of diamonds collected worldwide (Fig. 4A) represent the mantle, the bulk silicate Earth has an average δ13C value of −7.2‰; carbonates and organic matter in the crust have much smaller reservoirs (∼1021 g of C) (21). Experimental studies on C solubility in Fe−C phases suggest that the core (mainly, liquid outer core) may contain 20–200 × 1023 g of C (1, 19). Thus, our current knowledge suggests that the mantle may contain only 10–50% of C that was originally accreted from protoplanetary materials. Much of reequilibration between metallic cores of planetesimals and Earth’s silicate mantle took place in a deep magma ocean at temperatures as high as the solidus of the mantle (∼2,800 °C and >30 GPa) (48). If this carbon extraction from the silicate melts and metal−silicate reequilibration occurred in isotopic equilibrium at 2,400–2,800 °C, carbon in the Fe melts (assumed to have the same β-factors as Fe3C) is depleted in 13C by 1.8–2.4‰ and 2.1–2.8‰ compared with diamond and CaCO3 in magma ocean, respectively (Fig. 2). Under the deep magma ocean and heterogeneous accretion model (48), the core formation could be modeled by a Rayleigh-type fractional carbon segregation, rather than by a single-stage batch equilibrium process with the magma ocean. This scenario yields δ13C values of −15‰ to −10‰ for the core, and of −14‰ to −8.6‰ for the bulk Earth, depending on the degree of carbon extraction from the mantle to the core and the temperature (2,400−2,800 °C) (Fig. 5). No carbon addition (late veneer) is considered after the mantle−core segregation. These estimated δ13C values for the bulk Earth are moderately to significantly lower than the currently accepted mantle value (−5 ± 3‰). The range of δ13C values for the bulk Earth is in fact more consistent with those observed for chondrites and other meteorites (−24‰ to +5‰) (2, 49, 50). Recently, a conceptually similar, but qualitative model has been presented (48).
Fig. 5.
Modeling of δ13C values of the bulk Earth and the core as a function of (i) fraction of remaining C in the mantle during carbon sequestration to the core and (ii) temperature of Fe melt segregation from magma ocean. The δ13C of the bulk silicate Earth is assumed to be a mean δ13C value of the worldwide diamond (−7.2‰, Fig. 4A). The 13C/12C fractionation between CaCO3 or diamond in magma ocean and Fe3C in Fe-melt is 2–3‰ at 2,400–2,800 °C (Fig. 2). The fraction of remaining C in the mantle is estimated to be in the range 0.1–0.5 (gray area).
The carbon cycle involving carbides (SiC and Fe−C phases) has potentially significant implications for the study of early life on the Earth. Low δ13C values of carbonaceous materials (graphite) present in ancient rocks, particularly of Eoarchean (∼3.8 Ga) mineral associations from southern West Greenland, has been used as a proxy of ancient life (isotope biosignatures) (51). Several experimental studies in the past decade suggest that various abiotic reactions in the early atmosphere−oceans−crust could have produced hydrocarbons and other carbonaceous materials with such low δ13C values (52). Our calculations and modeling here show that carbide minerals and dissolved C in Fe−Ni produced even under deep-Earth conditions can have very low δ13C values (Figs. 2 and 3). Metamorphism of Eoarchean rocks in reducing environments could also have produced carbide minerals with low δ13C. SiC could also be produced by adiabatic decompression of volcanic eruption near the surface (12). These carbide phases might have later been oxidized to graphite. Thus, the “light carbon” of Hadean−Eoarchean rock record alone is not a viable biosignature. Abiogenic processes that can produce carbonaceous materials with low δ13C values, both near surface and in deep Earth, challenge our notion of carbon isotope biosignatures for early life on Earth and possibly other planets.
Materials and Methods
For a compound consisting of two elements such as cementite, the β-factor of one element can be calculated from the kinetic vibration energies of the entire compound and the other element (53–56):
where Ktotal and KFe are the kinetic energies of cementite and its iron sublattice, respectively. The , , and are the atomic masses of 12C, 13C, and carbon. The R and T are gas constant and temperature (K), respectively. The Ktotal and KFe were obtained, respectively, from the lattice contribution to the total heat capacity (57) and from 57Fe partial density of state determined by means of nuclear resonant inelastic X-ray scattering experiments with synchrotron radiation (58) (see SI Text). To estimate 13C β-factors for silicon carbide (SiC), we used a cubic 3C modification of a rigid-ion lattice dynamic model, combining short-range central and noncentral interactions, and long-range Coulomb interactions (59). The 13C β-factors of SiC were then calculated from the modal β-factor value for a given vector, using six frequencies of Si12C and Si13C isotopologues and averaging over the number of the wave vectors (see SI Text):
where is the dimensionless phonon frequency with ν the phonon frequencies, h is Planck’s constant, and kB is the Boltzmann constant. The asterisk denotes Si13C isotopologue.
Supplementary Material
Acknowledgments
We thank Pierre Cartigny and Edwin Schauble for providing δ13C values of natural diamonds and unpublished density functional theory calculations of Fe−C phases, respectively. Tom Chacko’s comments helped improve the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1401782112/-/DCSupplemental.
References
- 1.Dasgupta R, Hirschmann MM. The deep carbon cycle and melting in Earth's interior. Earth Planet Sci Lett. 2010;298(1-2):1–13. [Google Scholar]
- 2.Marty B, Alexander CMOD, Raymond SN. Primordial origins of Earth's carbon. Rev Mineral Geochem. 2013;75:149–181. [Google Scholar]
- 3.Keppler H, Wiedenbeck M, Shcheka SS. Carbon solubility in olivine and the mode of carbon storage in the Earth’s mantle. Nature. 2003;424(6947):414–416. doi: 10.1038/nature01828. [DOI] [PubMed] [Google Scholar]
- 4.Frost DJ, et al. Experimental evidence for the existence of iron-rich metal in the Earth’s lower mantle. Nature. 2004;428(6981):409–412. doi: 10.1038/nature02413. [DOI] [PubMed] [Google Scholar]
- 5.Lord OT, Walter MJ, Dasgupta R, Walker D, Clark SM. Melting in the Fe-C system to 70 GPa. Earth Planet Sci Lett. 2009;284(1-2):157–167. [Google Scholar]
- 6.Nakajima Y, Takahashi E, Suzuki T, Funakoshi K-i. “Carbon in the core” revisited. Phys Earth Planet Inter. 2009;174(1-4):202–211. [Google Scholar]
- 7.Rohrbach A, Ghosh S, Schmidt MW, Wijbrans CH, Klemme S. The stability of Fe-Ni carbides in the Earth's mantle: Evidence for a low Fe-Ni-C melt fraction in the deep mantle. Earth Planet Sci Lett. 2014;388:211–221. [Google Scholar]
- 8.Mikhail S, et al. Empirical evidence for the fractionation of carbon isotopes between diamond and iron carbide from the Earth's mantle. Geochem Geophys Geosyst. 2014;15(4):855–866. [Google Scholar]
- 9.Jacob DE, Kronz A, Viljoen KS. Cohenite, native iron and troilite inclusions in garnets from polycrystalline diamond aggregates. Contrib Mineral Petrol. 2004;146(5):566–576. [Google Scholar]
- 10.Kaminsky FV, Wirth R. Iron carbide inclusions in lower-mantle diamond from Juina, Brazil. Can Mineral. 2011;49(2):555–572. [Google Scholar]
- 11.Hazen RM, Downs RT, Jones AP, Kah L. 2013. Carbon mineralogy and crystal Chemistry. Rev Mineral Geochem 75:7–46.
- 12.Shiryaev AA, Gaillard F. Local redox buffering by carbon at low pressures and the formation of moissanite − natural SiC. Eur J Mineral. 2014;26(1):53–59. [Google Scholar]
- 13.Sen S, et al. Carbon substitution for oxygen in silicates in planetary interiors. Proc Natl Acad Sci USA. 2013;110(40):15904–15907. doi: 10.1073/pnas.1312771110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cartigny P. Stable isotopes and the origin of diamond. Elements. 2005;1(2):79–84. [Google Scholar]
- 15.Deines P. The carbon isotope geochemistry of mantle xenoliths. Earth Sci Rev. 2002;58(3):247–278. [Google Scholar]
- 16.Galimov EM. Isotope fractionation related to kimberite magmatism and diamond formation. Geochim Cosmochim Acta. 1991;55(6):1697–1708. [Google Scholar]
- 17.Stachel T, Harris JW, Muehlenbachs K. Sources of carbon in inclusion bearing diamonds. Lithos. 2009;112(Suppl 2):625–637. [Google Scholar]
- 18.Cartigny P, Palot M, Thomassot E, Harris JW. Diamond formation: A stable isotope perspective. Annu Rev Earth Planet Sci. 2014;42:699–732. [Google Scholar]
- 19.Wood BJ, Li J, Shahar A. 2013. Carbon in the core: Its influence on the properties of core and mantle. Rev Mineral Geochem 75:231–250.
- 20.Mikhail S, et al. Constraining the internal variability of the stable isotopes of carbon and nitrogen within mantle diamonds. Chem Geol. 2014;366:14–23. [Google Scholar]
- 21.Des Marais DJ. Isotopic evolution of the biogeochemical carbon cycle during the Precambrian. In: Valley JW, Cole DR, editors. Stable Isotope Geochemistry. Mineral Soc America,Chantilly; VA: 2001. pp. 555–578. [Google Scholar]
- 22.Cartigny P, Harris JW, Javoy M. Eclogitic diamond formation at Jwaneng: No room for a recycled component. Science. 1998;280(5368):1421–1424. doi: 10.1126/science.280.5368.1421. [DOI] [PubMed] [Google Scholar]
- 23.Deines P. The carbon isotopic composition of diamonds: Relationship to diamond shape, color, occurrence and vapor composition. Geochim Cosmochim Acta. 1980;44(7):943–961. [Google Scholar]
- 24.Smart KA, et al. Diamond growth from oxidized carbon sources beneath the Northern Slave Craton, Canada: A δ13C−N study of eclogite-hosted diamonds from the Jericho kimberlite. Geochim Cosmochim Acta. 2011;75(20):6027–6047. [Google Scholar]
- 25.Trumbull RB, et al. The carbon isotope composition of natural SiC (moissanite) from the Earth's mantle: New discoveries from ophiolites. Lithos. 2009;113(3-4):612–620. [Google Scholar]
- 26.Polyakov VB, Kharlashina NN. Effect of pressure on equilibrium isotopic fractionation. Geochim Cosmochim Acta. 1994;58(21):4739–4750. [Google Scholar]
- 27.Deines P. Carbon isotope effects in carbonate systems. Geochim Cosmochim Acta. 2004;68(12):2659–2679. [Google Scholar]
- 28.Deines P, Eggler DH. Experimental determination of carbon isotope fractionation between CaCO3 and graphite. Geochim Cosmochim Acta. 2009;73(24):7256–7274. [Google Scholar]
- 29.Schauble EA. 2009. Equilibrium carbon and hydrogen isotope fractionation in iron. Eos Trans AGU 90(52), Fall Meet Suppl, #V11C-1967.
- 30.Palyanov YN, et al. Mantle-slab interaction and redox mechanism of diamond formation. Proc Natl Acad Sci USA. 2013;110(51):20408–20413. doi: 10.1073/pnas.1313340110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reutsky VN, Borzdov YM, Palyanov YN. Effect of diamond growth rate on carbon isotope fractionation in Fe-Ni-C system. Diamond Relat Mater. 2012;21:7–10. [Google Scholar]
- 32.Satish-Kumar M, So H, Yoshino T, Kato M, Hiroi Y. Experimental determination of carbon isotope fractionation between iron carbide melt and carbon: C-12-enriched carbon in the Earth's core? Earth Planet Sci Lett. 2011;310(3-4):340–348. [Google Scholar]
- 33.Craig H. The geochemistry of the stable carbon isotopes. Geochim Cosmochim Acta. 1953;3(2-3):53–92. [Google Scholar]
- 34.Sugiura N. Ion probe measurements of carbon and nitrogen in iron meteorites. Meteorit Planet Sci. 1998;33(3):393–409. [Google Scholar]
- 35.Deines P, Wickman FE. A contribution to the stable carbon isotope geocehmistry of iron meteorites. Geochim Cosmochim Acta. 1975;39(5):547–557. [Google Scholar]
- 36.Grady MM, Pillinger CT. Carbon isotope relationships in winonaites and foresterite chondrites. Geochim Cosmochim Acta. 1986;50(2):255–263. [Google Scholar]
- 37.Di Pierro S, et al. Rock-forming moissanite (natural alpha-silicon carbide) Am Mineral. 2003;88(11-12):1817–1821. [Google Scholar]
- 38.Leung I, Guo WX, Friedman I, Gleason J. Natural occurrence of silicon-carbide in a diamondiferous kimberlite from Fuxian. Nature. 1990;346(6282):352–354. [Google Scholar]
- 39.Rohrbach A, Schmidt MW. Redox freezing and melting in the Earth’s deep mantle resulting from carbon-iron redox coupling. Nature. 2011;472(7342):209–212. doi: 10.1038/nature09899. [DOI] [PubMed] [Google Scholar]
- 40.Stagno V, Ojwang DO, McCammon CA, Frost DJ. The oxidation state of the mantle and the extraction of carbon from Earth’s interior. Nature. 2013;493(7430):84–88. doi: 10.1038/nature11679. [DOI] [PubMed] [Google Scholar]
- 41.Zhang B, Wu X. Calculation of self-diffusion coefficients in diamond. Appl Phys Lett. 2012;100(5):051901. [Google Scholar]
- 42.Lobanov SS, et al. Carbon precipitation from heavy hydrocarbon fluid in deep planetary interiors. Nat Commun. 2013;4:2446. doi: 10.1038/ncomms3446. [DOI] [PubMed] [Google Scholar]
- 43.Stachel T, Harris JW. The origin of cratonic diamonds—Constraints from mineral inclusions. Ore Geol Rev. 2008;34(1-2):5–32. [Google Scholar]
- 44.Condie KC, Pease V. When did plate tectonics begin on planet Earth? Geol Soc Am Spec Pap. 2008;440:281−294. [Google Scholar]
- 45.Foley SF, Buhre S, Jacob DE. Evolution of the Archaean crust by delamination and shallow subduction. Nature. 2003;421(6920):249–252. doi: 10.1038/nature01319. [DOI] [PubMed] [Google Scholar]
- 46.Heaman LM, Pearson DG. Nature and evolution of the Slave Province sucontinental lithospheric mantle. Can J Earth Sci. 2010;47(4):369–388. [Google Scholar]
- 47.Shirey SB, Richardson SH. Start of the Wilson cycle at 3 Ga shown by diamonds from subcontinental mantle. Science. 2011;333(6041):434–436. doi: 10.1126/science.1206275. [DOI] [PubMed] [Google Scholar]
- 48.Wood BJ, Walter MJ, Wade J. Accretion of the Earth and segregation of its core. Nature. 2006;441(7095):825–833. doi: 10.1038/nature04763. [DOI] [PubMed] [Google Scholar]
- 49.Grady MM, Wright IP. Elemental and isotopic abundances of carbon and nitrogen in meteorites. Space Sci Rev. 2003;106(1-4):231–248. [Google Scholar]
- 50.Pearson VK, Sephton MA, Franchi IA, Gibson JM, Gilmour I. Carbon and nitrogen in carbonaceous chondrites: Elemental abundances and stable isotopic compositions. Meteorit Planet Sci. 2006;41(12):1899–1918. [Google Scholar]
- 51.Mojzsis SJ, et al. Evidence for life on Earth before 3,800 million years ago. Nature. 1996;384(6604):55–59. doi: 10.1038/384055a0. [DOI] [PubMed] [Google Scholar]
- 52.Horita J. Some perspectives on isotope biosignatures for early life. Chem Geol. 2005;218(1-2):171–186. [Google Scholar]
- 53.Cole DR, et al. An experimental and theoretical determination of oxygen isotope fractionation in the system magnetite-H2O from 300 to 800 °C. Geochim Cosmochim Acta. 2004;68(17):3569–3585. [Google Scholar]
- 54.Polyakov VB, Mineev SD. Mossbauer spectroscopy as applied to isotopic geochemistry: II. The beta S-34-factor in pyrite and the beta O-18-factor in hematite. Geochem Int. 2000;38(9):831–837. [Google Scholar]
- 55.Polyakov VB, et al. Oxygen isotope fractionation factors involving cassiterite (SnO2): I. Calculation of reduced partition function ratios from heat capacity and X-ray resonant studies. Geochim Cosmochim Acta. 2005;69(5):1287–1300. [Google Scholar]
- 56.Polyakov VB, et al. The use of Mossbauer spectroscopy and calorimetry for determining isotopic equilibrium constants. Pyrite. Russ J Phys Chem. 2001;75(6):907–911. [Google Scholar]
- 57.Hallstedt B, et al. Thermodynamic properties of cementite (Fe3C) Calphad: Comput Coupling Phase Diagrams Thermochem. 2010;34(1):129–133. [Google Scholar]
- 58.Gao L, et al. Pressure-induced magnetic transition and sound velocities of Fe(3)C: Implications for carbon in the Earth's inner core. Geophys Res Lett. 2008;35(17):L17306. [Google Scholar]
- 59.Vetelino JE, Mitra SS. Lattice dynamic of cubic SiC. Phys Rev. 1969;178:1349–1352. [Google Scholar]
- 60.Richet P, Bottinga Y, Javoy M. A review of hydrogen, carbon, nitrogen, oxygen, sulphur, and chlorine stable isotope fractionation among gaseous molecules. Annu Rev Earth Planet Sci. 1977;5:65–110. [Google Scholar]
- 61.Polyakov VB, Kharlashina NN. The use of heat capacity data to calculate carbon-isotope fractionation between graphite, diamond, and carbon-dioxide—A new approach. Geochim Cosmochim Acta. 1995;59(12):2561–2572. [Google Scholar]
- 62.Galimov EM. 1973. Izotopy Ugleroda v Neftegazovoy Geologii (Nedra Press, Moscow) trans (1975) [Carbon Isotopes in Oil-Gas Geology] (NASA Goddard Space Flight Center, Greenbelt, MD), NASA TT F-682.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.