Significance
Amber, fossil tree resin, preserves organisms in microscopic fidelity, and frequently fossils preserved in amber are otherwise absent in the entire fossil record. Plant remains, however, are rarely entrapped in amber, compared with the vast amount of insects and other animals. Our newly discovered fossils from Eocene Baltic amber are the only documented case of fossilized carnivorous plant traps and represent the first fossil evidence of the carnivorous plant family Roridulaceae, which is today a narrow endemic of South Africa. Hence, our results shed light onto the paleobiogeography of the Roridulaceae, indicating a wide Eocene distribution of the roridulid ancestors and challenging previous notions about a Gondwanan origin of this plant family.
Keywords: plant carnivory, Roridulaceae, Eocene, Ericales
Abstract
The fossil record of carnivorous plants is very scarce and macrofossil evidence has been restricted to seeds of the extant aquatic genus Aldrovanda of the Droseraceae family. No case of carnivorous plant traps has so far been reported from the fossil record. Here, we present two angiosperm leaves enclosed in a piece of Eocene Baltic amber that share relevant morphological features with extant Roridulaceae, a carnivorous plant family that is today endemic to the Cape flora of South Africa. Modern Roridula species are unique among carnivorous plants as they digest prey in a complex mutualistic association in which the prey-derived nutrient uptake depends on heteropteran insects. As in extant Roridula, the fossil leaves possess two types of plant trichomes, including unicellular hairs and five size classes of multicellular stalked glands (or tentacles) with an apical pore. The apices of the narrow and perfectly tapered fossil leaves end in a single tentacle, as in both modern Roridula species. The glandular hairs of the fossils are restricted to the leaf margins and to the abaxial lamina, as in extant Roridula gorgonias. Our discovery supports current molecular age estimates for Roridulaceae and suggests a wide Eocene distribution of roridulid plants.
Plant carnivory is traditionally defined as the attraction, capture, and digestion of prey by vegetative traps, with the subsequent uptake of nutrients (1, 2). Some carnivorous plants, however, challenge the boundary of the botanical carnivory concept because they depend on commensal organisms for the digestion of their prey (2, 3). The most famous representative of those plants is Roridula, placed in the monogeneric family Roridulaceae that is endemic to a few localities in the southwestern Cape of South Africa (4, 5).
The resinous glandular leaves of both extant species, Roridula dentata and Roridula gorgonias, capture plenty of arthropods. The sticky trapping glue of Roridula is a viscous lipophilic resin containing triterpenoids as major component, which does not allow dissolution of digestive enzymes (6). Consequently, the secretory glands of Roridulaceae lack enzymatic activity (7, 8). For prey-derived nutrient uptake, Roridula depends on two obligately associated heteropteran Pameridea species (family Miridae, “capsid bugs”), which feed on the trapped animals (5, 9). In this “digestive mutualism” (10), the nutrient-rich fecal compounds of these “Roridula bugs” are incorporated by Roridula through nanometer-sized cuticular gaps and serve for a better alimentation in a nutrient-poor habitat (7, 8, 10, 11). The benefit of nutrient uptake from captured prey is the essential criterion for the concept of botanical carnivory (1, 2) and thus includes Roridulaceae (11, 12).
Here, we report two leaf fossils from Eocene Baltic amber possessing the relevant morphological features of an adhesive flypaper trap plant that we assign to the Roridulaceae lineage (Figs. 1–3). Both specimens originate from the Jantarny amber mine near Kaliningrad (Russia). The amber-bearing sediments of this fossil site date to 35–47 million years ago (13, 14).
Fig. 1.
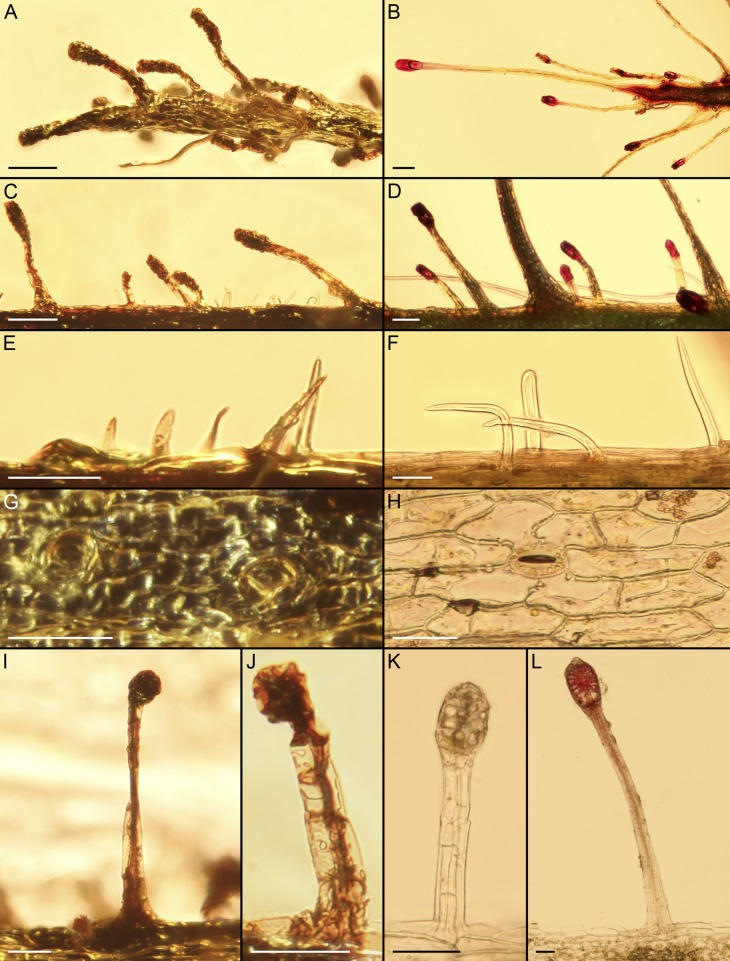
Carnivorous leaves from Eocene Baltic amber. (A) Overview of the leaf enclosed in amber specimen GZG.BST.27310 showing the adaxial tentacle-free side in slightly oblique view and stalked glands at the margin and on the abaxial side; arrowhead points to the exceptional long tentacle stalk with several branched oak trichomes attached. (B) Overview of the leaf enclosed in amber specimen GZG.BST.27311, showing abundant tentacles on the abaxial side. (C) Margin of abaxial leaf surface with tentacles of different size classes and nonglandular hyaline trichomes. (D) Leaf apex tapering into a sole tentacle. (E and F) Glandular heads with central pore (arrowheads) from both leaves. (Scale bars: A and B, 1 mm; C and D, 100 µm; E, 10 μm; F, 40 μm.)
Fig. 3.
Carnivorous leaf from Eocene Baltic amber (A and B; GZG.BST.27310) and leaves of extant Roridula gorgonias (C and D). (A) Exceptionally long tentacle stalk (with several branched oak trichomes attached) of the fossil leaf representing the fifth size class of stalked glands. (B and C) Overviews showing the tentacle-free adaxial surface and tentacles along the leaf margins. (D) Partial leaf tip showing different size classes of stalked glands. (Scale bars: A, 100 µm; B, 500 µm; C and D, 1 mm.)
Results
The linear-lanceolate leaves are 5 and 4.5 mm long and 0.2 mm wide at the base, and they narrow gradually toward the leaf tip, which terminates in a stalked gland (tentacle; Fig. 1). The leaves possess two trichome types: tentacles and nonglandular hyaline hairs (Figs. 1 and 2). The hyaline trichomes are located on both sides and the margins of the lamina, whereas the tentacles are exclusively found along the margins and on the abaxial side without a definite arrangement (Fig. 1). The tentacles are multicellular, consisting of a tapering stalk and a clavate to ovoid glandular head, which shows a small pore at the center of its distal side (Fig. 1 E and F). The stalks of the glands measure between 20 and 350 µm in length (Figs. 1 and 2 A and C), whereas an exceptional stalk exceeds this size, reaching 1.4 mm (Fig. 3A). As with the stalks, the glandular heads vary in size (20–120 µm long, 10–40 µm wide). Adhered organic remains as well as trichomes of other plants attached to the glandular tentacle heads (Figs. 1 A and D and 3A) indicate that they excreted a sticky secretion, as known from adhesive traps of extant carnivorous plants. The nonglandular trichomes are hyaline, unicellular, and arcuate to straight, tapering toward an acute apex (Figs. 1C and 2E). Their length ranges from 10 to 80 µm, and their width reaches up to 12 µm.
Fig. 2.
Morphological comparison of the carnivorous leaf fossils from Baltic amber (Left) and extant Roridula species (Right). (A and B) Leaf tip ending in a sole tentacle. (C and D) Stalked glands of different size classes. (E and F) Hyaline unicellular nonglandular trichomes. (G and H) Epidermal cells and stomata. (I–L) Multicellular tentacles. (A, C, E, and G) GZG.BST.27310. (I and J) GZG.BST.27311. (B, D, K, and L) R. gorgonias. (F and H) R. dentata. (Scale bars: A–D, 100 µm; E–L, 50 µm.)
Both leaves exhibit a well-preserved epidermis with small tetragonal cells at the leaf base and elongated larger cells from the middle part toward the leaf tip. These cells measure 3–54 × 6–18 µm. Stomata of 20–38 × 15–25 µm are present on the abaxial leaf side (Figs. 1C and 2G).
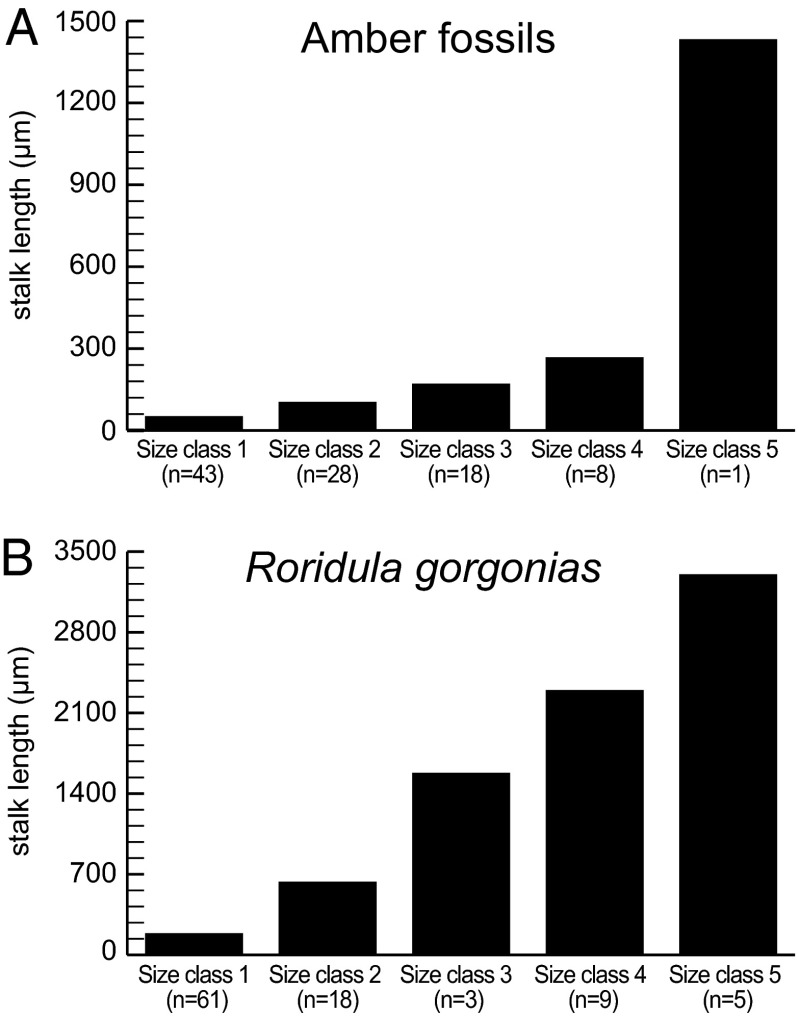
Our statistical cluster analyses (SI Text) revealed that the fossil specimens and Roridula gorgonias show the same morphological pattern among the tentacles. R. gorgonias exhibits five size clusters of tentacle stalk lengths whereas four clusters were detected for the amber inclusions. As an outlier, the longest tentacle was excluded from the cluster analyses of the fossils, but its presence indicates that a fifth size class was present in the Eocene leaves, as in extant Roridulaceae (Fig. 4).
Fig. 4.
Tentacle size classes of the fossil leaves and extant Roridula gorgonias based on the results of the cluster analyses and the tentacle stalk length. (A) Size classes of the fossil leaves, including the outlier which we interpret to represent size class 5. (B) Size classes of Roridula gorgonias. n indicates the number of tentacles per size class.
Discussion
Although glandular secreting trichomes appear in about 30% of vascular plants (15), the unique character combination in the fossils bears most similarities to extant representatives of the Roridulaceae. They share the long, narrow, and perfectly tapered leaf lamina ending in a single tentacle, the presence and morphology of two trichome types (tentacles and nonglandular hairs), the possession of glandular hairs along the leaf margins and on the abaxial lamina, the tentacle head with a central pore, and the size and shape of the epidermal cells and stomata. The glabrous adaxial side of the amber inclusions and the hyaline trichomes being located on both leaf surfaces only appear in the sepals of extant Roridula gorgonias. Besides smaller tentacle sizes, the fossils are distinguished from leaves of extant Roridula species by the absence of a prominent midrib on the abaxial leaf side, and are thus most similar to sepals of Roridula gorgonias.
Extant Roridula plants are very effective traps for all kinds of arthropods due to the sticky resinous trapping glue and the hierarchical organization of the tentacles into functional units for effective prey capture (16, 17). The longest tentacles make the first contact with the prey. Due to the high flexibility of these prominent tentacles, the moving prey then gets stuck to the medium-sized tentacles, which slow down the caught animal. Finally, the smallest and stiffest tentacles immobilize the prey (16). As in modern Roridulaceae, the leaf fossils have different size classes of tentacles that fulfill the functional roles for prey capture (entanglement, slow-down, and immobilization) and comply with the requirements for a carnivorous nature. In addition, the pore of the tentacle heads distinguishes the fossils from any other extant carnivorous plants with glandular adhesive traps such as sundews (Drosera) (3, 18, 19).
In the fossil record, evidence of carnivorous plants is exceedingly rare and macrofossils are restricted to seeds of the aquatic carnivore Aldrovanda (Droseraceae), which are recorded since the Eocene (20, 21). Hence, the fossil leaves from Baltic amber are (to our knowledge) the first documented case of carnivorous plant traps being fossilized.
The occurrence of Eocene Roridulaceae is consistent with recent divergence time estimates for a split of Sarraceniaceae (carnivorous American pitcher plants) from the Roridulaceae–Actinidiaceae clade about 48.6 million years ago, whereas the most recent common ancestor of Roridulaceae and Actinidiaceae was estimated at 38.1 million years ago (22). The age of these Ericales lineages is further supported by Late Cretaceous fossil flowers with affinities to the Actinidiaceae and Clethraceae families (23). The sediments containing the majority of Baltic amber are 35–47 million years old (13, 14). Thus, the amber fossils probably represent an early representative of the Roridulaceae lineage.
The geologic setting of the Baltic amber deposit and the paleobotanical record suggest that coastal areas with carbonate-free, nutrient-poor soils and swamp depressions harbored well-structured mixed forests of angiosperm and conifer trees with intermixed open habitats growing in a subtropical to warm-temperate climate (24–27).
The presence of Eocene roridulid plants in the northern hemisphere challenges notions about the biogeographical history of extant Roridulaceae, which were previously assumed to represent “old Cape elements,” paleoendemics of Gondwanan origin, dating back to up to 90 million years (28, 29). Thus, the leaf fossils represent an example of pseudo-Gondwanan relicts, extinct in Europe today and restricted to particular areas of the southern continents. With respect to the distinctive distribution areas of the closely related extant families Sarraceniaceae (North and South America) and Actinidiaceae (tropical Asia and America), the restriction of extant Roridulaceae to small patches in the Cape region can be regarded as relictual, probably resulting from post-Eocene extinction events.
Materials and Methods
Provenance of the Amber Piece.
The leaf inclusions were discovered in an amber piece that derives from Jantarny mine near Kaliningrad (Russia). Amber in this locality is mined in the “Blue Earth” layer, which is Priabonian in age (late Eocene, 35 million years minimum age) (13, 14). The amber piece was obtained from the collection of Christel and Hans Werner Hoffeins (Hamburg, Germany).
Microscopy and Imaging.
The original 39 × 21 × 5-mm piece of amber was ground and polished manually with wet silicon carbide papers (grit from 25.8- to 5-µm particle size; firm Struers). Two amber fragments measuring 21 × 14 × 3 and 24 × 19 × 3 mm with one leaf inclusion each were obtained by cutting the amber piece with a dental drill. The amber pieces are housed in the Geoscientific Collections of the Georg August University Göttingen (Göttingen, Germany) (collection numbers GZG.BST.27310 and GZG.BST.27311). Leaves of extant Roridula gorgonias and R. dentata (Roridulaceae) were obtained from cultured specimens of Thomas Carow (Nüdlingen, Germany) and A.R.S. The leaf inclusions and the extant Roridulaceae plant material were examined under a Carl Zeiss Stemi 2000 dissection microscope and a Carl Zeiss AxioScope A1 compound microscope, each equipped with a Canon 60D digital camera. In most instances, incident and transmitted light were used simultaneously. The images of Figs. 1 A–D, 2, and 3 A and B are digitally stacked photomicrographic composites of up to 130 individual focal planes obtained using the software package HeliconFocus 5.0 for a better illustration of the 3D structures.
Statistics.
According to Voigt et al. (16), Roridula gorgonias possesses three tentacle size classes that allow a very effective capture of prey. To test whether the tentacle size classes are present in the amber specimens, the tentacle measurements of the amber specimens, Roridula gorgonias and R. dentata were statistically evaluated, applying hierarchical cluster analyses with the statistics package IBM SPSS 21. In total, measurement values of 103 tentacles from both leaf inclusions and 103 tentacles from each extant Roridula species were used, comprising the stalk length, the stalk base and tip width, the gland length, and the gland width. The hierarchical cluster analyses were computed using Ward's method. The resulting clusters were optimized with the nonhierarchical k-means method and tested statistically, using three criteria suggested by Bacher (30), which are η-squared (η2), F-max, and the proportional reduction of error (PRE). The best cluster solution is the one where the values of η2 and PRE do not show any considerable improvement in the subsequent solution. Furthermore, the F value and η2 should be maximal, whereas PRE is supposed to be low. The number of clusters also should be selected with regard to the content and the underlying theoretical model (30). Results of the statistical analyses are provided in SI Text.
Supplementary Material
Acknowledgments
We thank Christel and Hans Werner Hoffeins (Hamburg) for providing the amber specimen and Thomas Carow (Nüdlingen) for providing a plant of Roridula dentata for study. We are grateful to Julia Gundlach (Bielefeld University), Dorothea Hause-Reitner, and Gerhard Hundertmark (University of Göttingen) for assistance, and to two anonymous reviewers for constructive suggestions.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 10.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414777111/-/DCSupplemental.
References
- 1.Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD. Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. Am Nat. 1984;124(4):479–497. [Google Scholar]
- 2.Adamec L. Foliar mineral nutrient uptake in carnivorous plants: what do we know and what should we know? Front Plant Sci. 2013;4:1–13. doi: 10.3389/fpls.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juniper BE, Robins RJ, Joel DM. The Carnivorous Plants. Academic; London: 1989. [Google Scholar]
- 4.Obermeyer AA. Roridulaceae. In: Codd LE, de Winter B, Killick DJB, Rycroft HB, editors. Flora of Southern Africa. Vol 13. Government Printer; Pretoria, South Africa: 1970. pp. 201–204. [Google Scholar]
- 5.Anderson B, Olivieri I, Lourmas M, Stewart BA. Comparative population genetic structures and local adaptation of two mutualists. Evolution. 2004;58(8):1730–1747. doi: 10.1111/j.0014-3820.2004.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 6.Simoneit BRT, Medeiros PM, Wollenweber E. Triterpenoids as major components of the insect-trapping glue of Roridula species. Z Naturforsch C. 2008;63(9):625–630. doi: 10.1515/znc-2008-9-1001. [DOI] [PubMed] [Google Scholar]
- 7.Ellis AG, Midgley JJ. A new plant-animal mutualism involving a plant with sticky leaves and a resident hemipteran insect. Oecologia. 1996;106:478–481. doi: 10.1007/BF00329705. [DOI] [PubMed] [Google Scholar]
- 8.Anderson B, Midgley JJ. It takes two to a tango but three is a tangle: mutualists and cheaters on the carnivorous plant Roridula. Oecologia. 2002;132:369–373. doi: 10.1007/s00442-002-0998-1. [DOI] [PubMed] [Google Scholar]
- 9.Dolling WR, Palmer JM. Pameridea (Hemiptera: Miridae): predaceous bugs specific to the highly viscid plant genus Roridula. Syst Entomol. 1991;16(3):319–328. [Google Scholar]
- 10.Anderson B, Midgley JJ. Digestive mutualism, an alternate pathway in plant carnivory. Oikos. 2003;102(1):221–224. [Google Scholar]
- 11.Anderson B. Adaptations to foliar absorption of faeces: a pathway in plant carnivory. Ann Bot. 2005;95(5):757–761. doi: 10.1093/aob/mci082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Midgley JJ, Stock WD. Natural abundance of δ 15N confirms insectivorous habit of Roridula gorgonias, despite it having no proteolytic enzymes. Ann Bot. 1998;82(3):387–388. [Google Scholar]
- 13.Standke G. The Tertiary geologic profiles of the Samland amber coast near Rauschen. Schriftenr Geowiss. 1998;7:93–133. German. [Google Scholar]
- 14.Standke G. Is Bitterfeld amber identical with Baltic amber? A geologic survey in space and time and genetic conclusions. Exkurs f und Verofftl DGG. 2008;236:11–33. German. [Google Scholar]
- 15.Wagner GJ, Wang E, Shepherd RW. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann Bot. 2004;93(1):3–11. doi: 10.1093/aob/mch011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voigt D, Gorb E, Gorb S. Hierarchical organisation of the trap in the protocarnivorous plant Roridula gorgonias (Roridulaceae) J Exp Biol. 2009;212(19):3184–3191. doi: 10.1242/jeb.034280. [DOI] [PubMed] [Google Scholar]
- 17.Voigt D, Gorb S. Desiccation resistance of adhesive secretion in the protocarnivorous plant Roridula gorgonias as an adaptation to periodically dry environment. Planta. 2010;232(6):1511–1515. doi: 10.1007/s00425-010-1270-2. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd FE. Is Roridula a carnivorous plant? Can J Res. 1934;10:780–786. [Google Scholar]
- 19.Lloyd FE. In: The Carnivorous Plants. Verdoorn F, editor. Chronica Botanica Company; Waltham, MA: 1942. [Google Scholar]
- 20.Degreef JD. Fossil Aldrovanda. Int Carn Pl Newsletter. 1997;26:93–97. [Google Scholar]
- 21.Heřmanová Z, Kvaček J. Late Cretaceous Palaeoaldrovanda, not seeds of a carnivorous plant, but eggs of an insect. Journal of the National Museum (Prague) 2010;179(9):105–118. [Google Scholar]
- 22.Ellison AM, et al. Phylogeny and biogeography of the carnivorous plant family Sarraceniaceae. PLoS One. 2012;7(6):e39291. doi: 10.1371/journal.pone.0039291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schönenberger J, et al. Glandulocalyx upatoiensis, a fossil flower of Ericales (Actinidiaceae/Clethraceae) from the Late Cretaceous (Santonian) of Georgia, USA. Ann Bot (Lond) 2012;109(5):921–936. doi: 10.1093/aob/mcs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohlman-Adamska A. In: A graphic reconstruction of an “amber” forest. The Amber Treasure Trove. Part 1. The Tadeusz Giecewicz’s Collection at the Museum of the Earth, Polish Academy of Sciences, Warsaw. Kosmowska-Ceranowicz B, editor. Oficyna Wydawnicza Sadyba; Warsaw: 2001. pp. 15–18. [Google Scholar]
- 25.Mosbrugger V, Utescher T, Dilcher DL. Cenozoic continental climatic evolution of Central Europe. Proc Natl Acad Sci USA. 2005;102(42):14964–14969. doi: 10.1073/pnas.0505267102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collinson ME. Vegetational and floristic changes around the Eocene/Oligocene boundary in western and central Europe. In: Prothero DR, Berggren WA, editors. Eocene-Oligocene Climatic and Biotic Evolution. Princeton Univ Press; Princeton: 1992. pp. 437–450. [Google Scholar]
- 27.Collinson ME, Hooker JJ. Paleogene vegetation of Eurasia: framework for mammalian faunas. Deinsea. 2003;10:41–83. [Google Scholar]
- 28.Warren BH, Hawkins JA. The distribution of species diversity across a flora’s component lineages: dating the Cape’s “relicts.”. Proc Biol Sci. 2006;273:2149–2158. doi: 10.1098/rspb.2006.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldblatt P, Manning JC. Plant diversity of the Cape Region of southern Africa. Ann Mo Bot Gard. 2002;89(1):281–302. [Google Scholar]
- 30.Bacher J. 2001. [Test statistics to determine the number of clusters in Quick Cluster]. ZA-Information/Zentralarchiv für Empirische Sozialforschung 48:71–97. German.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.