Significance
Endothelial cells (ECs) form vasculature to provide vital elements, such as nutrients and oxygen, to tissues and organs in the body. Thus, creating ECs from nonvascular cells by transducing some transcription factors not only leads to the development of new strategies for patient-specific therapeutic angiogenesis, but also facilitates the maintenance of the solid organs that are regenerated from pluripotent stem cells. In this paper, we show that the single transcription factor ETV2, which is lentivirally transduced, induces expression of the multiple EC-specific molecules in coordination with endogenous FOXC2 in the fibroblasts, resulting in the conversion of primary human adult skin fibroblasts into functional ECs that form mature perfused vessels in vivo.
Keywords: endothelial cells, angiogenesis, ETV2, direct conversion
Abstract
Transplantation of endothelial cells (ECs) is a promising therapeutic approach for ischemic disorders. In addition, the generation of ECs has become increasingly important for providing vascular plexus to regenerated organs, such as the liver. Although many attempts have been made to generate ECs from pluripotent stem cells and nonvascular cells, the minimum number of transcription factors that specialize in directly inducing vascular ECs remains undefined. Here, by screening 18 transcription factors that are important for both endothelial and hematopoietic development, we demonstrate that ets variant 2 (ETV2) alone directly converts primary human adult skin fibroblasts into functional vascular endothelial cells (ETVECs). In coordination with endogenous FOXC2 in fibroblasts, transduced ETV2 elicits expression of multiple key endothelial development factors, including FLI1, ERG, and TAL1, and induces expression of endothelial functional molecules, including EGFL7 and von Willebrand factor. Consequently, ETVECs exhibits EC characteristics in vitro and forms mature functional vasculature in Matrigel plugs transplanted in NOD SCID mice. Furthermore, ETVECs significantly improve blood flow recovery in a hind limb ischemic model using BALB/c-nu mice. Our study indicates that the creation of ETVECs provides further understanding of human EC development induced by ETV2.
Human vascular endothelial cells (ECs) generated from pluripotent stem cells (PSCs), including embryonic stem cells and induced PSCs (iPSCs), or nonvascular cells have great therapeutic potential for treating ischemic vascular diseases (1, 2). In addition, the generation of ECs has become increasingly important for providing vascular plexus to regenerated organs, such as the liver (3). iPSCs are an especially promising source for creating ECs, although the main limitation of applying iPSCs is the risk of incomplete differentiation and tumorigenicity (4, 5). To bypass the fully pluripotent state, multiple research groups have generated ECs by culturing fibroblasts transduced with iPSC-inducing factors (OCT4, SOX2, KLF4, and c-MYC) under defined EC culture conditions (6, 7). The long-term stability of these ECs remains to be established, however; the PSC-derived ECs often show poor proliferative abilities and drift into nonvascular lineages (8).
Combined expression of multiple transcription factors specific to a particular lineage has been demonstrated to change somatic cell fate by bypassing pluripotency; for example, Gata4, Mef2c, and Tbx5 convert murine cardiac fibroblasts into functional cardiomyocytes (9), and Ascl1, Brn2, and Myt1 induce neurons from murine fibroblasts (10). This “direct lineage conversion” approach offers promising prospects for creating cells of biomedical interest for cellular replacement therapies. This approach is also useful for studying the physiological mechanisms of transcriptional reprogramming, such as the establishment of cellular identity, and the transcriptional regulatory networks that drive terminal differentiation and functional maturation (11).
ECs and hematopoietic cells both originate from a common precursor, the hemogenic endothelium, which is present in the yolk sac, the aorta-gonad-mesonephros region, and the placenta (12). Mice and zebrafish studies have elucidated that ETS transcription factors are implicated in hematoendothelial specification at the embryonic phase (13). Erg (v-ets avian erythroblastosis virus E26 oncogene homolog) associates with Klf2 to induce proto-oncogene Flk1 expression during vascular development (14); Fli1 acts early in hemangioblast development by functioning upstream of many early endothelial genes, including Tal1 and Gata (15); and ets variant 2 (Etv2) is essential for the specification of endothelial and hematopoietic lineages early in gestation (16). Thus, the ETS family factors are more likely candidates for direct induction of ECs from nonvascular cells. It was recently reported that a combination of three ETS factors—ETV2, FLI1, and ERG—with short-term TGFβ inhibition directly converts human amniotic cells to functional ECs that show stable EC phenotypes (8). The amniotic cell-derived ECs can overcome PSC-derived EC issues, such as poor proliferation and lineage instability. This strategy of generating amniotic cell-derived ECs is rather complex, however. More importantly, it hardly induces ECs from human adult fibroblasts (8), which are readily accessible EC materials for facilitating autologous therapeutic angiogenesis.
Thus, transcription factors specialized in direct conversion of human fibroblasts into ECs need to be explored. Inthis study, by screening 18 transcription factors, including the ETS family factors, we demonstrate that the single ETS factor ETV2 is sufficient for directly converting primary human adult skin fibroblasts into functional ECs (ETVECs).
Results
Screening for Endothelial-Inducing Transcription Factors.
To identify human endothelial fate-inducing factors, we cloned a total of 18 transcription factors (TFs) (SI Appendix, Table S1) that are important for both endothelial and hematopoietic development. To do so, we infected the human embryonic lung fibroblast cell line HFL-1 with a pool of 18 TF lentiviruses. Fourteen days later, an EC marker, CD31−, and an EC and hemogenic endothelium marker, vascular endothelial growth factor (VEGF) receptor 2 (VEGF-R2)-expressing cells (12, 17), emerged from the Venus+ HFL-1 cells (SI Appendix, Fig. S1A). In addition, some of the Venus+ HFL-1 cells demonstrated acetylated low-density lipoprotein (AcLDL) uptake in a punctuate staining pattern characteristic of ECs (2) (SI Appendix, Fig. S1B). Sorted CD31+ HFL-1 cells multiplied under EC culture conditions, and 28 d after infection, the expanding Venus+ cells exhibited a cobblestone-like morphology featuring vascular ECs (SI Appendix, Fig. S1C). Flow cytometry analysis revealed that the CD31+ HFL-1 cells expressed multiple EC surface markers, including CD31, VEGF-R2, CD34, Tie2, neuropilin-1 (NRP1), and CXCR4, but not the hematopoietic cell marker CD45 (SI Appendix, Fig. S1D). Because they down-regulated the expression of a fibroblast marker, COL1A2 (SI Appendix, Fig. S1E), and formed an EC-specific marker, VE cadherin-positive lumens in the capillary-like structures on Matrigel-coated plates (SI Appendix, Fig. S1 F and G), we conclude that the Venus+CD31+ HFL-1 cells are ECs (HFL-ECs).
Vascular ECs are classified into three subsets according to the objective of their development: venous, arterial, and lymphatic ECs. Each EC subset is known to express specific markers (18, 19). We found that HFL-ECs preferentially express venous EC markers (NRP2, NR2F2, and EPHB4) at levels comparable to those in human umbilical vein ECs (HUVECs) (SI Appendix, Fig. S1E). Conversely, their expression levels of arterial (JAG1, EFNB2, and HEY1) and lymphatic (PROX1 and SOX18) EC markers does not surpass those of HUVECs (SI Appendix, Fig. S1E). These results suggest that at least one of the 18 TFs directly converts HFL-1 cells into ECs.
To identify which of the 18 TFs are involved in the induction of HFL-ECs, we used PCR to explore the factors integrated into the HFL-EC genome. We designed two types of primer pairs for the PCR analyses. One of these primer pairs amplified all of the factors integrated along with internal ribosomal entry site (IRES) sequences at the same time [SI Appendix, Fig. S2A (B) and Table S2]. Consequently, the type of factor was identified using the PCR product size (SI Appendix, Table S1). In the other primer pair, the forward primers were designed for each target and the reverse primer was shared [SI Appendix, Fig. S2A (C) and Tables S1 and S2]. Both of these PCR strategies revealed that ETV2 and HOPX are integrated into the HFL-EC genome (SI Appendix, Fig. S2 B and C).
To verify whether the two factors convert HFL-1 cells into HFL-ECs, we transduced ETV2 and HOPX alone and together into HFL-1 cells. Whereas ETV2 alone induced CD31+ cells from HFL-1 cells, HOPX did not contribute to EC induction (SI Appendix, Fig. S2D). Thus, we consider HOPX to be incidentally expressed in HFL-ECs. In addition, ETV2 alone also induced CD31+ cells from not only the human neonatal skin fibroblast cell line NB1RGB, but also primary human adult skin fibroblasts (HAFs) (SI Appendix, Figs. S2E and S3). Taken together, these results demonstrate that ETV2 alone directly induces ECs from human fibroblasts.
Basic fibroblast growth factor (bFGF) and VEGF are essential for EC development and survival (20). Thus, we investigated the effects of external addition of these two growth factors on ETVEC induction from ETV2-transduced HAFs. Compared with ordinary EGM-2 medium, EGM-2 medium replenished with VEGF and bFGF (both 10 ng/mL) gave rise to more CD31+ clones and significantly increased the numbers of ETVECs from ETV2-transduced HAFs (SI Appendix, Fig. S3 A and B). In addition, these growth factors up-regulated CD31 and VEGF-R2 expression levels on ETVECs (SI Appendix, Fig. S3C). These results suggest that VEGF and bFGF supplementation improves both the efficiency of the direct conversion of ETV2-transduced HAFs to ETVECs and the proliferation of ETVECs. Thus, we used EGM-2 medium supplemented with both factors in our subsequent experiments.
The ETS family member ETV2 consists of the transactivation domain (TAD) at its N terminus and the DNA-binding ETS domain at its C terminus (21). To determine which ETV2 domain is essential for the EC induction, we constructed four types of HA-tagged ETV2 truncations for lentivirus vectors (SI Appendix, Fig. S4A). At 15 d after the infection of HAFs with these lentiviruses, EC induction was evaluated by flow cytometry analysis. TAD- or ETS domain-fully deleted ETV2 failed to convert HAFs to ETVECs (SI Appendix, Fig. S4B). Moreover, both types of TAD-partially deleted ETV2 induced VEGF-R2 expression but failed to induce CD31 expression. These results collectively demonstrate that ETV2 directly converts human fibroblasts into ECs, and that its whole TAD is essential for the EC induction.
Optimal ETV2 Expression Level Is Essential for EC Conversion from HAFs.
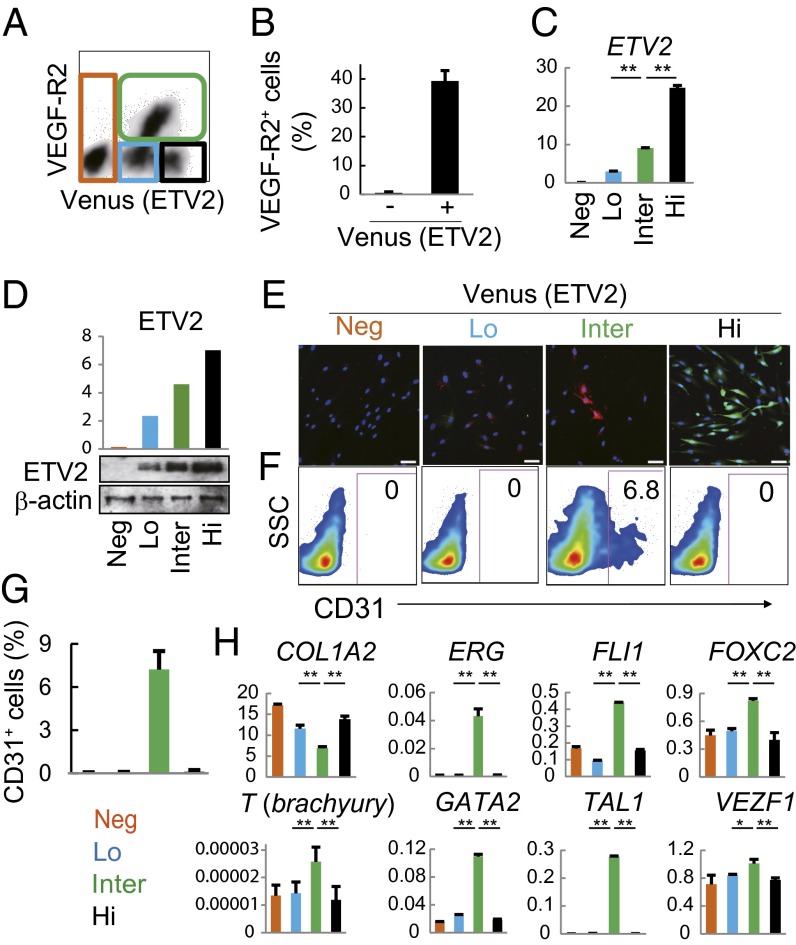
To further investigate how ETV2 induces EC transformation from HAFs, we transduced ETV2 alone into HAFs via lentivirus infection. Fourteen days later, ∼40% of the Venus (ETV2)-positive HAFs expressed VEGF-R2 (Fig. 1 A and B). According to the Venus (ETV2) and VEGF-R2 expression, at day 14, the ETV2-transduced HAFs consisted of four distinct populations (Fig. 1A), each of which expressed particular levels of ETV2 mRNA and protein (Fig. 1 C and D). The VEGF-R2+ population (Fig. 1A, green line) that expressed ETV2 at intermediate levels yielded some AcLDL uptake-positive and CD31+ cells (Fig. 1 C–G), whereas the ETV2-low and -high populations (Fig. 1 C and D), indicated in blue and black lines, respectively, in Fig. 1A, did not express VEGF-R2 or generate CD31+ cells (Fig. 1 A, F, and G). To explore the causes of such differences among these populations, we focused on the expression of the transcription factors that are important for hematoendothelial development (1, 13). Mesodermal progenitor marker T (Brachyury) and multiple endothelial development factors (ERG, FLI1, FOXC2, GATA2, TAL1, and VEZF1) (Fig. 1H), but not hematopoietic cell markers PTPRC (CD45) and ITGA2B (CD41) (SI Appendix, Fig. S5), were significantly up-regulated only in the VEGF-R2+ population. Moreover, COL1A2 was significantly down-regulated in this population (Fig. 1H).
Fig. 1.
Optimal ETV2 expression levels evoke endothelial properties from HAFs. HAFs at 14 d after ETV2 infection were analyzed. (A, B, F, and G) Flow cytometry analysis. The percentage of CD31+ cells in each population is shown in F and G. (C and H) Quantitative RT-PCR. Gene expression levels are relative to HPRT1. *P < 0.05; **P < 0.01, two-sided Student t test. (D) Whole-cell lysates subjected to Western blot analysis. ETV2 expression levels are relative to β-actin. (E) Dil-AcLDL uptake assay. Red, Dil-AcLDL; green, Venus. Data are representative of four independent cell cultures [mean ± SD; n = 4 cultures (B and G) or triplicate (C and H)]. Neg, negative; Lo, low; Inter, intermediate, Hi, high. (Scale bar: 50 μm.)
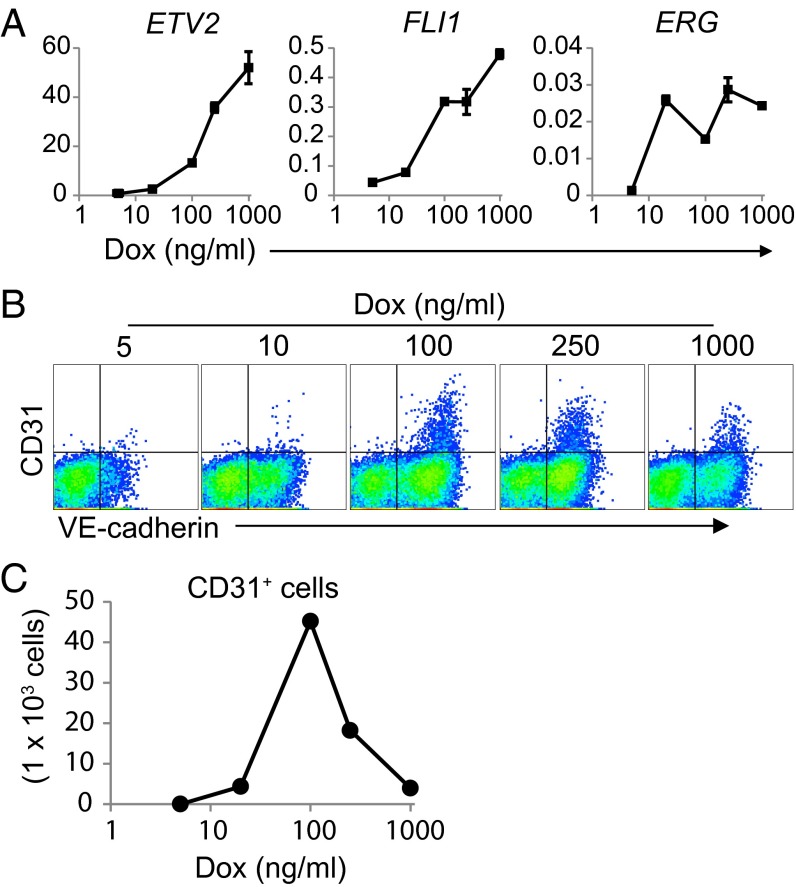
To clarify the relationship between the intensity of ETV2 expression in HAFs and EC induction from HAFs, we regulated ETV2 expression levels in HAFs using a doxycycline (Dox)-inducible system. Consequently, whereas the expression intensity of induced ETV2 and endogenous FLI1 in HAFs depended on the Dox concentration, endogenous ERG expression levels in HAFs did not increase at higher Dox concentrations (Fig. 2A). Interestingly, a defined Dox concentration (100 ng/mL) most efficiently induced ETVECs from ETV2-induced HAFs, and lower and higher concentrations than this level both reduced ETVEC induction somewhat (Fig. 2 B and C). Collectively, these results suggest that ETV2 expression at optimal levels most likely is required for the direct conversion of HAFs into ETVECs.
Fig. 2.
Higher ETV2 expression levels suppress ETVEC induction from HAFs. Dox-inducible ETV2 and rtTA-transduced HAFs were cultured in the presence of various Dox concentrations. (A) At 14 d after culture, Venus+ cells were subjected to quantitative RT-PCR. Gene expression levels are relative to HPRT1 (mean ± SD; triplicate). (B and C) At 14 d after the culture, EC induction was determined by flow cytometry analysis. Gated on 7-AAD−Venus+ cells. Absolute numbers of ETVECs are shown in C. Data are representative of three independent cell cultures.
Transient ETV2 Expression Is Sufficient to Directly Convert Part of HAFs into ETVECs.
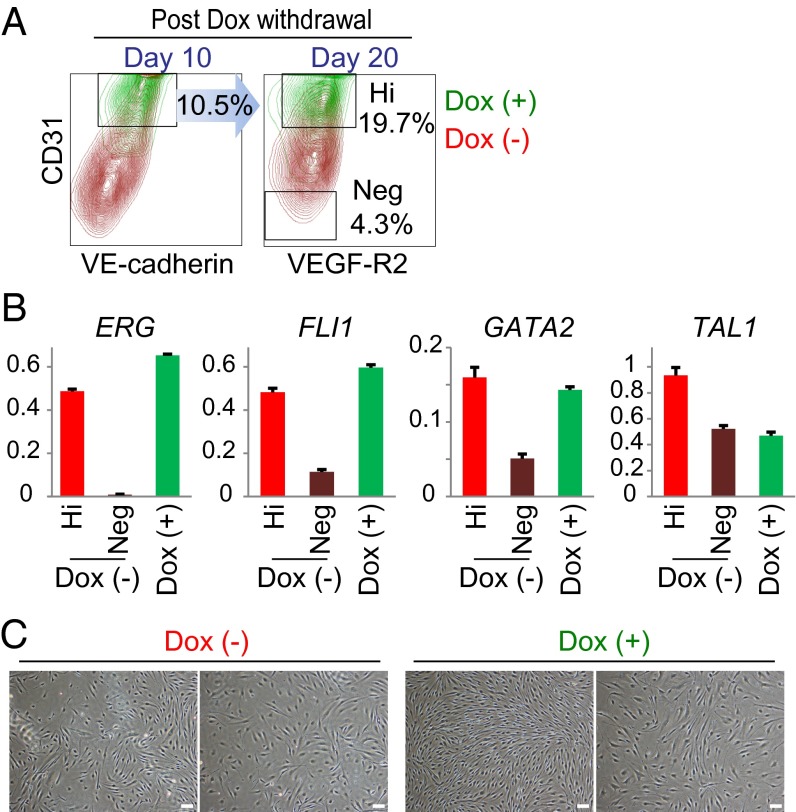
To investigate whether ETVECs require continuous ETV2 expression for retaining their EC phenotypes, we shut off ETV2 expression in ETVECs using the Dox-inducible system. Consequently, at 10 d after ETVECs were cultured without Dox on day 21, ∼10% of these ETVECs expressed CD31 and VE-cadherin at the same levels as ETVECs continuously exposed to Dox (Fig. 3A, Left). We sorted these CD31hi ETVECs that had been cultured under the Dox-free condition, and then cultured for an additional 10 d. Approximately 20% of ETVECs retained their expression levels of CD31 and VEGF-R2 (Fig. 3A, Right). Quantitative RT-PCR revealed that CD31hi ETVECs that had been cultured without Dox for 20 d retained similar expression levels of ERG, FLI1, GATA2, and TAL1 as the Dox-exposed ETVECs, although CD31− cells lost the expression of these molecules (Fig. 3B). In addition, multiple EC colonies were observed at 20 d after Dox withdrawal (Fig. 3C). These results demonstrate that transient ETV2 expression is sufficient to directly convert part of HAFs into ETVECs that stably maintain EC properties.
Fig. 3.
Transient ETV2 expression is sufficient to directly convert part of HAFs into ETVECs. (A) Dox-inducible ETV2 and rtTA-transduced HAFs were cultured in the presence of 100 ng/mL Dox for 21 d. The sorted CD31+ cells were cultured for another 10 d in the presence or absence of Dox. CD31hi cells were sorted again, then cultured for an additional 10 d under the same culture conditions. Numbers on the contour plots indicate the percentage of cells under a Dox-free culture condition. (B) At 20 d after the Dox withdrawal, CD31hi and CD31− cells were subjected to quantitative RT-PCR. Gene expression levels relative to HPRT1 (mean ± SD; triplicate). (C) Photos of the two representative EC colonies at 20 d after the culture with or without Dox. Data are representative of three independent cell cultures. Hi, CD31hi cells; Neg, CD31− cells. (Scale bar: 50 μm.)
Endogenous Forkhead Box C2 Expression in HAFs Is Essential for ETVEC Induction.
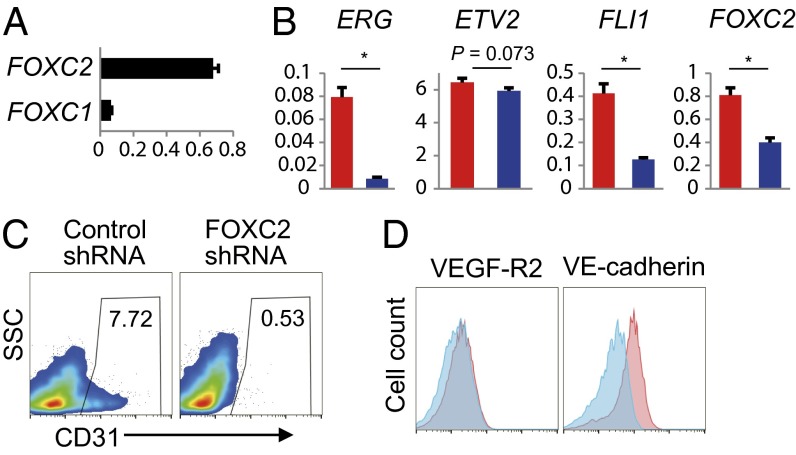
Etv2 is known to require its combination with FoxC for vascular gene expression in Xenopus and zebrafish embryos (13, 22), evoking the possibility of FOXC involvement in ETVEC induction. Thus, we first investigated FOXC1 and FOXC2 mRNA expression in HAFs. Quantitative RT-PCR analysis revealed that HAFs expressed both endogenous FOXC1 and FOXC2 mRNAs, and that FOXC2 expression was higher than FOXC1 expression (Fig. 4A). Knockdown of FOXC2 in HAFs resulted not only in significantly reduced ERG and FLI1 expression in ETV2-transduced HAFs, but also in markedly suppressed ETVEC induction (Fig. 4 B–D). These results demonstrate that endogenous FOXC2 in HAFs enables ETV2 to directly convert HAFs into ETVECs.
Fig. 4.
Endogenous FOXC2 in HAFs is essential for ETVEC induction. (A) Expression levels of FOXC genes in HAFs. (B–D) ETV2 was transduced into FOXC2 shRNA- and control shRNA-expressing HAFs. (B–D) Fifteen days later, Venus+ cells were subjected to quantitative RT-PCR analysis (B) and flow cytometry analysis (C and D). (B) Blue and red bars indicate FOXC2 and control shRNA, respectively. Gene expression levels are relative to HPRT1 (mean ± SD; triplicate). *P < 0.01, two-sided Student t test. (C and D) Plots represented by gates on 7-AAD−Venus+ cells. Results reported as percentage of CD31+ cells in the Venus+ cells. (D) Blue and red histograms indicate FOXC2 and control shRNA, respectively. Data are representative of three independent cell cultures.
ETVECs Represent Proliferative ECs.
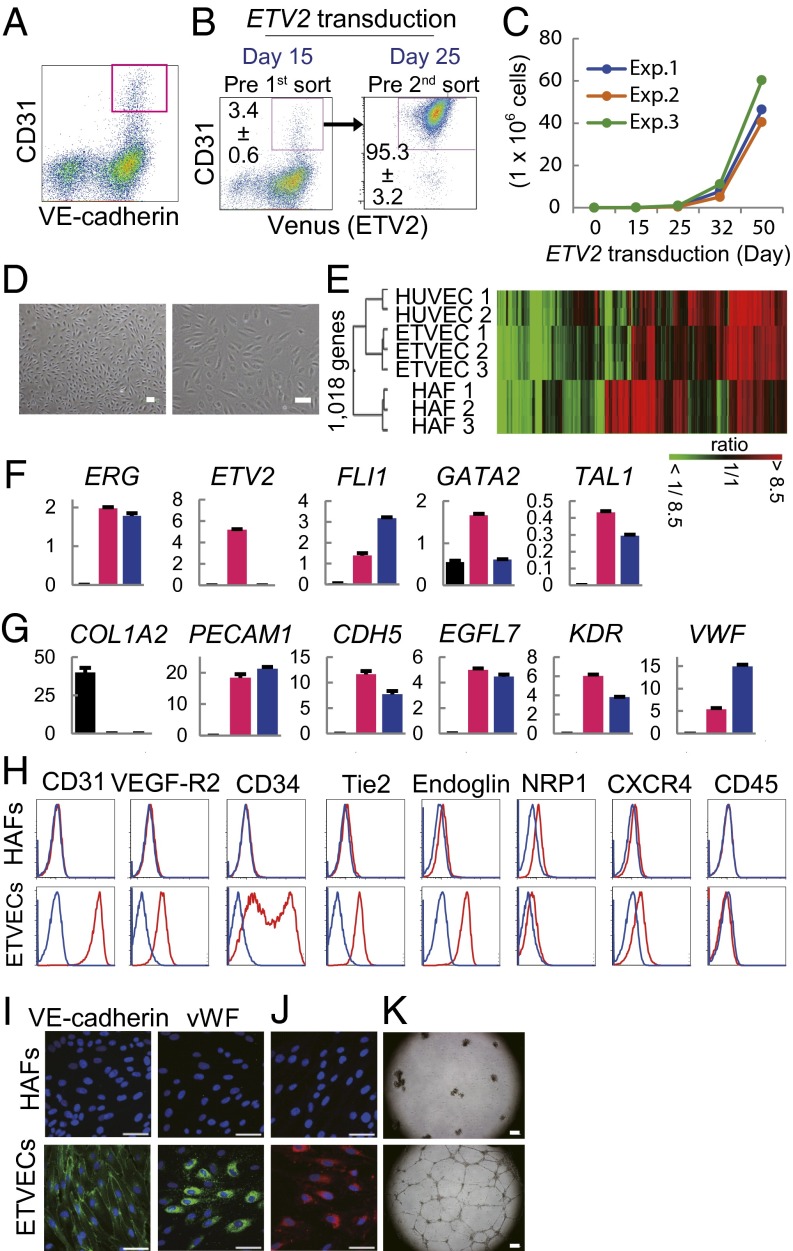
To verify ETVEC properties, we purified CD31+ cells from the ETV2-transduced HAFs using a cell sorter. Fifteen days after ETV2 induction into HAFs, CD31+ cells that also expressed VE-cadherin were isolated, and on day 25, the expanding CD31+ cells were purified again (Fig. 5 A and B). ETVECs continued to multiply beyond 50 d after ETV2 transduction (Fig. 5C) and exhibited a cobblestone-like morphology (Fig. 5D and SI Appendix, Fig. S6A). Global gene expression analysis revealed that ETVECs clustered with HUVECs rather than with their HAFs of origin, as illustrated by hierarchal clustering (Fig. 5E). Many EC phenotype-representative genes, including ESMA and SCARF1, were highly enriched, and the fibroblast markers TWIST2 and ZEB2 were down-regulated in ETVECs (SI Appendix, Fig. S6 B and C).
Fig. 5.
ETVECs represent proliferative ECs. (A) HAFs at 15 d after ETV2 infection were subjected to flow cytometry analysis. The dot plots are represented by gates on 7-AAD−Venus+ cells. The pink quadrangle indicates ETVECs. (B) Purifying ETVECs through sorting CD31+ cells. (C) Absolute numbers of ETVECs. Three representative experiments are presented. (D) Microscopic images of ETVECs (Exp.1) at 32 d after ETV2 transduction. (Left) Low-magnification image. (Right) High-magnification image. (E) Heat-map image and hierarchical clustering of the DNA microarray data. (F and G) Quantitative RT-PCR for transcription factors (F) and EC effector molecules (G) performed with HAFs (black), ETVECs (pink), and HUVECs (blue). Gene expression levels are relative to HPRT1. (H) Flow cytometry analysis of HAFs and ETVECs. Red and blue lines indicate targets and isotype controls, respectively. (I) Immunofluorescence cytostaining. (J) Dil-AcLDL uptake assay. (K) Capillary-like structure formation on Matrigel-coated plates. Data are representative of 10 independent cell cultures (B) or four independent cell cultures (A, C, and F–K). Data are mean ± SD. n = 10 cultures (B) or triplicate (F and G). (Scale bars: 50 μm in D, I, and J; 300 μm in K.)
Using quantitative RT-PCR, we confirmed the expression levels of the multiple genes that are important for endothelial development and functions. ETVECs expressed not only ETV2 at levels comparable to those of the VEGF-R2+ population (Figs. 1H and 5F) but also multiple endothelial developmental factors (ERG, FLI1, GATA2, and TAL1) at levels similar to those of HUVECs (Fig. 5F). ETVECs expressed a series of endothelial effector molecules including a mature endothelial marker, von Willebrand factor (vWF), at both mRNA and protein levels (23), but lacked COL1A2 and surface CD45 expression (Fig. 5 G–I and SI Appendix, Table S3). An analysis of the EC subset markers showed that ETVECs expressed the venous EC markers NRP2, NR2F2, and EPHB4, but not the arterial EC marker EFNB2 or lymphatic EC marker PROX1 (SI Appendix, Fig. S7). Although the expression levels of these venous EC markers were lower in ETVECs than in HFL-ECs (SI Appendix, Fig. S1E), ETVECs seem to be more likely a venous EC subset. Functionally, ETVECs showed uptake of AcLDL and formation of capillary-like structures on Matrigel-coated plates (Fig. 5 J and K). These results indicate that ETVECs represent proliferative ECs.
HFL-ECs expressed ERG and FLI1 at significantly higher levels than ETVECs (SI Appendix, Fig. S8). Interestingly, the constitutive expression levels of FOXC1 and FOXC2 were significantly higher in original HFL-1 cells than in HAFs (SI Appendix, Fig. S9). These results are consistent with the results shown in Fig. 4, supporting the importance of endogenous FOXCs in converting human fibroblasts into ECs by transducing ETV2.
ETVECs Are Engraftable and Form Mature Functional Perfused Vessels in Vivo.
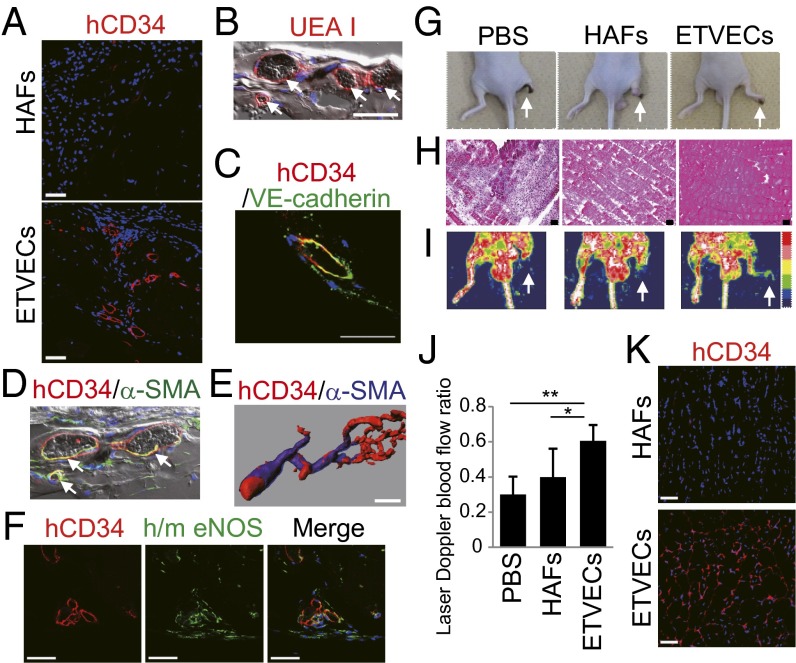
To evaluate the angiogenic potential of ETVECs in vivo, we implanted Matrigel-suspended ETVECs and HAFs s.c. into NOD SCID mice. At 28 d after implantation, ETVECs, but not HAFs, formed vasculature that was recognized not only by a human CD34-specific mAb, but also by Ulexeuropaeus agglutinin I (UEA I) that binds to human, but not murine, ECs (1, 24) (Fig. 6 A and B). The ETVEC-constituting vessels also expressed VE-cadherin (Fig. 6C). Nearly all of the ETVEC-constituting vessels allowed erythrocyte circulation through the vessels (Fig. 6 B and D). Moreover, approximately half of the ETVEC-constituting vessels were stabilized by association with α-smooth muscle actin (SMA)-positive mural cells for at least 42 d after implantation (Fig. 6 D and E). Notably, part of the ETVEC-constituting vessels were found to express endothelial nitric oxide synthase (eNOS) (Fig. 6F), which synthesizes nitric oxide to promote EC survival and prevent atherosclerosis (25).
Fig. 6.
ETVECs establish mature functional vasculature in vivo. (A–F) Images of Matrigel plugs extracted from NOD SCID mice 28 (A–D and F) and 42 (E) days after the implantation of ETVECs (A–F) and HAFs (A). (B and D) The fluorescence channels were merged over bright-field pictures. White arrows indicate that erythrocytes were circulating in ETVEC-constituting vasculature. (E) Three-dimensional structure of the ETVEC-constituting vasculature. (F) Immunofluorescence images showing human CD34 (hCD34) (Left), human/mouse eNOS (h/m eNOS) (Center), and a merged image (Right) (Hoechest 33342 in blue). (G–K) Hind limb ischemia model of BALB/c-nu mice at 14 d after transplantation. (G) Representative photographs. White arrows indicate the right ischemic hind limbs. (H) Hematoxylin and eosin staining of the adductor muscles of ischemic limbs. (I) Doppler images of superficial blood flow in lower limbs. Red to white color and dark-blue color on the image indicate high and low perfusion signals, respectively. White arrows indicate the right ischemic hind limbs. (J) Comparison of perfusion recovery in ischemic hind limbs. Data are mean ± SD (PBS, n = 10; HAFs, n = 10; ETVECs, n = 6). *P < 0.03; **P < 0.001, one-way ANOVA. (K) Immunofluorescence images of the cell-transplanted adductor muscles. Data are representative of three independent experiments. (Scale bars: 50 μm.)
To assess the therapeutic benefit of ETVEC transplantation, we then injected the ETVECs and HAFs i.m. into the ischemic hind limbs of BALB/c-nu mice that had been subjected to femoral artery ligation (26). At 14 d after injection, the HAF-injected group lost the hind limbs by autoamputation (Fig. 6G), caused by extensive muscle necrosis in the ischemic regions (Fig. 6H). In contrast, i.m. transplantation of ETVECs protected the ischemic muscles from necrosis (Fig. 6H), which protected the hind limbs from complete damage (Fig. 6G). Among the three groups, the PBS-injected group exhibited the most severe muscle deterioration of the ischemic limbs (Fig. 6H). Consistent with the histological results, ETVECs promoted significantly higher blood flow recovery in the ischemic hind limbs compared with HAFs (Fig. 6 I and J). ETVECs, but not HAFs, developed human CD34+ blood vessels in the muscles of the ischemic limbs (Fig. 6K and SI Appendix, Fig. S10A). Whereas HAFs expressed FGF2 and VEGFA, but not CSF3, ETVECs showed an opposite expression pattern of these angiogenic growth factors, as with the case of HUVECs (27) (SI Appendix, Fig. S10B). Despite the differences in expression pattern between HAFs and ETVECs, there were no apparent differences in murine EC incorporation into the muscles (SI Appendix, Fig. S10C). We think that the comprehensive expression of angiogenic growth factors, including these three factors, of ETVECs may be comparable to that of HAFs. In addition, proteases, especially cathepsins, secreted from ECs have been reported to have a crucial role in angiogenesis (28, 29). Quantitative RT-PCR analysis revealed that ETVECs expressed significantly higher amounts of various cathepsin mRNAs compared with HUVECs (SI Appendix, Fig. S11). These results support the capacity of ETVECs for constituting vessels in the ischemic regions.
Collectively, the foregoing observations demonstrate that ETVECs survive and form mature functional perfused vasculature after transplantation in vivo.
Discussion
Our study demonstrates that the ETS factor ETV2 is sufficient to induce expression of multiple key endothelial development factors, including FLI1 and ERG, in human fibroblasts; consequently, ETV2-expressing fibroblasts convert into functional ECs. ETV2 was originally found to regulate vascular development and angiogenesis at the embryonic phase (13). Several murine studies have shown that although ETV2 is a master regulator of hematoendothelial development from the primitive mesoderm, its expression is transient in early murine embryos, and that mature ECs no longer express ETV2 (16). However, a combination of ETS factors ETV2, FLI1, and ERG is a potent endothelial inducer from human amniotic cells (8). More recently, ETV2 has been reported to transdifferentiate skeletal muscle into functional ECs in zebrafish (30). Taken together, these findings indicate that ETV2 is a core factor for directly converting somatic cells into ECs.
ETV2 functions in combination with FOXC2 through a composite DNA-binding site, the FOX:ETS motif. The evolutionarily conserved FOX:ETS motif is identified within numerous endothelial enhancers and promoters, including those from Tal1, Tie2, Flk1, and Notch4 (22), and expression of these genes is strongly enhanced by simultaneous binding of ETV2 and FOXC2 to the cis-acting element. Our study indicates that human fibroblasts express substantial levels of endogenous FOXC2 under steady-state conditions, and that knockdown of FOXC2 in human fibroblasts results in loss of ETVEC generation, which clearly explains why transducing only ETV2 into human fibroblasts was sufficient to convert the fibroblasts into ETVECs. After binding to the FOX:ETS motif, ETV2 is thought to recruit cofactors to induce posttranscriptional modifications to ETV2 and/or alter DNA methylation states of the endothelial genes, resulting in facilitation of endothelial gene expression (31). We found that TAD-fully deleted ETV2 failed to convert human fibroblasts to ECs, which supports the foregoing hypothesis because the truncated ETV2 has no capacity to interact with another factor via the TAD. In addition, two different types of TAD-partially deleted ETV2 induced VEGF-R2 expression, but not CD31 expression; thus, both types of ETV2 truncate failed to induce ETVECs. These results suggest that ETV2 might require different cofactors to induce endothelial genes. A histone demethylase, Jmjd1a, has been shown to suppress ETV2 to activate transcription from the matrix metalloproteinase-1 promoter by directly interacting with ETV2 (21, 32). Thus, Jmjd1a is more likely to be a repressor of ETV2 during vasculogenesis. In contrast, coactivators that interact with ETV2 remain to be identified.
In general, direct lineage conversion requires high expression levels of the candidate factors to change the preexisting epigenetic state of target cells (11). However, we found that optimal ETV2 expression levels were essential for converting human fibroblasts into ETVECs; lower or higher ETV2 expression failed to surmount the fibroblastic state. In the case of amniotic cells, proper stoichiometric amounts of ETV2 relative to FLI1 and ERG are important to allow conversion to stable ECs (8), which is consistent with our results using a Dox-inducible system indicating that higher Dox concentrations efficiently induced expression of ETV2 and endogenous FLI1, but not of endogenous ERG, resulting in failure of ETVEC induction from HAFs (Fig. 2). To increase the efficiency of ETVEC induction from fibroblasts, how ETV2 expression levels affect the epigenetic state of endothelial genes needs to be elucidated.
Although ETVECs demonstrated their substantial maturity by producing vWF and expressing the angiocrine factor EGFL7 during in vitro culture, NOS3 was not detected in ETVECs at any time point (SI Appendix, Fig. S12). This fact suggests that, at least in the defined EC culture medium, ETV2 expression may be insufficient to induce expression of some endothelial genes independent of the FOX:ETS motif, such as NOS3 (22). Interestingly, in day 28 Matrigel plugs, some of the ETVECs that constituted vessels expressed eNOS (Fig. 6F). An intriguing explanation for this finding is that laminar shear stress and/or angiopoietin-1 produced by α-SMA+ mural cells may induce eNOS expression in the ETVECs (25, 33). Thus, ETVECs may obtain further maturity in the appropriate tissue microenvironment to establish stable and long-lasting vasculature.
Analyzing the expression pattern of the EC subset markers revealed that ETVECs had venous EC properties. In contrast, ETVECs constituted vessels that were associated with α-SMA+ mural cells in the Matrigel plugs, which characterizes the arteriole structure (34). As mentioned earlier, the tissue microenvironment and/or the association with pericytes may direct the characteristics of ETVECs to the arteriole or venule.
Our study demonstrates that stable ETV2 expression induces numerous endothelial-specific genes other than those evidently regulated by the FOX:ETS motif from human fibroblasts. However, whether expression of these genes is induced directly via unknown ETV2 actions, such as partnering transcription factors other than FOXC2, or secondarily by other gene products, remains unclear. Our novel EC induction system may extensively elucidate the mechanism of endothelial-specific gene expression by ETV2 and provide further understanding of human endothelial development. It also could facilitate the exploration of innovative strategies for patient-specific therapeutic angiogenesis.
Materials and Methods
Details are provided in SI Appendix, SI Text.
Cell Culture and Lentivirus Infection.
After transduction of ETV2, fibroblasts were cultured on type I collagen-coated dishes in EGM-2 medium supplemented with 10 ng/ml VEGF and bFGF. At day 15 and 25 after the infection, CD31+ cells were sorted.
Angiogenesis Assay in Vivo.
For the Matrigel plus assay, cells suspended in Matrigel were subcutaneously implanted in NOD SCID mice, the plugs were removed 28 and 42 days later, and they were subjected to immunofluorescence staining. For the hind-limb ischemic model, cells or PBS were injected into the adductor muscle of the ischemic thigh. Fourteen days later, neoangiogenesis in the ischemic limbs was evaluated.
Supplementary Material
Acknowledgments
We thank Y. Takihara (Hiroshima University) and K. Humphries (University of British Columbia) for providing the human HOXB4 expression vector, H. Miyoshi (RIKEN) and A. Miyawaki (RIKEN) for the lentivirus vectors, S. A. Mani (University of Texas M.D. Anderson Cancer Center) for the human FOXC2 shRNA vectors, and R. Komine for determining CD31 expression of clones. This study was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Japan Society for the Promotion of Science [Grant-in-Aid for Scientific Research A 22249009 (to A.Y.)]; the Core Research for Evolutional Science and Technology Program (“Reprograming of immune system by modulation of intracellular signal transduction”) of the Japan Science and Technology Agency (A.Y.); the Takeda Science Foundation (R.M., T. Shichita, and T. Sekiya); the Uehara Memorial Foundation (A.Y.); the Mochida Memorial Foundation (R.M.); the SENSHIN Medical Research Foundation (R.M.); and Keio Gijuku Academic Development Funds (R.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE48980).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413234112/-/DCSupplemental.
References
- 1.Kurian L, et al. Conversion of human fibroblasts to angioblast-like progenitor cells. Nat Methods. 2013;10(1):77–83. doi: 10.1038/nmeth.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu SJ, et al. Generation of functional hemangioblasts from human embryonic stem cells. Nat Methods. 2007;4(6):501–509. doi: 10.1038/nmeth1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takebe T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499(7459):481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 4.Sancho-Martinez I, Baek SH, Izpisua Belmonte JC. Lineage conversion methodologies meet the reprogramming toolbox. Nat Cell Biol. 2012;14(9):892–899. doi: 10.1038/ncb2567. [DOI] [PubMed] [Google Scholar]
- 5.Vierbuchen T, Wernig M. Direct lineage conversions: Unnatural but useful? Nat Biotechnol. 2011;29(10):892–907. doi: 10.1038/nbt.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margariti A, et al. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Natl Acad Sci USA. 2012;109(34):13793–13798. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, et al. Conversion of human fibroblasts to functional endothelial cells by defined factors. Arterioscler Thromb Vasc Biol. 2013;33(6):1366–1375. doi: 10.1161/ATVBAHA.112.301167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsberg M, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFβ suppression. Cell. 2012;151(3):559–575. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ieda M, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vierbuchen T, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vierbuchen T, Wernig M. Molecular roadblocks for cellular reprogramming. Mol Cell. 2012;47(6):827–838. doi: 10.1016/j.molcel.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirschi KK. Hemogenic endothelium during development and beyond. Blood. 2012;119(21):4823–4827. doi: 10.1182/blood-2011-12-353466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell. 2009;16(2):180–195. doi: 10.1016/j.devcel.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meadows SM, Salanga MC, Krieg PA. Kruppel-like factor 2 cooperates with the ETS family protein ERG to activate Flk1 expression during vascular development. Development. 2009;136(7):1115–1125. doi: 10.1242/dev.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F, Walmsley M, Rodaway A, Patient R. Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr Biol. 2008;18(16):1234–1240. doi: 10.1016/j.cub.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 16.Lee D, et al. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2(5):497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa G, Kouskoff V, Lacaud G. Origin of blood cells and HSC production in the embryo. Trends Immunol. 2012;33(5):215–223. doi: 10.1016/j.it.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Atkins GB, Jain MK, Hamik A. Endothelial differentiation: Molecular mechanisms of specification and heterogeneity. Arterioscler Thromb Vasc Biol. 2011;31(7):1476–1484. doi: 10.1161/ATVBAHA.111.228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dejana E. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res. 2010;107(8):943–952. doi: 10.1161/CIRCRESAHA.110.223750. [DOI] [PubMed] [Google Scholar]
- 20.Marcelo KL, Goldie LC, Hirschi KK. Regulation of endothelial cell differentiation and specification. Circ Res. 2013;112(9):1272–1287. doi: 10.1161/CIRCRESAHA.113.300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Haro L, Janknecht R. Functional analysis of the transcription factor ER71 and its activation of the matrix metalloproteinase-1 promoter. Nucleic Acids Res. 2002;30(13):2972–2979. doi: 10.1093/nar/gkf390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Val S, et al. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell. 2008;135(6):1053–1064. doi: 10.1016/j.cell.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108(6):2095–2105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skovseth DK, Yamanaka T, Brandtzaeg P, Butcher EC, Haraldsen G. Vascular morphogenesis and differentiation after adoptive transfer of human endothelial cells to immunodeficient mice. Am J Pathol. 2002;160(5):1629–1637. doi: 10.1016/S0002-9440(10)61110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10(3):165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki K, et al. Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy. Proc Natl Acad Sci USA. 2006;103(39):14537–14541. doi: 10.1073/pnas.0604144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rackwitz L, et al. Stem cell- and growth factor-based regenerative therapies for avascular necrosis of the femoral head. Stem Cell Res Therapy. 2012;3(1):7. doi: 10.1186/scrt98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbich C, et al. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med. 2005;11(2):206–213. doi: 10.1038/nm1182. [DOI] [PubMed] [Google Scholar]
- 29.van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2006;26(4):716–728. doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- 30.Veldman MB, et al. Transdifferentiation of fast skeletal muscle into functional endothelium in vivo by transcription factor Etv2. PLoS Biol. 2013;11(6):e1001590. doi: 10.1371/journal.pbio.1001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lammerts van Bueren K, Black BL. Regulation of endothelial and hematopoietic development by the ETS transcription factor Etv2. Curr Opin Hematol. 2012;19(3):199–205. doi: 10.1097/MOH.0b013e3283523e07. [DOI] [PubMed] [Google Scholar]
- 32.Knebel J, De Haro L, Janknecht R. Repression of transcription by TSGA/Jmjd1a, a novel interaction partner of the ETS protein ER71. J Cell Biochem. 2006;99(1):319–329. doi: 10.1002/jcb.20945. [DOI] [PubMed] [Google Scholar]
- 33.Searles CD. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression. Am J Physiol Cell Physiol. 2006;291(5):C803–C816. doi: 10.1152/ajpcell.00457.2005. [DOI] [PubMed] [Google Scholar]
- 34.Armulik A, Genové G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.