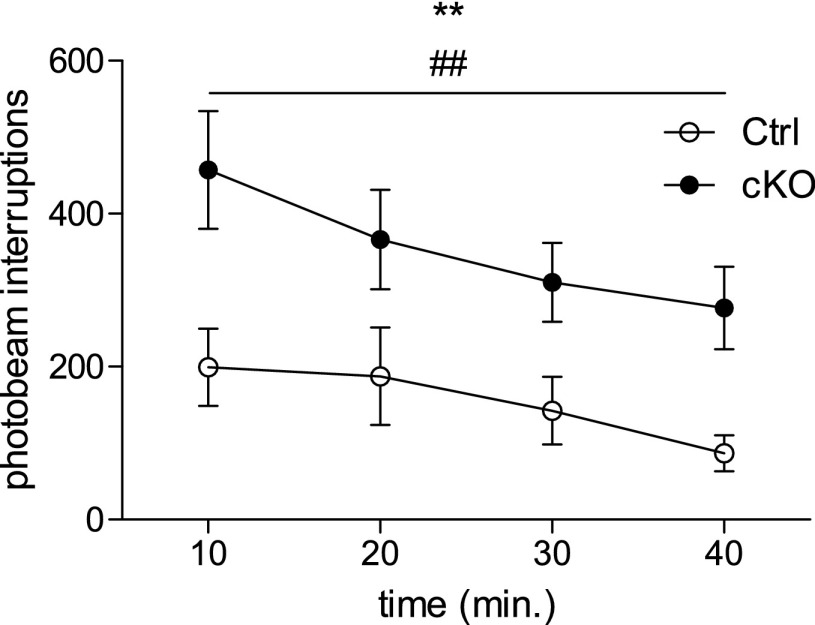
We would like to thank Konsolaki and Skaliora for their interest in our paper (1). In their view, our interpretation for the increased locomotion found in the knockout animals is incorrect because, as they argue, it could be a result of different reactions to exposure to novel environments, or to a disturbed circadian rhythm (2). First, it should be established that the method through which we have measured locomotion—open field test, 1-h-long sessions during the day—is quite standard in the literature (see refs. 3 and 4 for recent examples involving knockout animals; further references are excluded because of space restrictions). In our analysis, using the open field test (in 2-d experiments), we chose to illustrate data in bar graphs in our original publication (1). However, we agree with Konsolaki and Skaliora (2) that it can be of interest to plot locomotion over time. In response to their comment, we therefore show here that by plotting locomotion (recorded as photobeam interruptions) vs. time (each 10 min) (Fig. 1: preinjection data plotted over time provided as example), it is evident that both control and knockout mice decrease their activity with time, but that the locomotion of the knockout mice is higher than the controls throughout the test. Plotting the data in this way, thus, further supports our conclusion that the knockout mice show a hyperlocomotion phenotype. Furthermore, our data (Fig. 1) appear similar to that in the example provided by Konsolaki and Skaliora (figure 1B in ref. 2), which they choose to refer to as “genuine hyperlocomotion.” Thus, we seem to agree that our knockout mice indeed are hyperlocomotive. Konsolaki and Skaliora further refer to what they find is an ambiguity in terms of experimental method; however, because we use a protocol of more than 1 d of testing, it follows that the environment is less new to the animals on the second compared with the first day of testing. Regarding circadian rhythm disturbances, Konsolaki and Skaliora (2) offer a single reference (5) for their claim that the subthalmic nucleus (STN) is involved in “sleep patterns,” as they put it. This reference merely shows that c-fos activation of the STN is correlated with the sleep cycle and cites another study (6), which shows no alteration in the sleep-wake cycle after lesions of the STN. We do not believe there is adequate evidence to suggest that our results, which were collected at different points within the day-cycle, should be interpreted as a consequence of disturbances in the circadian rhythm.
Fig. 1.
Automated assessment of locomotion in an open field setting in activity boxes over 40 min of time (preinjection) showed that subtype 2 Vesicular glutamate transporter (Vglut2)f/f;Pitx2-Cre conditional knockout animals (cKO), as well as control littermates (Ctrl), habituate to the environment (change over time P = 0.0081; F = 4.330; df = 3); marked with **), whereas cKO mice preserve a generally higher level of locomotion than Ctrls throughout the test (difference between Ctrl and cKO groups P = 0.0038; F = 10.86; df = 3; marked with ##). For Ctrl: n = 10; for cKO: n = 11. Data analyzed with repeated-measures ANOVA (Graphpad Prism 5.02).
Our interpretation is based on a careful evaluation of the evidence we gathered, which shows that the STN was uniquely affected, with severe disturbances in the basal ganglia circuitry that complement our behavioral observations. As always, different interpretations are possible, and welcome, but we stand by our interpretation as expressed in our original paper (1): our intervention was the cause of a hyperlocomotive phenotype, explained by alterations in the STN and connected structures.
Acknowledgments
We thank all authors of our original paper: Nadine Schweizer (Department of Neuroscience, Biomedical Center, Uppsala University), Stéfano Pupe (Department of Neuroscience, Biomedical Center, Uppsala University and Brain Institute, Federal University of Rio Grande do Norte), Emma Arvidsson (Department of Neuroscience, Biomedical Center, Uppsala University), Karin Nordenankar (Department of Neuroscience, Biomedical Center, Uppsala University), Casey J.A. Smith-Anttila (Department of Neuroscience, Biomedical Center, Uppsala University), Souha Mahmoudi (Faculty of Pharmacy, Université de Montréal), Anna Andrén (Department of Neuroscience, Biomedical Center, Uppsala University), Sylvie Dumas (Oramacell), Aparna Rajagopalan (Department of Neuroscience, Biomedical Center, Uppsala University), Daniel Lévesque (Faculty of Pharmacy, Université de Montréal), Richardson N. Leão (Department of Neuroscience, Biomedical Center, Uppsala University and Brain Institute, Federal University of Rio Grande do Norte), and Åsa Wallén-Mackenzie (Department of Neuroscience, Biomedical Center, Uppsala University). This work was supported by the same financiers as in the original publication.
Footnotes
The authors declare no conflict of interest.
References
- 1.Schweizer N, et al. Limiting glutamate transmission in a Vglut2-expressing subpopulation of the subthalamic nucleus is sufficient to cause hyperlocomotion. Proc Natl Acad Sci USA. 2014;111(21):7837–7842. doi: 10.1073/pnas.1323499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konsolaki E, Skaliora I. Motor vs. cognitive elements of apparent ”hyperlocomotion”: A conceptual and experimental clarification. Proc Natl Acad Sci USA. 2015;112:E3–E4. doi: 10.1073/pnas.1413820112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paumier KL, et al. Behavioral characterization of A53T mice reveals early and late stage deficits related to Parkinson’s disease. PLoS ONE. 2013;8(8):e70274. doi: 10.1371/journal.pone.0070274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medvedev IO, et al. D1 dopamine receptor coupling to PLCβ regulates forward locomotion in mice. J Neurosci. 2013;33(46):18125–18133. doi: 10.1523/JNEUROSCI.2382-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vetrivelan R, Qiu MH, Chang C, Lu J. Role of basal ganglia in sleep-wake regulation: Neural circuitry and clinical significance. Front Neuroanat. 2010;4:145. doi: 10.3389/fnana.2010.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu MH, Vetrivelan R, Fuller PM, Lu J. Basal ganglia control of sleep-wake behavior and cortical activation. Eur J Neurosci. 2010;31(3):499–507. doi: 10.1111/j.1460-9568.2009.07062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]