Abstract
Neuropeptides and their receptors are present in human skin, and their importance for cutaneous homeostasis and during wound healing is increasingly appreciated. However, there is currently a lack of understanding of the molecular mechanisms by which their signaling modulates keratinocyte function. Here, we show that δ-opioid receptor (DOPr) activation inhibits proliferation of human keratinocytes, resulting in decreased epidermal thickness in an organotypic skin model. DOPr signaling markedly delayed induction of keratin intermediate filament (KRT10) during in vitro differentiation and abolished its induction in the organotypic skin model. This was accompanied by deregulation of involucrin (IVL), loricrin, and filaggrin. Analysis of the transcription factor POU2F3, which is involved in regulation of KRT10, IVL, and profilaggrin expression, revealed a DOPr-mediated extracellular signal-regulated kinase (ERK)-dependent downregulation of this factor. We propose that DOPr signaling specifically activates the ERK 1/2 mitogen-activated protein kinase pathway to regulate keratinocyte functions. Complementing our earlier studies in DOPr-deficient mice, these data suggest that DOPr activation in human keratinocytes profoundly influences epidermal morphogenesis and homeostasis.
Introduction
The epidermis is a stratified epithelium constantly undergoing self-renewal, which is temporally and spatially coordinated by the balanced expression of genes regulating proliferation and differentiation of keratinocytes, the main cell type present (Blanpain and Fuchs, 2009). The transition of basal keratinocytes toward the spinous layer is accompanied by repression of the synthesis of intermediate filament proteins keratin 5 (KRT5) and KRT14 (Fuchs and Green, 1980) and the upregulation of early differentiation markers KRT1 and KRT10. Differentiation toward the granular layer involves upregulation of cornified envelope precursor proteins such as involucrin (IVL) and loricrin (LOR), as well as filaggrin (FLG).
This sequence of epidermal gene regulation required for appropriate differentiation of keratinocytes is regulated by several transcription factors, including POU domain, class 2, transcription factor 3 (POU2F3, also known as Skn-1, Epoc-1, and Oct-11). POU2F3 belongs to a family of POU domain transcription factors, which are preferentially expressed in specific epidermal layers and are involved in regulation of multiple keratinocyte differentiation genes. POU2F3 protein seems to be expressed throughout all epidermal layers with highest expression in the suprabasal layers (Andersen et al., 1993; Goldsborough et al., 1993; Yukawa et al., 1993; Faus et al., 1994; Andersen et al., 1997), and mice lacking POU2F3 express normal levels of KRT1 and KRT10 but show marked abnormalities in KRT14 gene expression during wound healing. POU2F3 gene expression is spatially regulated at the wound front, corresponding to altered KRT1, LOR, FLG, KRT14, and SPR1 gene expression, which suggests a role for POU2F3 in facilitating reepithelialization at the wound front (Andersen et al., 1997).
In view of the constant environmental assaults that the skin must endure, such a delicate balance of gene expression might easily become derailed in the absence of robust stabilizing mechanisms. Recently, attention has focused on the local skin neuroendocrine system as a potential player in regulating epidermal differentiation. Expression of several neurohormones, neurotransmitters, and neuropeptides, including β-endorphin and enkephalins, has been shown in human skin (Slominski et al., 2000; Slominski and Wortsman, 2000; Bigliardi-Qi et al., 2000; Kauser et al., 2003; Bigliardi-Qi et al., 2004; Slominski et al., 2011, 2012, 2013). We are particularly interested in the role of the receptor for methionine5 (Met-) and leucine-enkephalin, the δ-opioid receptor (DOPr), in skin healing and homeostasis (Bigliardi et al., 2009). Following specific activation at the cell surface, the DOPr transmits the signal across the membrane via an associated G-protein, leading to intracellular signaling cascade activation. The DOPr can regulate extracellular signal-regulated kinase (ERK) 1/2 activity (Burt et al., 1996; Fukuda et al., 1996; Belcheva et al., 1998; Xu et al., 2010), which has been linked to cell differentiation and proliferation (Eckert et al., 2002; Shaul and Seger, 2007; Gazel et al., 2008). Expression of the DOPr in murine and cultured human skin has been documented, and mice lacking the DOPr exhibit aberrant epidermal phenotypes and delayed wound healing (Bigliardi-Qi et al., 2006). Although the mechanisms underlying these phenotypes remain unclear, the importance of the DOPr in these processes is only now beginning to be appreciated: DOPr-deficient mice exhibit markedly increased expression of KRT10, alongside an atrophic epidermis, alluding to a role for DOPr during stratification and skin homeostasis (Bigliardi-Qi et al., 2006, 2009).
In this study, we sought to identify the molecular consequences of DOPr activation in keratinocytes and to understand how this influences skin differentiation and homeostasis.
Results
DOPr is expressed in suprabasal and granular layers of human epidermis
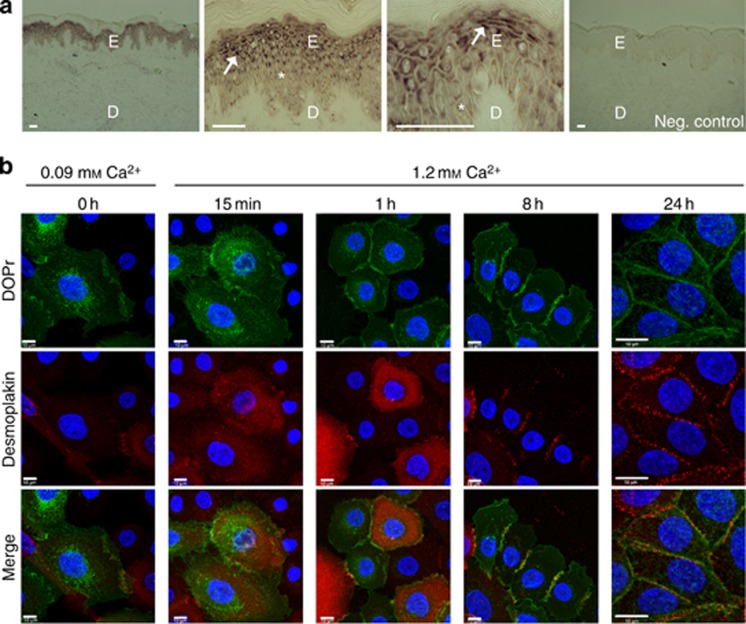
We have previously documented the expression of DOPr mRNA in cultured human keratinocytes (Bigliardi-Qi et al., 2006, 2009), and have now gone on to confirm this expression in vivo by in situ hybridization on human corporal skin sections. Positive hybridization signals were detected in the stratum granulosum and, to a lesser extent, in the stratum spinosum. However, it was apparent that not all keratinocytes express the same amount of DOPr, reflected in the heterogeneous staining pattern (Figure 1a).
Figure 1.
δ-Opioid receptor (DOPr) is primarily expressed in suprabasal layers of normal human skin and exhibits Ca2+-dependent membrane localization in vitro. (a) In situ hybridization with digoxygenin-labeled antisense riboprobes showed prominent DOPr mRNA expression in spinous and granular layer keratinocytes (arrows) of normal human epidermis. Basal, sporadically, suprabasal layer keratinocytes (asterisk) express DOPr at lower levels. Bar = 50 μm. (b) Confocal fluorescence image stacks of DOPr (green) and desmoplakin (red) were obtained at 0.1 μm intervals in Z-section. Nuclei are counterstained with Hoechst (blue). N/TERT-1 cells overexpressing C-terminal green fluorescent protein (GFP)-tagged DOPr cultured in 0.09 mM Ca2+ medium exhibit an almost complete loss of desmosomal junctions while DOPr gets internalized (column 1). After change to 1.2 mM Ca2+ medium desmosomes gradually reform. DOPr starts to translocate to the membrane 15 minutes after Ca2+ addition and concentrates at the cell–cell junctions with progressive desmosome maturation. Bar = 10 μm.
Further, to reliably identify the localization of the receptor, a lentiviral overexpression system was used to introduce a DOPr–green fluorescent protein (GFP) fusion protein into N/TERT-1 keratinocytes. In low Ca2+ (0.09 mM) medium, DOPr in cultured keratinocytes was almost completely localized in intracellular compartments, with little expression at the cell surface (Figure 1b—column 1). Upon shifting DOPr-overexpressing keratinocytes to higher Ca2+concentrations (1.2 mM), the majority of DOPr translocated to the cell surface, and a smaller fraction was detected in intracellular compartments (Figure 1b—column 5). Within 1 hour of addition of Ca2+, the opioid receptor was found on the membrane, despite the cells having not yet fully established desmosomal junctions, marked by desmoplakin labeling at areas of cell–cell contact (Figure 1b—column 3). Eight hours after addition of high Ca2+, both desmosomal junction formation and DOPr membrane localization had stabilized (Figure 1b—column 4).
Overexpression and activation of the DOPr results in reduced proliferation of keratinocytes
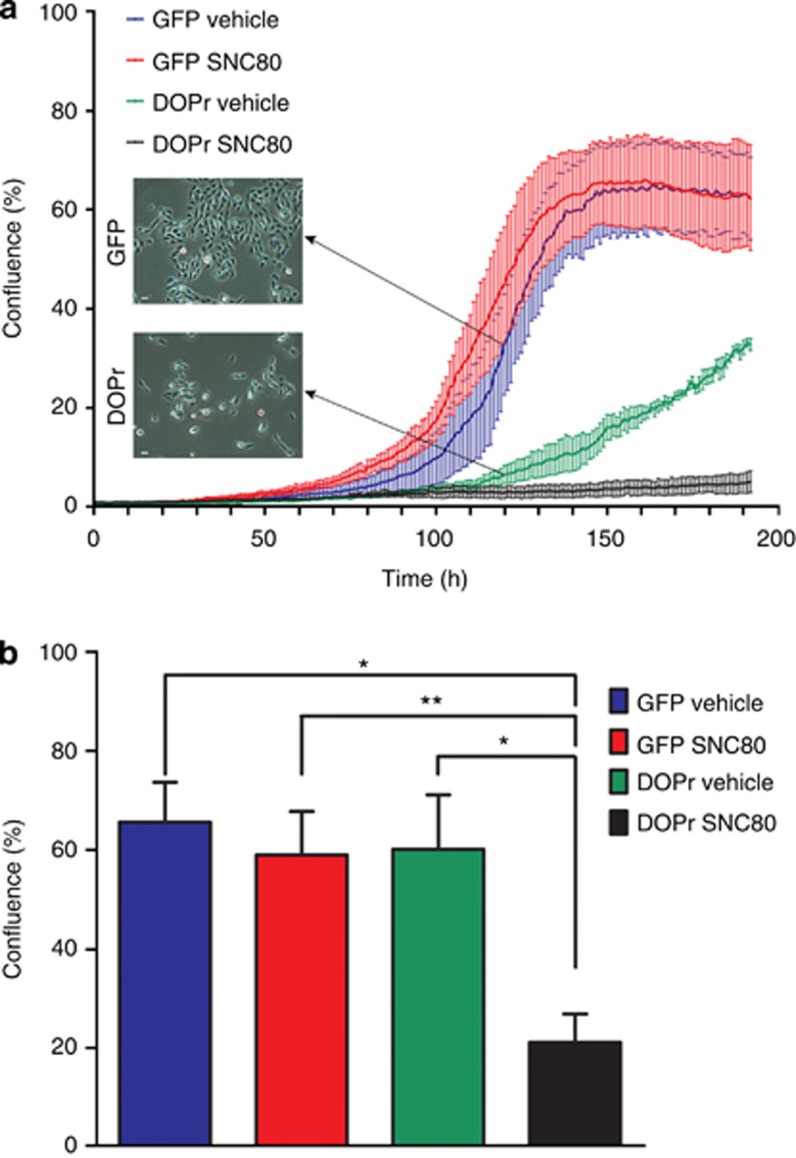
DOPr overexpression markedly changed the phenotype of N/TERT-1 keratinocyte cultures. Colonies of DOPr-overexpressing cells were more spread out than control cell colonies and appeared to have reduced cell proliferation rates. Although control cells entered an exponential growth phase, before plateauing after about 6 days in culture, DOPr-overexpressing cells showed markedly reduced proliferation (Figure 2a). The addition of the DOPr ligand SNC80 significantly and specifically reduced the level of confluence of DOPr-overexpressing cell cultures (Figure 2b).
Figure 2.
δ-Opioid receptor (DOPr) overexpression inhibits keratinocyte proliferation. (a) Proliferation curves of DOPr-overexpressing and control cells, in either vehicle control medium (0.001% DMSO) or 100 nM SNC80-containing medium, were obtained from the images captured hourly with a 10x objective lens by the Incucyte machine. The graph depicts the mean+/−SEM percentage confluence per field of view of a representative experiment (n=3 per culture condition). Phase-contrast images of control and DOPr-overexpressing N/TERT-1 cultures were captured at 20x magnification, 5 days after plating the same amount of N/TERT-1 cells into standard culture vessels. Bar = 20 μm. (b) The percentage confluence after 5 days (120 hours) of culture is displayed as mean +/−SEM of three independent experiments. One-way ANOVA with the Newman-Keuls post hoc test, *P<0.05, **P<0.01. GFP, green fluorescent protein.
DOPr activation alters keratinocyte differentiation
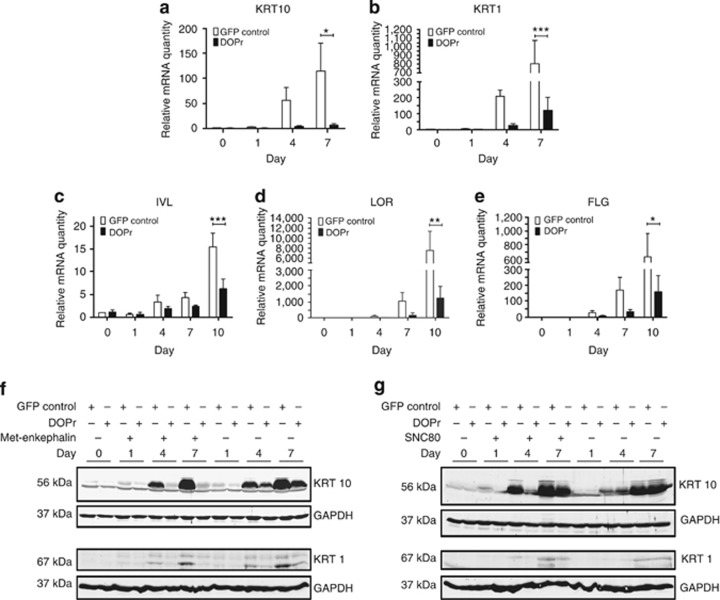
We further went on to analyze the changes in expression of KRT10 and KRT1 in an in vitro model of keratinocyte differentiation using N/TERT-1 keratinocytes (Dickson et al., 2000). Cells were grown to confluence before differentiation was induced by growth factor withdrawal for up to 10 days in the presence of the DOPr peptide agonist Met-enkephalin. Quantitative real-time PCR analysis of mRNA levels demonstrated that continued differentiation was associated with 56- (KRT10) and 114-fold (KRT1) upregulation of these transcripts in control cells, whereas DOPr-overexpressing cells exhibited significantly lower inductions; just 4- (KRT10) and 13-fold (KRT1) increases were observed (Figures 3a and b). With advancing differentiation, the mRNA levels of the cornified envelope precursor proteins, IVL, LOR, and FLG, increased markedly in control cells, but in DOPr-overexpressing cells only at day 10 was a comparable increase in expression of these genes detected (Figures 3c–e).
Figure 3.
δ-Opioid receptor (DOPr) overexpression and activation impairs keratinocyte differentiation. Cells were grown to confluence and differentiation induced by removal of growth factors under the influence of methionine5 (Met)-enkephalin for up to 10 days. Differentiation marker gene expression was analyzed by quantitative real-time PCR. Data are represented after normalization to RPL13a expression as mean ±SEM (n=3 keratin intermediate filament (KRT10), n=4 KRT1, n=5 involucrin (IVL), loricrin (LOR), and filaggrin (FLG)). Expression levels of (a) KRT10, (b) KRT1, (c) IVL, (d) LOR, and (e) FLG are shown after normalization to vehicle-treated control cells. Two-way analysis of variance, ***P<0.001, **P<0.01, and *P<0.05. (f, g) Whole-cell lysates from confluent cultures (day 0) and day 1, 4, and 7 of differentiation with and without addition of 100 nM Met-enkephalin (f) or SNC80 (g) were analyzed by immunoblot for KRT10 and KRT1 expression, with equal loading verified by glyceraldehyde-3-phosphate dehydrogenase (GAPDH). GFP, green fluorescent protein.
The delay in KRT1 and KRT10 induction during early differentiation under the influence of DOPr signaling was confirmed at the protein level. The increase in KRT1 and KRT10 observed in control cells at day 4 and 7 of differentiation was not detected in DOPr-activated cells in the presence of the endogenous agonist Met-enkephalin (Figure 3f). When DOPr-overexpressing cells were differentiated without addition of an agonist, a minor reduction in KRT10 protein expression was also detected (Figure 3f, lanes 11–14). Similar results were obtained using the exogenous DOPr agonist SNC80 (Figure 3g, lanes 5–8), further illustrating the importance of the DOPr pathway irrespective of the ligand used to stimulate signaling.
The transcription factor POU2F3 is involved in DOPr-mediated regulation of differentiation
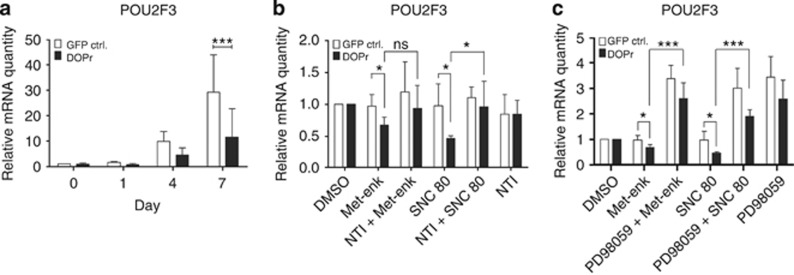
POU2F3 is one transcription factor involved in the transition from basal to suprabasal keratinocyte phenotype (Lena et al., 2010) and in the regulation of KRT10 and IVL expression (Andersen et al., 1993; Welter et al., 1996; Andersen et al., 1997). Our observations in HaCaT cells (Supplementary Figures S3a and S3c online), DOPr−/− mice, and activated DOPr-overexpressing N/TERT-1 keratinocytes (Figure 4a) confirm these published reports. After 1 day of differentiation, DOPr activation by Met-enkephalin was associated with a 1.3-fold downregulation of POU2F3 mRNA, whereas control cells induced POU2F3 transcription 1.5-fold. The relative mRNA expression level of POU2F3 at day 7 was only ninefold greater in DOPr-overexpressing cells but increased 23-fold in control cells (Figure 4a). POU2F3 regulation, thus, appeared to be DOPr specific. Accordingly, during 24 hours of incubation, POU2F3 inhibition could be reversed by naltrindole, resulting in similar expression levels in control and DOPr-overexpressing cells (Figure 4b).
Figure 4.
POU domain, class 2, transcription factor 3 (POU2F3) is a target of δ-opioid receptor (DOPr)-mediated signaling. Keratinocytes were grown to confluence and differentiation induced by removal of growth factors. POU2F3 expression was analyzed by quantitative real-time PCR. Relative quantity is represented after normalization to RPL13a and the respective vehicle control as reference. (a) POU2F3 expression under influence of methionine5 (Met)-enkephalin during 7 days of differentiation. Graphs show mean ±SEM (n=5). Two-way analysis of variance, ***P<0.001. (b) Keratinocytes were differentiated for 24 hours in the presence of 100 nM Met-enkephalin, 100 nM SNC80, 10 μM naltrindole, or vehicle control. Graph represents mean ±SEM (n=4). t-Test, *P<0.05. (c) Keratinocytes were differentiated for 24 hours in the presence of 100 nM Met-enkephalin, 100 nM SNC80, 20 μM of extracellular signal-regulated kinase (ERK) inhibitor PD98059, or vehicle control. The graph represents mean ±SEM (n=4). t-Test, *P<0.05 and ***P<0.001. GFP, green fluorescent protein.
To test whether the regulation of POU2F3 was mediated by ERK activation, we blocked the activation of ERK. Incubation with 20 μM of the mitogen-activated protein kinase/ERK kinase 1 (MEK 1) inhibitor PD98059 revealed a strong correlation between POU2F3 expression and ERK 1/2 activity. In DOPr-overexpressing cells, PD98059 abolished POU2F3 repression induced by either Met-enkephalin or SNC80 (Figure 4c; Supplementary Figure S4c online). The complete inhibition of ERK 1/2 mitogen-activated protein kinase signaling induced POU2F3 mRNA expression in both control and DOPr-overexpressing cells, indicating the regulation of a POU2F3 repressor by activated ERK 1/2.
DOPr impairs stratification and homeostasis in a reconstructed epidermis model
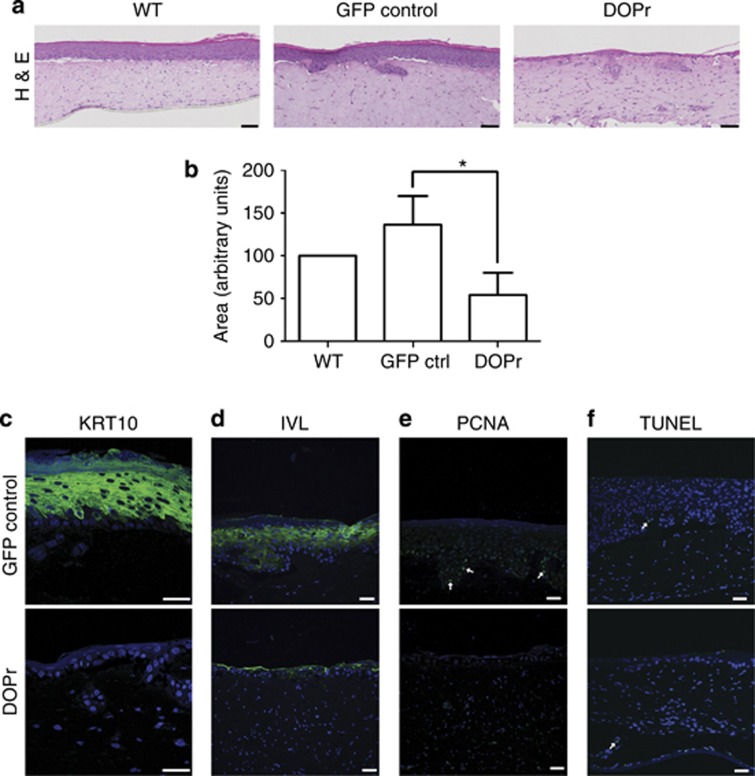
To further investigate the influence of DOPr on epidermal regeneration and homeostasis, we generated reconstructed skin with features of epidermal differentiation and morphogenesis using organotypic skin cultures (Bell et al., 1983; Stark et al., 1999). DOPr-overexpressing N/TERT-1 cells, in contrast to control cells, exhibited a poor capacity to form a fully differentiated epidermis (Figures 5a and c). The previously observed slow proliferation rate of DOPr-overexpressing keratinocytes (Figure 2b) correlated with an atrophic (thinner) epidermis in organotypic cultures. Quantification of the epidermal area generated during stratification showed significantly lower epidermal thickness in DOPr-overexpressing cultures compared with controls (Figure 5b). Immunofluorescence analysis of KRT10 revealed the absence of this early differentiation marker in DOPr-overexpressing cells (Figure 5c), indicating an impairment of epidermal differentiation as observed in the keratinocyte monolayer differentiation experiments (Figures 3a and f). Although only two to three cell layers were present, and despite the absence of KRT10, slight expression of IVL was detected in DOPr-overexpressing organotypic cultures (Figure 5c). Accordingly, proliferating cell nuclear antigen could not be detected in DOPr-overexpressing cells but was present in control cultures (Figure 5e). No increase in the number of TUNEL-positive cells was observed under DOPr overexpression, indicating that the epidermal atrophy was not linked to apoptosis (Figure 5f) but more likely to the decreased proliferation rate evidenced in Figure 2.
Figure 5.
δ-Opioid receptor (DOPr) activity results in decreased epidermal thickness and atypical stratification. Organotypic cultures of N/TERT-1 cells non-transduced or transduced with control or DOPr-green fluorescent protein (GFP) lentivirus were generated. (a) Hematoxylin and eosin (H&E) staining sections of organotypic raft cultures show decreased epidermal thickness when the DOPr was overexpressed. Bar = 100 μm. (b) Epidermal areas from H&E staining were quantified and normalized to the respective wild-type (WT) control. Graph represents the mean±SD (n=3) with duplicates per experimental condition. One-way analysis of variance, *P<0.05. Tissue sections were labeled for differentiation markers (c) keratin intermediate filament (KRT10) and (d) involucrin (IVL) by immunofluorescence. (e) Proliferating cell nuclear antigen (PCNA) immunofluorescence labeling marks proliferating cells in the basal layer of control, and (f) the TUNEL assay shows only single apoptotic cells in both control and DOPr-overexpressing cultures. This correlates well with the atrophic phenotype. Nuclei were counterstained with Hoechst. Bar=50 μm.
We also confirmed the importance of the DOPr system in fully differentiated human skin samples. Upon treatment with SNC80 or Met-enkephalin for 7 days, we observed DOPr-mediated epidermal overexpression of KRT10 and POU2F3, which could be inhibited by the specific DOPr antagonist naltrindole (Supplementary Figure S5 online). These data clearly indicate a responsiveness of human skin to DOPr stimulation and warrant more detailed investigation to refine our knowledge of its likely physiological role.
Discussion
The presence of opioid receptors in human skin has been known for years. Their function in epidermal proliferation and differentiation is only just beginning to be understood. We show here that the DOPr is predominantly expressed in the more differentiated layers of the human epidermis, which correlates well with the expression of the endogenous ligand enkephalin (Slominski et al., 2011). This raises the question of whether receptor activation triggers molecular signaling mechanisms influencing the differentiation or activation state of keratinocytes (Freedberg et al., 2001). From previous studies in other overexpression systems––e.g., with HEK293 cells––it is known that DOPr activation is capable of causing downstream mitogen-activated protein kinase signaling and especially ERK 1/2 phosphorylation and activation (Fryer et al., 2001; Audet et al., 2005; Eisinger and Ammer, 2008a,2008b;2009). But different cell types may vary in their availability of downstream molecules and might then experience a different outcome following DOPr activation (Chen et al., 2006; Gross et al., 2006; Shahabi et al., 2006).
The current study confirms that both, the endogenous DOPr peptide agonist Met-enkephalin and the synthetic DOPr alkaloid ligand SNC80, mediate a transient ERK 1/2 mitogen-activated protein kinase activation in different keratinocyte cell lines with DOPr overexpression (Supplementary Figures S1 and S2 online). As well as activating DOPr homomers, SNC80 can also activate heteromers of μ-opioid receptor and DOPr and induce potent downstream signaling (Metcalf et al., 2012);however, the extent of DOPr overexpression in our system effectively shifts the equilibrium toward DOPr homomer formation and renders it unlikely that heteromer signaling upon SNC80 binding contributes to the effects seen (Law et al., 2005). Moreover, despite this, and the differing affinities of the natural and synthetic DOPr ligands used, a conserved pattern of response is observed in DOPr-overexpressing cells. DOPr-mediated signaling in undifferentiated keratinocytes in monolayer tissue culture not only inhibits proliferation but also impairs differentiation, leading to a deregulation of the epidermal stratification process. The abundant expression of the DOPr in the suprabasal layers correlates well with the knowledge that these cells, in contrast to those in the basal layer, have a low proliferative potential. During normal skin homeostasis, the DOPr could interfere at the transition from spinous to granular layer keratinocytes, where KRT10 expression is downregulated. This is consistent with the observation that the expression pattern of DOPr in non-wounded skin (Figure 1a) partially correlates with the repression of KRT10 on mRNA level (Roop, 1987). In monolayer cultures, a tendency toward higher IVL expression in DOPr-overexpressing cells under basal growth conditions was observed, which might partially reflect this transition process. DOPr activation and signaling in undifferentiated keratinocytes might be more harmful than helpful. Our monolayer keratinocyte culture represented basal layer epidermal conditions and might not reflect the actual state of a keratinocyte expressing functional DOPr, and the time frame of DOPr expression during differentiation is not necessarily the same as found in the skin culture models. Of note, this cannot be mimicked in 2D cell cultures. Because of the deregulated early differentiation phase in DOPr-overexpressing N/TERT-1 cells, subsequent differentiation processes are altered in the 2D monolayer in vitro model. According to the DOPr expression pattern in vivo, the early phase might be executed normally, and DOPr only influences subsequent processes during epidermal differentiation. Human skin organ culture experiments with fully differentiated and developed skin confirmed the regulation of KRT10 and POU2F3 in intact epidermis (Supplementary Figure S5 online) and revealed the complex regulatory mechanism of the opioid system in vivo. On the other hand, DOPr-deficient mice showed increased expression of KRT10, which might reflect the absence of the DOPr “off switch” during their transition to the granular layer (Bigliardi-Qi et al., 2006).
Previous studies have suggested that POU2F3 is exclusively expressed in the epidermis and acts as a bifunctional transcription factor activating the expression of KRT10 and small proline-rich protein 2A (Cabral et al., 2003), while simultaneously repressing transcription of KRT14, IVL, and profilaggrin (Welter et al., 1996; Jang et al., 2000; Sugihara et al., 2001). The competition between the transcription factor ΔNp63 and POU2F3 in the regulation of KRT10 and KRT14 expression was thereby suggested to act as a molecular switch for differentiation (Lena et al., 2010). Our study indicates that POU2F3 is a critical factor in the complex regulatory network of differentiation. It was further suggested that POU2F3 enhances epidermal stratification by promoting keratinocyte proliferation (Hildesheim et al., 2001). In agreement with Hildesheim et al. (2001), we observed low proliferation and reduced POU2F3 expression, accompanied by poor stratification of DOPr-activated keratinocytes.
Andersen et al. (1997) reported that POU2F3-deficient mice showed no obvious deviation from normal skin phenotype, probably due to compensatory mechanisms, and therefore its function was proposed to be more important for wound healing. Wound edge keratinocytes are more migratory and flexible compared with homeostatic keratinocytes in order to efficiently close the wound. POU2F3 was suggested to be repressed in these cells to enable this phenotype change, and DOPr might be involved in this process. DOPr activity at the wound edge could facilitate the phenotype change of keratinocytes in order to promote the early reepithelialization process (Andersen et al., 1997; Usui et al., 2005; Patel et al., 2006). This observation is in line with the observations from DOPr-deficient mice, which exhibit a delay in the wound healing process with formation of hypertrophic wound edges (Bigliardi-Qi et al., 2006). The wound edge keratinocytes in these mice might not have undergone the necessary molecular changes, such as DOPr-mediated downregulation of POU2F3, in order to migrate properly and close the wound efficiently (Bigliardi-Qi et al., 2006). Recently, POU2F3 downregulation in superficial cutaneous wounds at day 3 of the wound healing process in humans has been reported, which strongly supports the data from our DOPr mouse models (Nuutila et al., 2012). Further experiments are necessary to clarify the connection of DOPr activity as well as POU2F3 regulation during wound healing in humans. To our knowledge previously unreported, our study indicates a connection between ERK signaling and POU2F3 regulation. A possible transcription factor involved in ERK-mediated repression of POU2F3 might be c-Myc. The c-Myc protein is an important transcription regulator during the reepithelialization process of wound healing (Tsuboi et al., 1990; Zanet et al., 2005) and might therefore be involved in the DOPr-mediated regulation of POU2F3 via ERK signaling. Thus, this signaling cascade is more complex and likely involves polycomb protein–mediated chromatin remodeling (Brien et al., 2012). Therefore, possible epigenetic changes occurring upon DOPr activation will need to be uncovered if we are to fully understand this system.
The present study suggests that DOPr is expressed and activated in a spatially and temporally controlled manner in human skin cells. Deregulation would then be predicted to lead to alterations in epidermal differentiation affecting skin physiology and pathology, whereas the therapeutic application of exogenous opioidergic drugs could prove invaluable to facilitate wound healing if we could define the right target cells, dose, and timing for treatment. This study represents the beginning of the process of achieving the required level of understanding. The DOPr-mediated molecular signaling mechanism, to our knowledge previously unreported, observed in both 2D and 3D culture models suggests that peripheral opioids have an important role in controlling processes of skin homeostasis, therefore contributing to the wound healing process. This mechanism and the good correlation between the mouse and human in vivo and in vitro system provide a basis for future clinical interventions and applications using opioid receptor ligands.
Materials and Methods
Cell culture
N/TERT-1 (Dickson et al., 2000) cells, developed at Dr J. Rheinwald's laboratory (Harvard Medical School, Boston, MA), were cultured in keratinocyte serum-free medium supplemented with 0.2 ng ml−1 EGF and 25 μg ml−1 bovine pituitary extract, grown to 50% confluence at 37 °C and 5% CO2, and subcultured using TrypLE Express (all purchased from Life Technologies, Singapore). HEK 293Ta cells were cultured in DMEM containing 10% fetal bovine serum, 0.1 mM minimum essential medium nonessential amino acids (Life Technologies), and penicillin/streptomycin. Differentiation was induced by subjecting cells at 95–100% confluence to withdrawal of growth factors (EGF, bovine pituitary extract).
Reagents
Antibodies used in this study were as follows: rabbit polyclonal anti-GFP antibody (no. ab290, Abcam, Hong Kong); mouse monoclonal anti-KRT10, clone DE-K10 (Ivanyi et al., 1989; no. ab9026, Abcam, or no. MS-611, Thermo Fisher Scientific, Fremont, CA); mouse monoclonal anti-KRT1 antibody, clone 34βB4 (no. NCL-CK1, Novocastra, New Castle Upon Tyne, UK); mouse monoclonal anti-IVL (Sy5, no. MS-126, Thermo Fisher Scientific), rabbit anti-proliferating cell nuclear antigen (FL-261, no. sc-7907, Santa Cruz Biotechnology, Heidelberg, Germany), mouse monoclonal anti-desmoplakin, clone 11-5F (kindly provided by D. Garrod, University of Manchester, UK (Parrish et al., 1987)); and mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (3E8AD9, no. A21994, Life Technologies). Secondary antibodies used were goat anti-mouse AlexaFluor 594, goat anti-rabbit AlexaFluor 488, and goat anti-rabbit AlexaFluor 594 (Life Technologies/Molecular Probes, Eugene, OR). Detection antibodies used for western blotting were goat anti-mouse IRDye 800 and goat anti-rabbit IRDye 700DX (Rockland, Gilbertsville, PA). SNC80 and naltrindole were from Tocris Biosciences (Bristol, UK), PD98059 from Promega (Madison, WI), and the solvent for SNC80 and PD98059, DMSO, as well as Met-enkephalin from Sigma-Aldrich (Singapore).
DNA construct
The plasmid used for all N/TERT-1 experiments was a lentiviral expression clone with pEZ-Lv122 vector backbone including the DOPr-GFP open reading frame (NCBI entry U10504) purchased from GeneCopoeia (no. EX-A1155-Lv122; Rockville, MD).
Lentivirus
For production of recombinant lentiviral particles HEK 293Ta cells were transfected with 1.5 μg of purified DOPr plasmid and 1 μg of each human lentiviral packaging vectors psPax, pMD2.G (Yang et al., 2004) using Qiagen Effectene Transfection Reagent (Qiagen, Singapore), according to the manufacturer's instructions. Two days after transfection virus-containing medium was harvested and concentrated by ultracentrifugation at 22,000 rpm at 4 °C for 2 hours 16 minutes in a Beckman Coulter JS-24.38 rotor. Cells were infected at 30% confluence with a multiplicity of infection of approximately 10, in the presence of 10 μg ml−1 Polybrene (Millipore, Singapore). After 24 hours, the viral particle–containing medium was replaced with fresh medium, and cells were cultured for at least 48 hours before use.
RNA extraction and quantitative real-time PCR
Total RNA from cultured cells was isolated using the “RNeasy Mini Kit” (Qiagen), according to the manufacturer's instructions. A volume of 1 μg RNA was reverse-transcribed with the “PrimeScript RT reagent Kit” (TaKaRa, Otsu, Shiga, Japan), according to the manufacturer's instructions. Quantitative PCR assays were carried out on a real-time PCR detection system (7500 Fast Real-Time PCR System; Applied Biosystems, Singapore) using the “QuantiFast SYBR Green PCR Kit” (Qiagen). To evaluate the expression level of human KRT1, KRT10, IVL, and POU2F3 mRNAs, commercially available QuantiTect Primer Assays (Qiagen, no. QT00014182, QT00017045, QT00082586, and QT00029057) were used. Primers for LOR detection were: 5′-TCATGATGCTACCCGAGGTTTG-3′ and 5′-CAGAACTAGATGCAGCCGGAGA-3′ and for FLG: 5′-GAAGACAAGGATCGCACCAC-3′ and 5′-ATGGTGTCCTGACCCTCTTG-3′. RPL13a expression was used for normalization. These primer sequences were as follows: 5′-CTCAAGGTCGTGCGTCTGAA-3′ and 5′-TGGCTGTCACTGCCTGGTACT-3′. Quantification was performed using the comparative 2−ΔΔCT method.
Immunoblotting
Protein extracts were prepared by cell lysis in buffer containing 10 mM TRIS-HCL, 5 mM EDTA, 5 mM EGTA at pH 7.5, supplemented with 4% SDS, 1% Triton X-100, 160 mM dithiothreitol, 0.8 mM phenylmethylsulfonyl fluoride, 0.8 mM Na3VO4, and 1x protease inhibitor (Roche, Singapore). Lysates were boiled for 5 minutes at 95 °C and subjected to SDS-PAGE. Proteins were transferred on to a nitrocellulose membrane (BioRad, München, Germany). For glyceraldehyde-3-phosphate dehydrogenase (1:8000), KRT10 (1:200), and KRT1 (1:200) antibodies, blots were incubated in tris-buffered saline containing 0.1% Tween-20 and 5% nonfat milk. After incubation with appropriate secondary antibody (1:5000), proteins were visualized with the Odyssey infrared imaging system (LICOR Biosciences, Lincoln, NE).
Immunolabeling and confocal microscopy
Cells were seeded on glass coverslips and subjected to treatments indicated in the figures. Cells were fixed in 4% paraformaldehyde for 15 minutes at room temperature, permeabilized, and blocked using 0.3% Triton X-100 and 10% goat serum in phosphate-buffered saline. Coverslips were incubated overnight at 4 °C with corresponding primary antibodies (anti-GFP 1:900, anti-desmoplakin 1:100). A 1:300 dilution of labeled secondary antibodies was used; nuclei were stained with 10 μg ml−1 Hoechst 33258 (Sigma-Aldrich, no. B2883) and mounted on to microscope slides (Fluorescence Mounting Medium, Dako). Organotypic skin cultures were fixed in 10% neutral buffered formalin. Paraffin embedding, sectioning, and hematoxylin and eosin staining were conducted by the Institute of Molecular and Cell Biology Core Histopathology Laboratory, Singapore. For immunofluorescence labeling, deparaffinized sections underwent heat-mediated antigen retrieval. After blocking, antibodies were added and incubated overnight at 4 °C. Secondary antibody labeling, counterstaining with Hoechst dye, and mounting were as above. Confocal images were captured as z-stacks and projected to a single image using an Olympus FV1000 microscope system with an UPLFLN 40x/1.3 NA oil-immersion lens and UPLSAPO 20x/0.75 NA dry lens. Images were processed and exported using FV1000 Viewer application software (FV10-ASW, Olympus, Singapore). Figures were assembled using Photoshop (CS3 Extended; Adobe, Singapore).
In situ hybridization
The digoxigenin-labeled riboprobes were generated from the full-length hDOPr clone in a pcDNA3 plasmid (a gift from B.L. Kieffer) and hybridized as described in Bigliardi et al. (1998). The biopsies were all taken from healthy individuals after donor approval at the University of Basel in 1997 and were approved according to the local ethical guidelines at that time. The use of surgical waste skin for skin organ cultures (Supplementary Information) has been approved under the NUS-IRB, Reference Code 13-25-IE of the University of Singapore. The study was conducted according to the Declaration of Helsinki Principles. Brightfield images were captured on a Nikon Diaphot 300 microscope equipped with a digital CCD color camera (CF 20 DXC, Kappa Messtechnik, Germany) using 10x/0.25 NA, 40x/0.55 NA, and 60x/0.7 NA dry lenses.
Proliferation assay
A total of 1000 N/TERT-1 cells were plated into 96-well plates and allowed to adhere overnight. Medium was changed to either DMSO vehicle control medium or 100 nM SNC80-containing medium, and the plate was placed into the Incucyte machine (Essen BioScience, Ann Arbor, MI). Hourly images were captured with the 10x/1.49 NA objective lens. Using the metrics from the Incucyte software, percentage confluence was determined.
Organotypic culture
A dermal equivalent was generated by preparing a gel containing rat tail collagen type I (BD, no. 354236) and dermal fibroblasts (100,000 cells per ml) in a cell culture insert (pore size 1 μm, BD, no. 353102). Dermal equivalents were cultured in FAD medium: 3:1 mixture of DMEM and Ham's F12 nutrient mix (Life Technologies) supplemented with penicillin/streptomycin (PAA Laboratories GmbH, Germany), 10% fetal bovine serum (JR Scientific, Woodland, CA), 1% Glutamax (Life Technologies), 0.4 μg ml−1 Hydrocortisone (Sigma-Aldrich; no. H0888), 5 μg ml−1 Insulin (Sigma-Aldrich, no. I2643), 1.8 × 10−4 M Adenine (Sigma-Aldrich; no. A2786), 10 ng ml−1 EGF (Sigma-Aldrich, no. E9644), 5 μg ml−1 Transferrin (Sigma-Aldrich, no. T2036-1G), and 2 × 10−11 M 3,3′,5-Triiodo-L-thyronine (T3; Sigma-Aldrich, no. T6397). After 48 hours, N/TERT-1 cells (200,000 per culture) were seeded on top of dermal equivalents and cultured under submerged conditions until a confluent monolayer had developed. Cultures were shifted to air–liquid interface, EGF supplementation stopped, and cultures allowed to stratify for 14 days.
Quantification of the epidermal area from organotypic cultures
Hematoxylin and eosin-stained tissue sections were imaged using an Ariol high-resolution fluorescence and brightfield slide scanner (Leica Microsystems, Wetzlar, Germany) at the Institute of Molecular and Cell Biology Core Histopathology Laboratory, Singapore. Using ImageJ, a threshold was defined, and the remaining area of dark hematoxylin and eosin staining representing the epidermis was quantified. GFP control and DOPr-overexpressing cultures were normalized to non-transduced wild-type controls and three independent experiments combined for statistical analysis. A one-way analysis of variance with the Newman-Keuls post hoc test was performed to determine the P-value.
Acknowledgments
We are grateful to Birgit Lane and the team from Institute of Medical Biology, A*STAR for their help and support, and Tommy Baumann, Ting Ting Seah, and Alicia Yap for their excellent technical assistance. These studies were supported by funding from Swiss National Science Foundation (SNSF) grant 31003A-116811 to MB-Qi and by a core grant from the Singapore Biomedical Research Council of the Singapore Agency for Science, Technology, and Research (A*STAR). We also thank Lucy Robinson of Insight Editing, London, for her assistance in revising the manuscript.
Glossary
- DOPr
δ-opioid receptor
- ERK
extracellular signal-regulated kinase
- FLG
filaggrin
- GFP
green fluorescent protein
- IVL
involucrin
- KRT
keratin intermediate filament
- LOR
loricrin
- Met
methionine5
- POU2F3
POU domain, class 2, transcription factor 3
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Andersen B, Schonemann MD, Flynn SE, et al. Skn-1a and Skn-1i: two functionally distinct Oct-2-related factors expressed in epidermis. Science. 1993;260:78–82. doi: 10.1126/science.7682011. [DOI] [PubMed] [Google Scholar]
- Andersen B, Weinberg WC, Rennekampff O, et al. Functions of the POU domain genes Skn-1a/i and Tst-1/Oct-6/SCIP in epidermal differentiation. Genes Dev. 1997;11:1873–1884. doi: 10.1101/gad.11.14.1873. [DOI] [PubMed] [Google Scholar]
- Audet N, Paquin-Gobeil M, Landry-Paquet O, et al. Internalization and Src activity regulate the time course of ERK activation by delta opioid receptor ligands. J Biol Chem. 2005;280:7808–7816. doi: 10.1074/jbc.M411695200. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Vogel Z, Ignatova E, et al. Opioid modulation of extracellular signal-regulated protein kinase activity is ras-dependent and involves Gbetagamma subunits. J Neurochem. 1998;70:635–645. doi: 10.1046/j.1471-4159.1998.70020635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E, Sher S, Hull B, et al. The reconstitution of living skin. J Invest Dermatol. 1983;81:2s–10s. doi: 10.1111/1523-1747.ep12539993. [DOI] [PubMed] [Google Scholar]
- Bigliardi PL, Bigliardi-Qi M, Buechner S, et al. Expression of mu-opiate receptor in human epidermis and keratinocytes. J Invest Dermatol. 1998;111:297–301. doi: 10.1046/j.1523-1747.1998.00259.x. [DOI] [PubMed] [Google Scholar]
- Bigliardi PL, Tobin DJ, Gavériaux-Ruff C, et al. Opioids and the skin—where do we stand. Exp Dermatol. 2009;18:424–430. doi: 10.1111/j.1600-0625.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- Bigliardi-Qi M, Bigliardi PL, Eberle AN, et al. Beta-endorphin stimulates cytokeratin 16 expression and downregulates mu-opiate receptor expression in human epidermis. J Invest Dermatol. 2000;114:527–532. doi: 10.1046/j.1523-1747.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- Bigliardi-Qi M, Gavériaux-Ruff C, Zhou H, et al. Deletion of delta-opioid receptor in mice alters skin differentiation and delays wound healing. Differentiation. 2006;74:174–185. doi: 10.1111/j.1432-0436.2006.00065.x. [DOI] [PubMed] [Google Scholar]
- Bigliardi-Qi M, Sumanovski LT, Buchner S, et al. Mu-opiate receptor and beta-endorphin expression in nerve endings and keratinocytes in human skin. Dermatology. 2004;209:183–189. doi: 10.1159/000079887. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien GL, Gambero G, O'Connell DJ, et al. Polycomb PHF19 binds H3K36me3 and recruits PRC2 and demethylase NO66 to embryonic stem cell genes during differentiation. Nat Struct Mol Biol. 2012;19:1273–1281. doi: 10.1038/nsmb.2449. [DOI] [PubMed] [Google Scholar]
- Burt AR, Carr IC, Mullaney I, et al. Agonist activation of p42 and p44 mitogen-activated protein kinases following expression of the mouse delta opioid receptor in Rat-1 fibroblasts: effects of receptor expression levels and comparisons with G-protein activation. Biochem J. 1996;320 (Pt 1:227–235. doi: 10.1042/bj3200227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral A, Fischer DF, Vermeij WP, et al. Distinct functional interactions of human Skn-1 isoforms with Ese-1 during keratinocyte terminal differentiation. J Biol Chem. 2003;278:17792–17799. doi: 10.1074/jbc.M300508200. [DOI] [PubMed] [Google Scholar]
- Chen YL, Law PY, Loh HH. Sustained activation of phosphatidylinositol 3-kinase/Akt/nuclear factor kappaB signaling mediates G protein-coupled delta-opioid receptor gene expression. J Biol Chem. 2006;281:3067–3074. doi: 10.1074/jbc.M506721200. [DOI] [PubMed] [Google Scholar]
- Dickson MA, Hahn WC, Ino Y, et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert RL, Efimova T, Dashti SR, et al. Keratinocyte survival, differentiation, and death: many roads lead to mitogen-activated protein kinase. J Investig Dermatol Symp Proc. 2002;7:36–40. doi: 10.1046/j.1523-1747.2002.19634.x. [DOI] [PubMed] [Google Scholar]
- Eisinger DA, Ammer H. Delta-opioid receptors activate ERK/MAP kinase via integrin-stimulated receptor tyrosine kinases. Cell Signal. 2008;20:2324–2331. doi: 10.1016/j.cellsig.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Eisinger DA, Ammer H. Delta-opioid receptors stimulate ERK1/2 activity in NG108-15 hybrid cells by integrin-mediated transactivation of TrkA receptors. FEBS Lett. 2008;582:3325–3329. doi: 10.1016/j.febslet.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Eisinger DA, Ammer H. Down-regulation of c-Cbl by morphine accounts for persistent ERK1/2 signaling in delta-opioid receptor-expressing HEK293 cells. J Biol Chem. 2009;284:34819–34828. doi: 10.1074/jbc.M109.042937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faus I, Hsu HJ, Fuchs E. Oct-6: a regulator of keratinocyte gene expression in stratified squamous epithelia. Mol Cell Biol. 1994;14:3263–3275. doi: 10.1128/mcb.14.5.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedberg IM, Tomic-Canic M, Komine M, et al. Keratins and the keratinocyte activation cycle. J Invest Dermatol. 2001;116:633–640. doi: 10.1046/j.1523-1747.2001.01327.x. [DOI] [PubMed] [Google Scholar]
- Fryer RM, Hsu AK, Gross GJ. ERK and p38 MAP kinase activation are components of opioid-induced delayed cardioprotection. Basic Res Cardiol. 2001;96:136–142. doi: 10.1007/s003950170063. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Kato S, Morikawa H, et al. Functional coupling of the delta-, mu-, and kappa-opioid receptors to mitogen-activated protein kinase and arachidonate release in Chinese hamster ovary cells. J Neurochem. 1996;67:1309–1316. doi: 10.1046/j.1471-4159.1996.67031309.x. [DOI] [PubMed] [Google Scholar]
- Gazel A, Nijhawan RI, Walsh R, et al. Transcriptional profiling defines the roles of ERK and p38 kinases in epidermal keratinocytes. J Cell Physiol. 2008;215:292–308. doi: 10.1002/jcp.21394. [DOI] [PubMed] [Google Scholar]
- Goldsborough AS, Healy LE, Copeland NG, et al. Cloning, chromosomal localization and expression pattern of the POU domain gene Oct-11. Nucleic Acids Res. 1993;21:127–134. doi: 10.1093/nar/21.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross ER, Hsu AK, Gross GJ. The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3 beta. Am J Physiol Heart Circ Physiol. 2006;291:H827–H834. doi: 10.1152/ajpheart.00003.2006. [DOI] [PubMed] [Google Scholar]
- Hildesheim J, Kuhn U, Yee CL, et al. The hSkn-1a POU transcription factor enhances epidermal stratification by promoting keratinocyte proliferation. J Cell Sci. 2001;114:1913–1923. doi: 10.1242/jcs.114.10.1913. [DOI] [PubMed] [Google Scholar]
- Ivanyi D, Ansink A, Groeneveld E, et al. New monoclonal antibodies recognizing epidermal differentiation-associated keratins in formalin-fixed, paraffin-embedded tissue. Keratin 10 expression in carcinoma of the vulva. J Pathol. 1989;159:7–12. doi: 10.1002/path.1711590105. [DOI] [PubMed] [Google Scholar]
- Jang SI, Karaman-Jurukovska N, Morasso MI, et al. Complex interactions between epidermal POU domain and activator protein 1 transcription factors regulate the expression of the profilaggrin gene in normal human epidermal keratinocytes. J Biol Chem. 2000;275:15295–15304. doi: 10.1074/jbc.275.20.15295. [DOI] [PubMed] [Google Scholar]
- Kauser S, Schallreuter KU, Thody AJ, et al. Regulation of human epidermal melanocyte biology by beta-endorphin. J Invest Dermatol. 2003;120:1073–1080. doi: 10.1046/j.1523-1747.2003.12242.x. [DOI] [PubMed] [Google Scholar]
- Law PY, Erickson-Herbrandson LJ, Zha QQ, et al. Heterodimerization of mu- and delta-opioid receptors occurs at the cell surface only and requires receptor–G protein interactions. J Biol Chem. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]
- Lena AM, Cipollone R, Amelio I, et al. Skn-1a/Oct-11 and DeltaNp63alpha exert antagonizing effects on human keratin expression. Biochem Biophys Res Commun. 2010;401:568–573. doi: 10.1016/j.bbrc.2010.09.102. [DOI] [PubMed] [Google Scholar]
- Metcalf MD, Yekkirala AS, Powers MD, et al. The delta opioid receptor agonist SNC80 selectively activates heteromeric mu-delta opioid receptors. ACS Chem Neurosci. 2012;3:505–509. doi: 10.1021/cn3000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuutila K, Siltanen A, Peura M, et al. Human skin transcriptome during superficial cutaneous wound healing. Wound Repair Regen. 2012;20:830–839. doi: 10.1111/j.1524-475X.2012.00831.x. [DOI] [PubMed] [Google Scholar]
- Parrish EP, Steart PV, Garrod DR, et al. Antidesmosomal monoclonal antibody in the diagnosis of intracranial tumours. J Pathol. 1987;153:265–273. doi: 10.1002/path.1711530311. [DOI] [PubMed] [Google Scholar]
- Patel GK, Wilson CH, Harding KG, et al. Numerous keratinocyte subtypes involved in wound re-epithelialization. J Invest Dermatol. 2006;126:497–502. doi: 10.1038/sj.jid.5700101. [DOI] [PubMed] [Google Scholar]
- Roop DR. Regulation of keratin gene expression during differentiation of epidermal and vaginal epithelial cells. Curr Top Dev Biol. 1987;22:195–207. doi: 10.1016/s0070-2153(08)60104-0. [DOI] [PubMed] [Google Scholar]
- Shahabi NA, McAllen K, Sharp BM. Delta opioid receptors stimulate Akt-dependent phosphorylation of c-jun in T cells. J Pharmacol Exp Ther. 2006;316:933–939. doi: 10.1124/jpet.105.091447. [DOI] [PubMed] [Google Scholar]
- Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Skobowiat C, et al. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Zbytek B, et al. Regulated proenkephalin expression in human skin and cultured skin cells. J Invest Dermatol. 2011;131:613–622. doi: 10.1038/jid.2010.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Zbytek B, et al. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34:827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark HJ, Baur M, Breitkreutz D, et al. Organotypic keratinocyte cocultures in defined medium with regular epidermal morphogenesis and differentiation. J Invest Dermatol. 1999;112:681–691. doi: 10.1046/j.1523-1747.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- Sugihara TM, Kudryavtseva EI, Kumar V, et al. The POU domain factor Skin-1a represses the keratin 14 promoter independent of DNA binding. A possible role for interactions between Skn-1a and CREB-binding protein/p300. J Biol Chem. 2001;276:33036–33044. doi: 10.1074/jbc.M103000200. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Yamaoka S, Maki M, et al. Soluble factors including proteinases released from damaged cells may trigger the wound healing process. Biochem Biophys Res Commun. 1990;168:1163–1170. doi: 10.1016/0006-291x(90)91151-h. [DOI] [PubMed] [Google Scholar]
- Usui ML, Underwood RA, Mansbridge JN, et al. Morphological evidence for the role of suprabasal keratinocytes in wound reepithelialization. Wound Repair Regen. 2005;13:468–479. doi: 10.1111/j.1067-1927.2005.00067.x. [DOI] [PubMed] [Google Scholar]
- Welter JF, Gali H, Crish JF, et al. Regulation of human involucrin promoter activity by POU domain proteins. J Biol Chem. 1996;271:14727–14733. doi: 10.1074/jbc.271.25.14727. [DOI] [PubMed] [Google Scholar]
- Xu C, Hong MH, Zhang LS, et al. Serine 363 of the {delta}-opioid receptor is crucial for adopting distinct pathways to activate ERK1/2 in response to stimulation with different ligands. J Cell Sci. 2010;123:4259–4270. doi: 10.1242/jcs.073742. [DOI] [PubMed] [Google Scholar]
- Yang JY, Michod D, Walicki J, et al. Partial cleavage of RasGAP by caspases is required for cell survival in mild stress conditions. Mol Cell Biol. 2004;24:10425–10436. doi: 10.1128/MCB.24.23.10425-10436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukawa K, Yasui T, Yamamoto A, et al. Epoc-1: a POU-domain gene expressed in murine epidermal basal cells and thymic stromal cells. Gene. 1993;133:163–169. doi: 10.1016/0378-1119(93)90634-f. [DOI] [PubMed] [Google Scholar]
- Zanet J, Pibre S, Jacquet C, et al. Endogenous Myc controls mammalian epidermal cell size, hyperproliferation, endoreplication and stem cell amplification. J Cell Sci. 2005;118:1693–1704. doi: 10.1242/jcs.02298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.