Abstract
The structure of LX7101, a dual LIM-kinase and ROCK inhibitor for the treatment of ocular hypertension and associated glaucoma, is disclosed. Previously reported LIM kinase inhibitors suffered from poor aqueous stability due to solvolysis of the central urea. Replacement of the urea with a hindered amide resulted in aqueous stable compounds, and addition of solubilizing groups resulted in a set of compounds with good properties for topical dosing in the eye and good efficacy in a mouse model of ocular hypertension. LX7101 was selected as a clinical candidate from this group based on superior efficacy in lowering intraocular pressure and a good safety profile. LX7101 completed IND enabling studies and was tested in a Phase 1 clinical trial in glaucoma patients, where it showed efficacy in lowering intraocular pressure.
Keywords: LX7101, glaucoma, kinase, ROCK inhibitor, LIM-kinase
LIM-kinases 1 and 2 (LIMK1 and LIMK2) regulate cytoskeletal dynamics by phosphorylating and deactivating cofilin, a protein that depolymerizes actin filaments.1 As cytoskeletal regulators, LIM-kinases have been investigated for their role in a variety of cellular processes2 including cell barrier integrity,3 axonal elongation,4 cell migration,5 and cell cycle arrest6 as they pertain to various disease states such as HIV infection7 and cancer.8 Along with growing interest in these kinases, a number of LIMK inhibitors have also been reported.9−11 We recently reported the discovery of a novel class of pyrrolopyrimidine LIMK inhibitors12 and demonstrated their use for the reduction of intraocular pressure (IOP) in a mouse model of ocular hypertension.13
Ocular hypertension is associated with open-angle glaucoma, a neurodegenerative disease of the inner retina and optic nerve head and a leading cause of blindness worldwide.14 Although many therapies exist for the treatment of glaucoma through the lowering of IOP, many patients fail to adequately control IOP or experience significant side effects with current medications, indicating a need for new, more efficacious therapies.15
LIMK inhibitors are believed to lower IOP by inducing depolymerization of actin filaments in the trabecular meshwork in the eye, resulting in relaxation of the tissue. Pressure in the eye is regulated by the fluid dynamics of aqueous humor in the anterior chamber. Aqueous humor is produced by the ciliary body, flows through the anterior chamber, and exits through trabecular meshwork. In patients suffering from ocular hypertension, structural changes in the trabecular meshwork can result in decreased outflow facility, resulting in an increase in pressure.16 Relaxation of the trabecular meshwork with LIMK inhibitors could counteract this effect, directly reversing the cause of ocular hypertension.
Several inhibitors of ROCK, a kinase upstream of LIMK in the signaling cascade that regulates actin filament dynamics, are being investigated in the clinic for reduction of IOP through relaxation of the trabecular meshwork. These compounds effectively lower IOP; however, they are associated with significant side effects, particularly conjunctival hyperemia, which may limit their use.17 Since LIM-kinases are downstream from ROCK in the signaling cascade and their effects are more specific to actin polymerization, inhibitors of LIMK may reduce IOP with fewer side effects than inhibitors of ROCK.
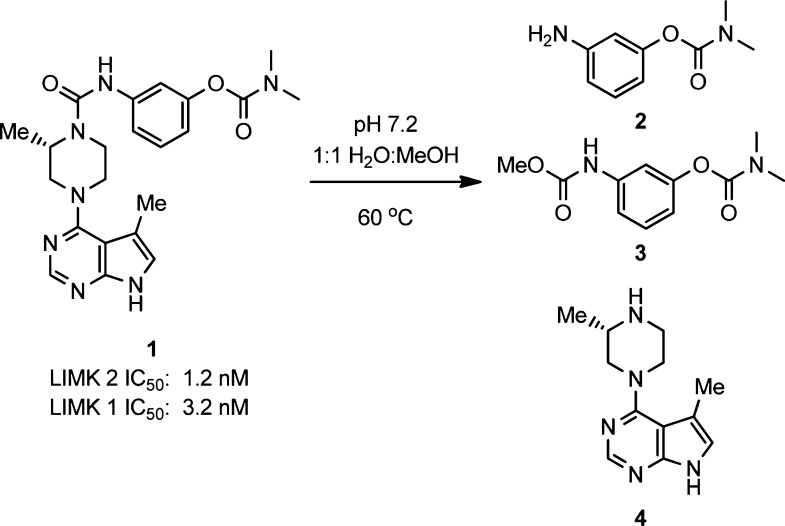
Our previous report detailed the discovery of a class of pyrrolopyrimidine LIMK inhibitors such as compound 1 (Figure 1). These compounds potently and selectively inhibit LIMK1 and LIMK2 in both in vitro and cell-based assays. Administration of an aqueous formulation of 1 to the eyes of ocular hypertensive mice resulted in reduced IOP.
Figure 1.
Observed products from solvolysis of compound 1.
In the process of investigating aqueous formulations of 1, we discovered that the compound slowly decomposes in aqueous solution (Figure 1). For example, when 1 was dissolved in pH 7.2 phosphate buffered H2O/MeOH at 60 °C, high-performance liquid chromatography (HPLC) purity of the compound decreased to 84% after 6 h and 58% after 24 h. Analysis of the decomposition products by liquid chromatography–mass spectrometry (LC–MS) revealed the formation of 2, 3, and 4, resulting from solvolysis of the central urea. The presence of 3 indicates reactivity of the bond between the piperazine nitrogen and the carbonyl, and no corresponding product was observed from the methanolysis of the aniline carbonyl bond.
Various conditions were examined to better understand this unusual reactivity (Table 1). At lower temperatures, the decomposition of 1 was greatly delayed; however, at 20 and 4 °C in 1:1 MeOH/H2O at pH 7.2, HPLC purity of 1 still decreased 1–2% over 5 days. Since topical ophthalmic formulations generally require 12 months stability,18 we did not believe this level of stability to be sufficient. Removal of the MeOH cosolvent also prevented degradation, even at 60 °C; however, without MeOH as a cosolvent, the compound was not soluble in water, so these conditions were not helpful for topical ophthalmic dosing. Additionally, replacement of MeOH with MeCN or removal of the buffer salts had only a small stabilizing effect on the degradation of 1. Finally, the effect of pH on compound stability was tested. Acidifying the solution to pH 5 had minimal effect, while basifying the solution with NaOH dramatically decreased stability. On the basis of these results, various compounds were tested for stability in 1:1 H2O/MeOH at pH 7.2 at 60 °C for 24 h to establish the structure–activity relationship (SAR) of the solvolysis reaction.
Table 1. Aqueous Instability of Compound 1 under Various Conditionsa.
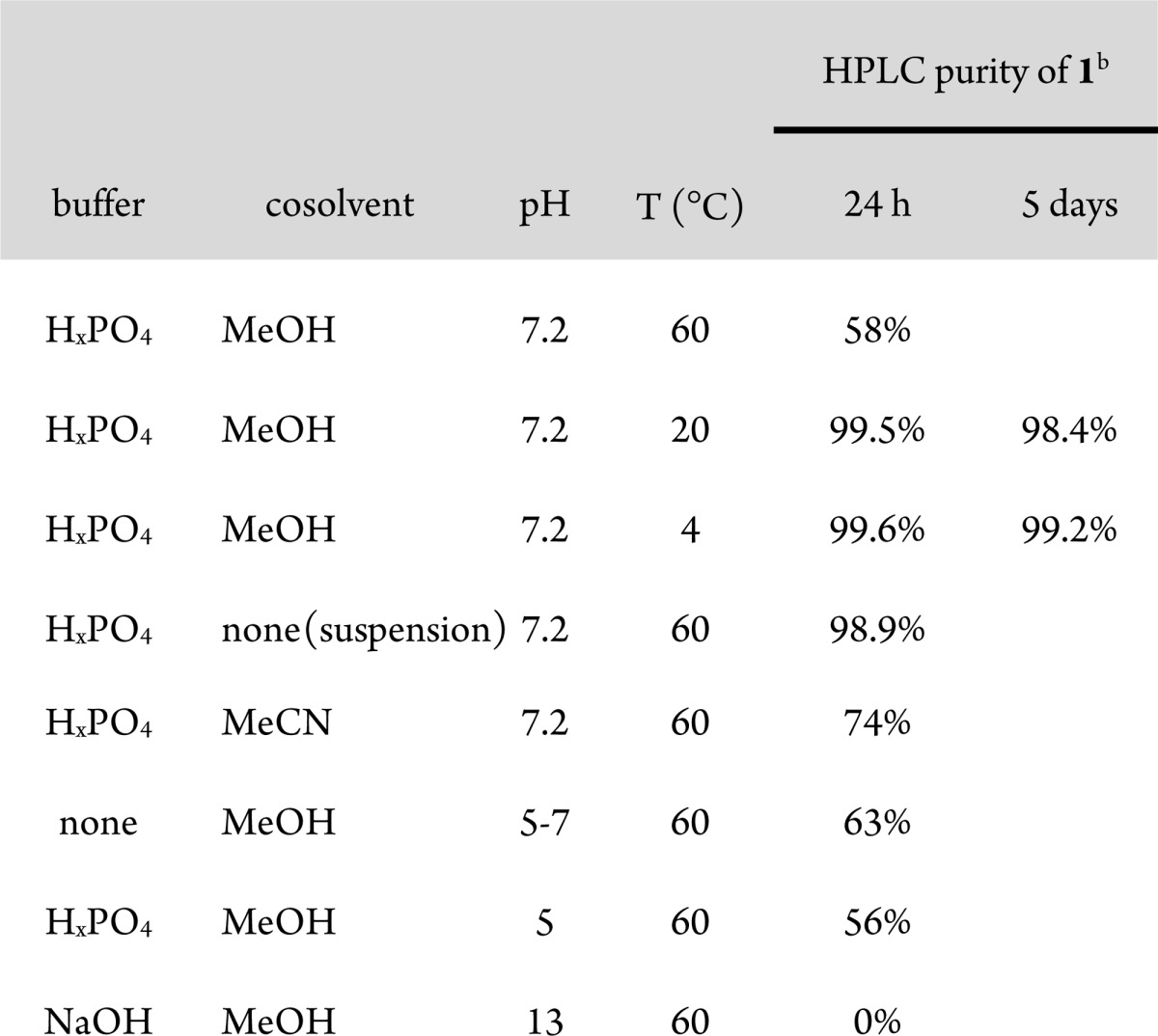
Stability of 1 tested at 2 mg/mL in 1:1 H2O/cosolvent.
Purity evaluated by HPLC using 10 mM aq. NH4OAc/MeCN gradient on a 4.6 × 50 mm 4.6 μm C18 column with 220 nm UV detection.
Modifications of the aniline portion of the molecule failed to produce stable, active compounds (Table 2). Substitution of the aryl ring with electron donating groups only slightly improved stability (compounds 5–7). Replacement of the aniline nitrogen with a methylene produced a stable compound (8) but resulted in a 500-fold reduction in activity for LIMK2. Benzyl ureas 9 and 10 also showed improved stability, but lost 8–150-fold activity.
Table 2. Modifications of Aniline.
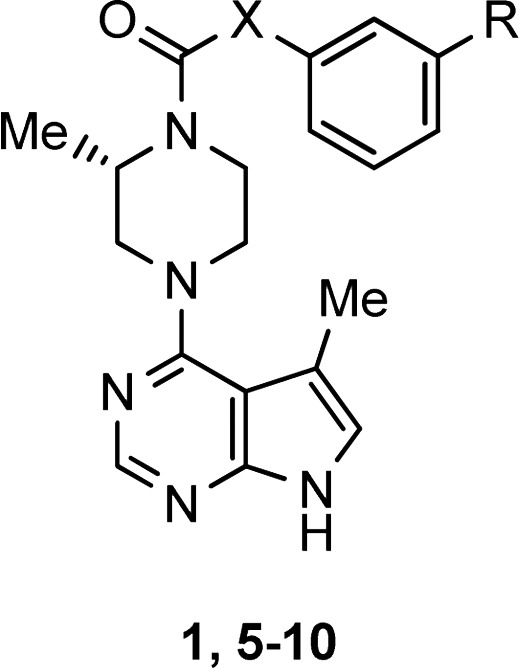
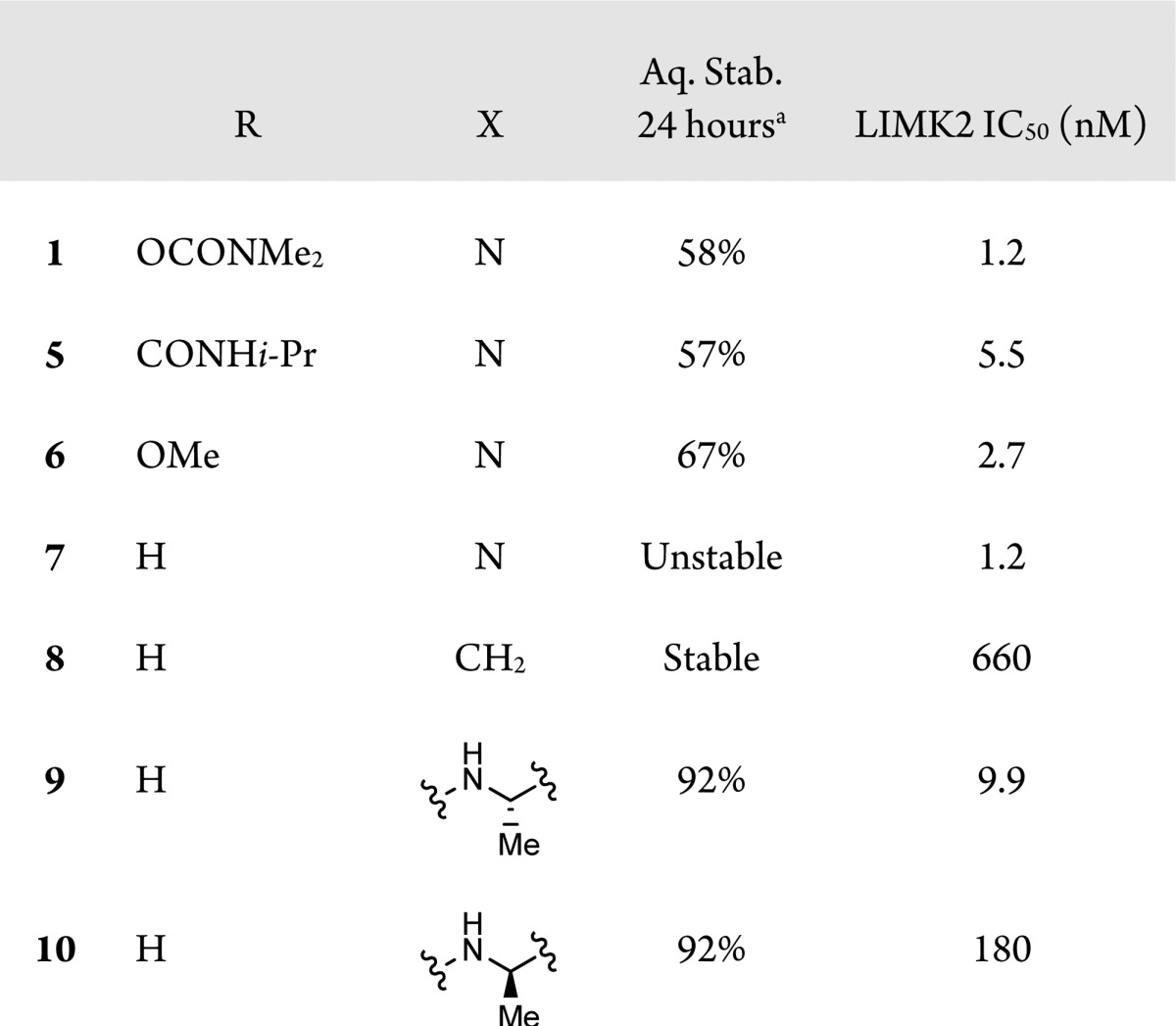
Stability tested at 2 mg/mL in 1:1 H2O/MeOH at 60 °C for 24 h. Purity evaluated by HPLC.
Modifications of the piperazine were examined as well (Table 3). Replacement of the piperazine nitrogen attached to the pyrimidine with a methylene resulted in a small increase in stability (12 compared to 11). Larger effects on the stability of 1 resulted from changes to the substitution alpha to the piperazinyl urea nitrogen. Removal of the alpha methyl (11) improved stability to 88%, and addition of a second methyl (13) decreased stability to 0.4%. Interestingly, replacement of the methyl with tert-butyl (14) had a small stabilizing effect on the compound, perhaps due to steric hindrance of nucleophilic attack. The effect of alpha substitution on compound stability could be attributed to steric destabilization of the planar, conjugated conformation of the piperazinyl N with the urea carbonyl, rendering the C–N bond susceptible to solvolysis.
Table 3. Modifications of Piperazine.
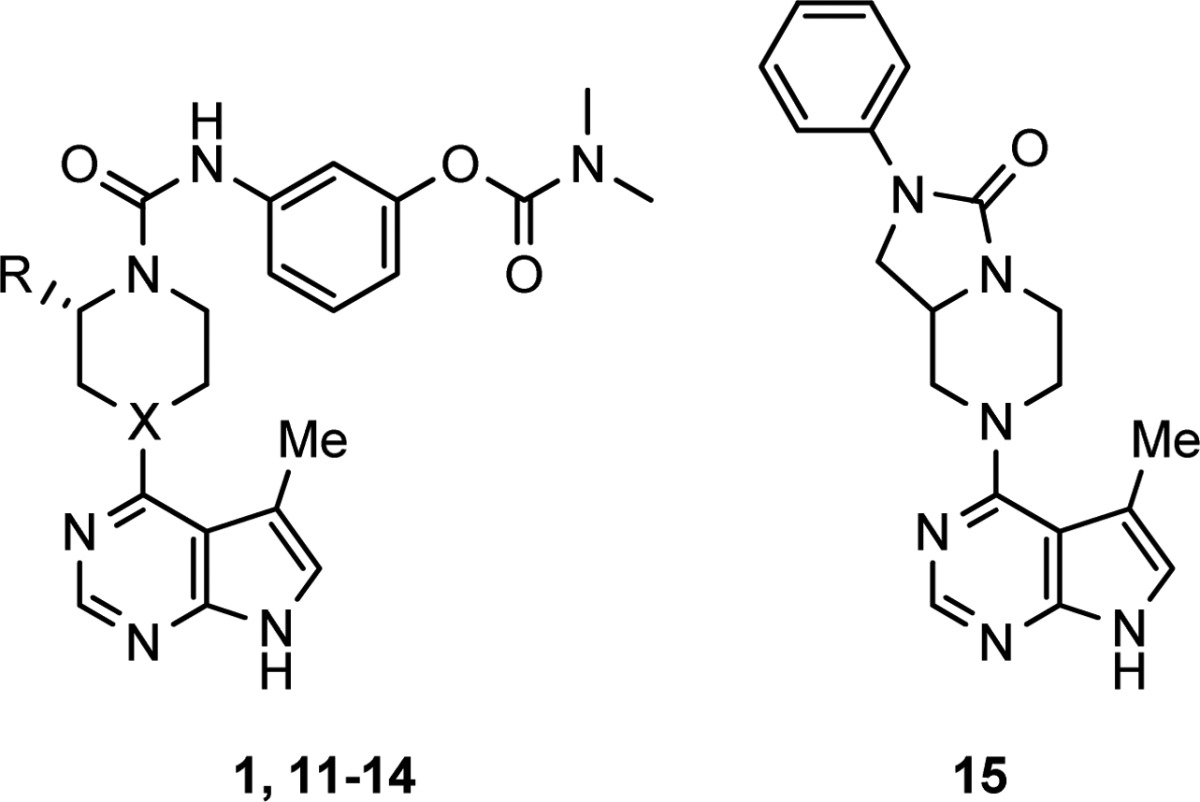
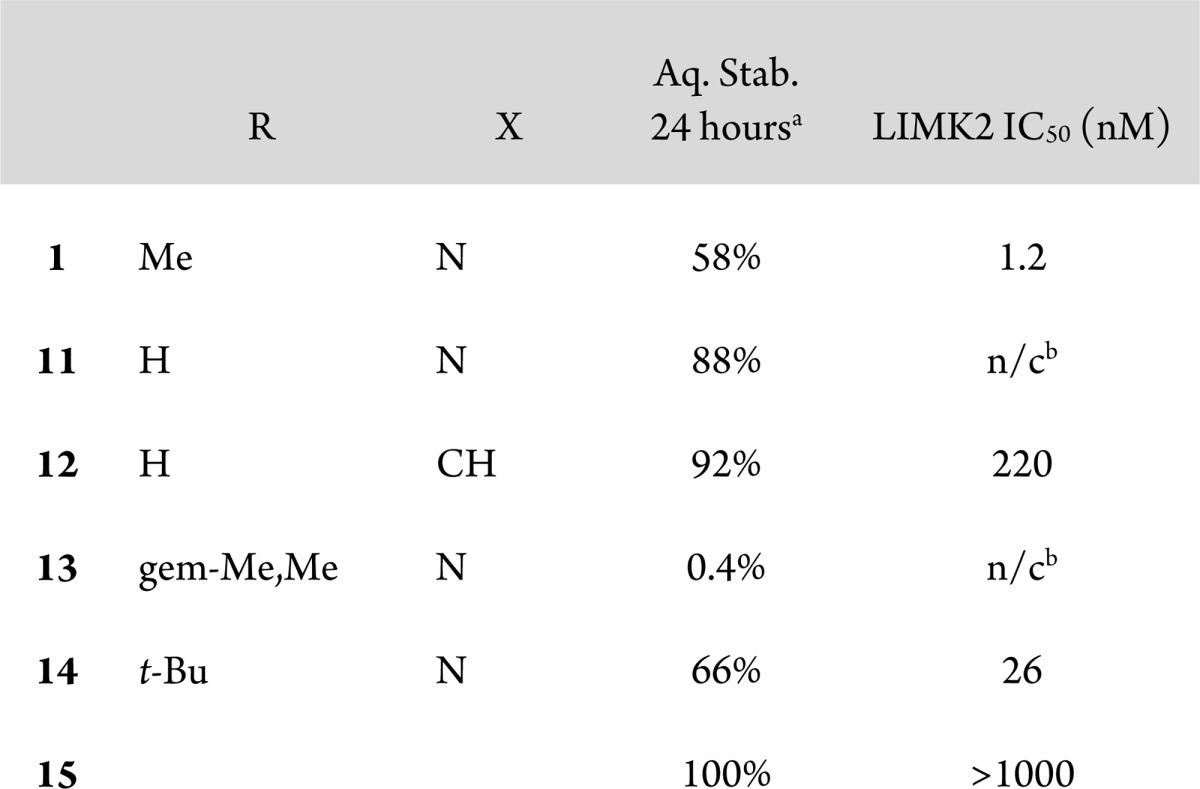
Stability tested at 2 mg/mL in 1:1 H2O/MeOH at 60 °C for 24 h. Purity evaluated by HPLC.
Not calculated.
This hypothesis was supported by molecular modeling done on compound 7.19 Although many conformations of similar energy were observed, the lowest energy conformation of the piperazine places the methyl substituent in an axial conformation that accommodates the planar, conjugated conformation of the nitrogen and carbonyl. We hypothesize that the energetic cost of this axial orientation might provide a driving force for breaking conjugation, resulting in compound instability.
To test the hypothesis empirically, we prepared compound 15. This compound was linked from the piperazine ring to the aniline nitrogen enforcing a planar, conjugated conformation. Not surprisingly, this compound showed no degradation in the stability assay, but it was also completely inactive at LIMK2.
This result suggested that the planar conformation of the urea was not the active one. Consequently, we returned to looking at amides of the type found in Table 4, in which the piperazine nitrogen was replaced by a carbon. With replacement of the labile nitrogen carbonyl bond with a stable carbon carbonyl bond, all amides of this form showed complete stability in our assay for up to 3 days. We reported in our previous communication amides of this type but had not evaluated them more fully because unsubstituted analogues such as 16 had low activity.
Table 4. Aqueous Stable Amidesa.
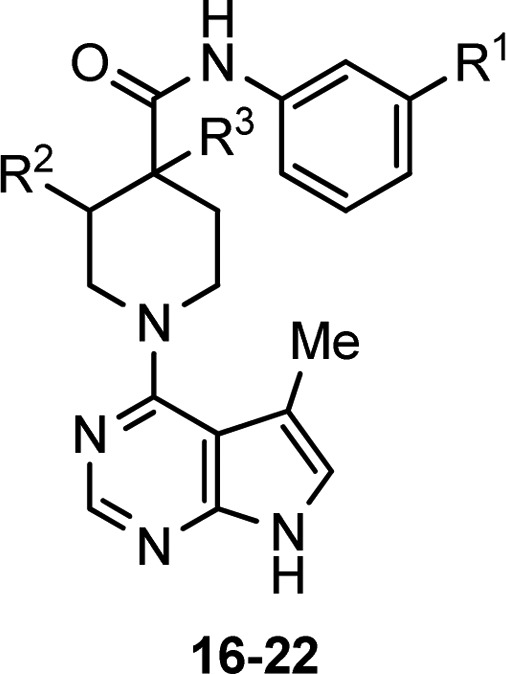
All compounds stable at 2 mg/mL in 1:1 H2O/MeOH at 60 °C for 24 h. Purity evaluated by HPLC.
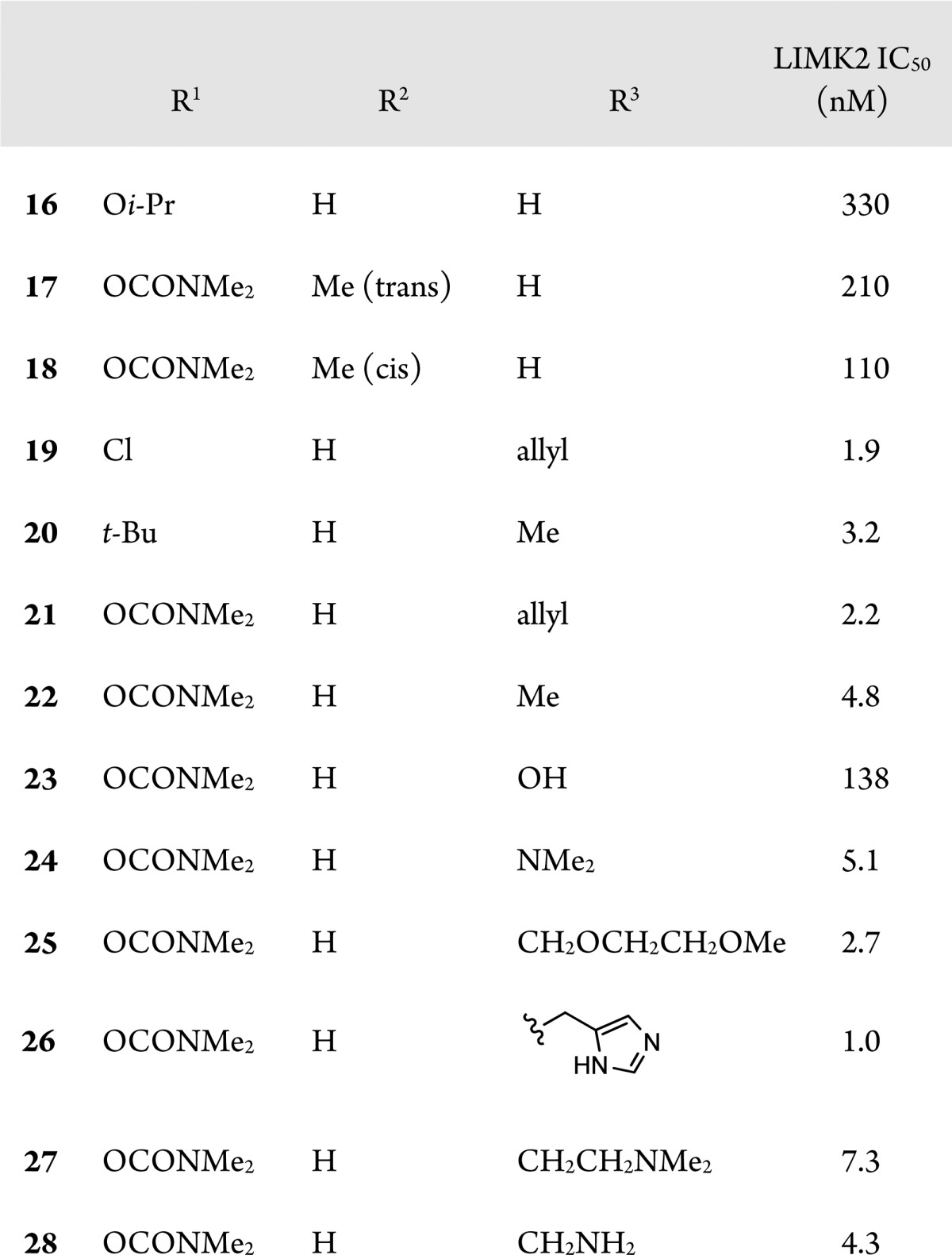
Consequently, we prepared analogues with substitution on the piperidine hoping to improve the potency. Substitution in the analogous position to compound 1 did not sufficiently improve potency (17 and 18). However, substitution alpha to the carbonyl did dramatically improve potency with a variety of substituents at the apha position and on the aniline phenyl ring (19–22). These compounds were aqueous stable and showed in vivo activity in the mouse IOP assay, but poor aqueous solubility made it difficult to achieve topical formulation.
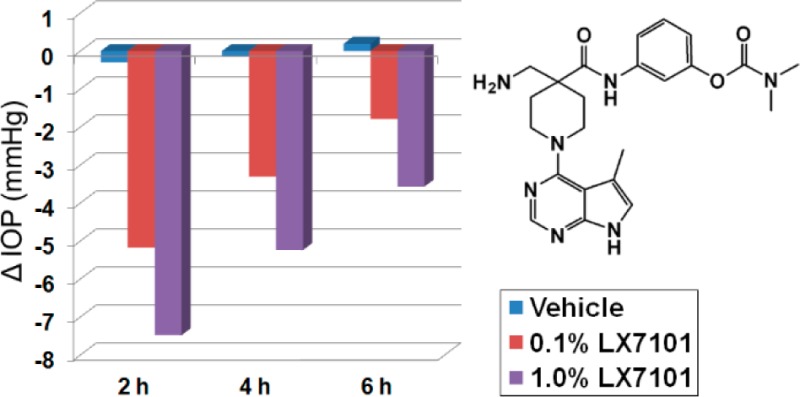
The addition of solubilizing groups to the position alpha to the carbonyl improved solubility while generally maintaining good LIMK2 activity (23–28). We compared many compounds of this type formulated as 0.1% HPMC based aqueous solutions in the ocular hypertensive mouse model. Results for compounds 25–28, along with selectivity for ROCK, are shown in Table 5. Compound 25 had the best selectivity for ROCK, but only moderate efficacy in lowering IOP. Compounds 26 and 27 had greater efficacy, perhaps due to improved aqueous solubility. Following this trend, compound 28, the most soluble compound (up to 10 mg/mL in HPMC based vehicle) exhibited the best efficacy at lowering IOP, although it was only moderately selective versus ROCK at low ATP concentrations. However, when the in vitro binding assays were repeated at high ATP concentrations, compound 28 proved significantly more selective for LIMK2 (300-fold vs ROCK1 and 45-fold vs ROCK2). As ATP has a relatively weak affinity for LIMK2 (Km = 85 μM) compared to ROCK 1 and 2 (Km = <20 μM), it is not surprising that the overall selectivity of compound 28 for LIMK2 increases at the higher physiological ATP concentrations.20 Consequently, we believe that under physiological conditions, the activity of 28 is primarily due to inhibition of LIMK2. The activity of 28 was slightly less than that observed with ROCK inhibitor Y-39983 in the same assay.13
Table 5. Profiles of Key Compounds.
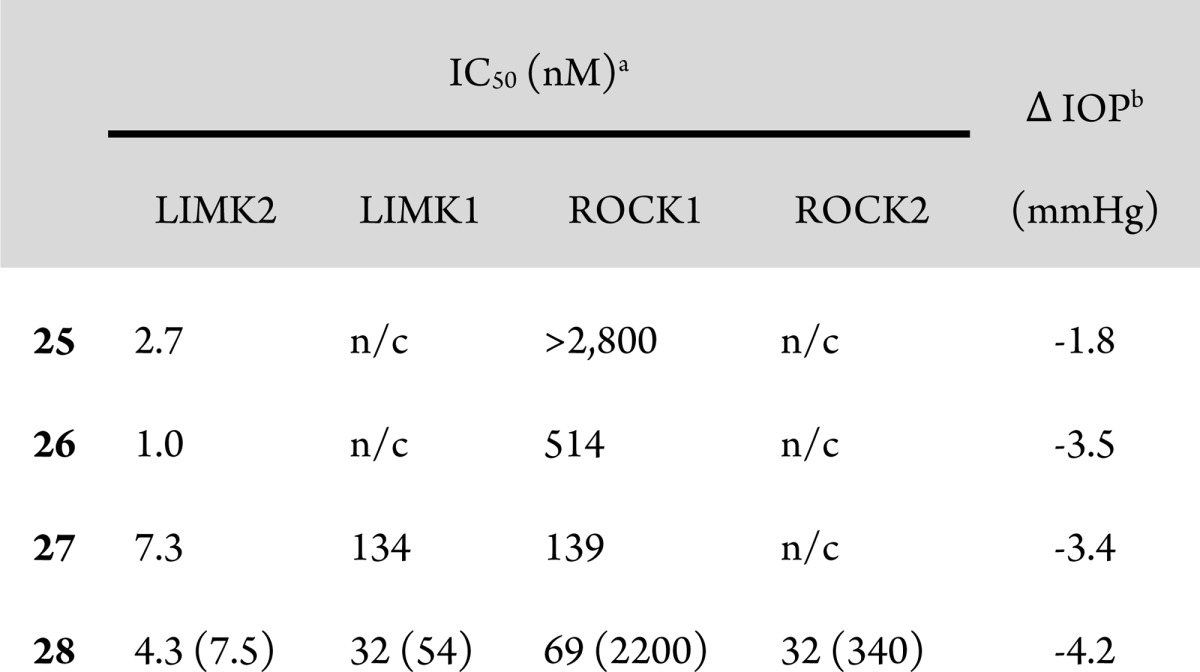
IC50 values at 2 μM ATP, in parentheses at 200 μM ATP.
Reduction in intraocular pressure at 2 h postdose in dexamethasone induced ocular hypertensive mice following topical instillation of 3 μL of 1 mg/mL HPMC based aqueous solution of compound, compared to vehicle.
In addition, compound 28 was screened against a panel of 403 kinases at Ambit Biosciences (now DiscoverRx). Moderate selectivity was observed in this screen (34 assays including LIMK2 and ROCK2 indicated that the Kd is most likely <1 μM; data not shown). Compound 28 was also evaluated in a panel of binding assays including 78 different receptors and transporters at Cerep where no significant cross reactivity was observed (only 5 assays had >60% inhibition at 10 μM; data not shown). On the basis of these results, we were unable to rule out the possibility that the improved efficacy of 28 relative to other LIMK inhibitors was due to inhibition of ROCK or other kinases in addition to LIMK2. However, the improved efficacy of 28 could also be from improved solubility, resulting in increased exposure on topical dosing (see Supporting Information for topical PK of 28). Unfortunately, we were not able to find equally soluble compounds with high LIMK2 selectivity and so were unable to resolve this question.
However, we considered that tolerability was more critical than selectivity, and consequently, topical doses of 28 were evaluated for tolerability on the eyes of mice, rats, and rabbits. The compound was found to be well tolerated at doses up to 0.5% in non-GLP single dose studies. In rats, systemic exposure following topical application of 28 was below limits of quantization for a single dose. Repeat topical dosing of 28 gave a systemic Cmax of 12–19 nM, which was well below the exposure of the NOAEL oral dose (500 mpk). Stability testing of 28 showed 99% stability in an aqueous solution formulation after 14 days at 60 °C.
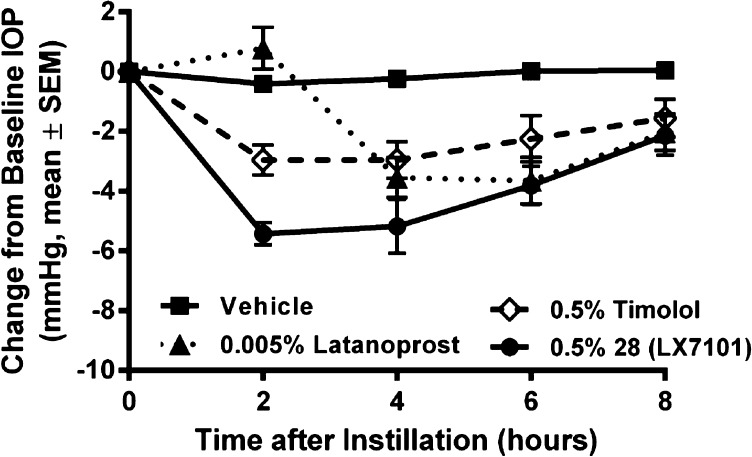
Finally, a well-tolerated dose of compound 28 (0.5%) was compared with the standard clinical doses of commercial glaucoma drugs timolol (0.5%) and latanoprost (0.005%) in the mouse IOP assay (Figure 2). At this dose, 28 achieved additional reduction of IOP (5.0 mmHg total reduction) compared to the 0.1% formulation and demonstrated a long duration of action, with IOP not returning to baseline until more than 8 h postdose. More critically, at these doses, 28 produced a significantly greater reduction of IOP than either timolol or latanoprost.
Figure 2.
Change in intraocular pressure in dexamethasone induced ocular hypertensive mice following topical installation of 3 μL of 5 mg/mL HPMC based aqueous solution of 28, a 5 mg/mL formulation of timolol, or a 0.05 mg/mL formulation of latanoprost.
On the basis of these results, we selected compound 28 as clinical candidate LX7101. IND enabling studies were completed for the compound, and it was evaluated in a phase 1 clinical trial in glaucoma patients. The compound was well tolerated and showed significant reductions in IOP in these patients. The results of these studies will be published at a future time.
In conclusion, we have disclosed the structure of clinical compound LX7101 (28) for the treatment of ocular hypertension and associated glaucoma. This compound is a potent inhibitor of LIM kinase 2, although we cannot rule out the possibility that some of the observed activity is due to weak inhibition of ROCK. LX7101 was derived from compound 1 by replacing the central piperazine ring of 1 with an aminomethylene substituted piperidine. The modifications were necessary to rectify the unusual aqueous instability of the central urea of 1 and to increase aqueous solubility. These changes produced a compound with high efficacy in a mouse model of ocular hypertension that, when progressed to phase 1 clinical trials, lowered IOP in glaucoma patients.
Glossary
Abbreviations
- LIMK2
LIM-kinase 2
- ROCK
Rho-associated protein kinase
- IOP
intraocular pressure
- HPMC
hydroxypropyl methylcellulose
- NOAEL
no observable adverse event level
- AUC
area under the curve
- Cmax
maximum concentration
- IND
investigational new drug application
Supporting Information Available
Experimental details and analytical data of the compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
⊥ Department of Medicinal Chemistry, FORUM Pharmaceuticals, 500 Arsenal Street, Watertown, Massachusetts 02472, United States.
The authors declare no competing financial interest.
Supplementary Material
References
- Bernard O. Lim Kinases, Regulators of Actin Dynamics. Int. J. Biochem. Cell Biol. 2007, 39, 1071–1076. [DOI] [PubMed] [Google Scholar]
- Manetti F. LIM kinases are attractive targets with many macromolecular partners and only a few small molecule regulators. Med. Res. Rev. 2012, 32, 968–998. [DOI] [PubMed] [Google Scholar]
- Slee J. B.; Lowe-Krentz L. J. Actin realignment and cofilin regulation are essential for barrier integrity during shear stress. J. Cell. Biochem. 2013, 114, 782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q.; Ji Y.-S.; Cai C.; Chen Z.-Y. LIM Kinase 1 (LIMK1) interacts with tropomyosin-related kinase B (TrkB) and mediates brain-derived neurotrophic factor (BDNF)-induced axonal elongation. J. Biol. Chem. 2012, 287, 41720–41731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.; Jiao D.; Hu H.; Song J.; Yan J.; Wu L.; Xu L.-Q. Downregulation of LIMK1 level inhibits migration of lung cancer cells and enhances sensitivity to chemotherapy drugs. Oncol. Res. 2013, 20, 491–498. [DOI] [PubMed] [Google Scholar]
- Gamell C.; Schofield A. V.; Suryadinata R.; Sarcevic B.; Bernard O. LIMK2 mediates resistance to chemotherapeutic drugs in neuroblastoma cells through regulation of drug-induced cell cycle arrest. PLoS One 2013, 8, e72850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetti F. HIV-1 proteins join the family of LIM kinase partners. New roads open up for HIV-1 treatment. Drug Discovery Today 2012, 17, 81–88. [DOI] [PubMed] [Google Scholar]
- Olson M. F. Follow the leader: LIM kinases pave the way for collective tumor cell invasion. Cell Cycle 2010, 9, 4417–4418. [DOI] [PubMed] [Google Scholar]
- He L.; Seitz S. P.; Trainor G. L.; Tortolani D.; Vaccaro W.; Poss M.; Tarby C. M.; Tokarski J. S.; Penhallow B.; Hung C.-Y.; Attar R.; Lin T.-A. Modulation of cofilin phosphorylation by inhibition of the Lim family kinases. Bioorg. Med. Chem. Lett. 2012, 22, 5995–5998. [DOI] [PubMed] [Google Scholar]
- Sleebs B. E.; Nikolakopoulos G.; Street I. P.; Falk H.; Baell J. B. Identification of 5,6-substituted 4-aminothieno[2,3-d]pyrimidines as LIMK1 inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 5992–5994. [DOI] [PubMed] [Google Scholar]
- Ross-Macdonald P.; de Silva H.; Guo Q.; Xiao H.; Hung C.-Y.; Penhallow B.; Markwalder J.; He L.; Attar R. M.; Lin T.; Seitz S.; Tilford C.; Wardwell-Swanson J.; Jackson D. Identification of a nonkinase target mediating cytotoxicity of novel kinase inhibitors. Mol. Cancer Ther. 2008, 7, 3490–3498. [DOI] [PubMed] [Google Scholar]
- Harrison B. A.; Whitlock N. A.; Voronkov M. V.; Almstead Z. Y.; Gu K.-J.; Mabon R.; Gardyan M.; Hamman B. D.; Allen J.; Gopinathan S.; McKnight B.; Crist M.; Zhang Y.; Liu Y.; Courtney L. F.; Key B.; Zhou J.; Patel N.; Yates P. W.; Liu Q.; Wilson A. G. E.; Kimball S. D.; Crosson C. E.; Rice D. S.; Rawlins D. B. Novel class of LIM-kinase 2 inhibitors for the treatment of ocular hypertension and associated glaucoma. J. Med. Chem. 2009, 52, 6515–6518. [DOI] [PubMed] [Google Scholar]
- Whitlock N. A.; McKnight B.; Corcoran K. N.; Rodriguez L. A.; Rice D. S. Increased intraocular pressure in mice treated with dexamethasone. Invest. Ophthalmol. Vis. Sci. 2010, 51, 6496–6503. [DOI] [PubMed] [Google Scholar]
- Quigley H. A. Glaucoma. Lancet 2011, 377, 1367–1377. [DOI] [PubMed] [Google Scholar]
- Bettin P.; Di Matteo F. Glaucoma: present challenges and future trends. Ophthalmic Res. 2013, 50, 197–208. [DOI] [PubMed] [Google Scholar]
- Toris C. B.; Koepsell S. A.; Yablonski M. E.; Camras C. B. Aqueous humor dynamics in ocular hypertensive patients. J. Glaucoma 2002, 11, 253–258. [DOI] [PubMed] [Google Scholar]
- Wang S. K.; Chang R. T. An emerging treatment option for glaucoma: Rho kinase inhibitors. Clin. Ophthalmol. 2014, 8, 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food Drug Administration Center for Drugs Evaluation Research (2014). Guidance for Industry: ANDAs: Stability Testing of Drug Substances and Products. Questions and Answers (FDA Maryland).
- The modeling was done with the CFF98 Force Field within the Discover and Insight programs (Biovia, Inc. (previously Accelrys, Inc.), San Diego, CA) and confirmed with ICM (Molsoft, LLC, San Diego, CA) employing the MMFF force field.
- Goodwin N. C.; Cianchetta G.; Burgoon H. A.; Healy J.; Mabon R.; Strobel E. D.; Allen J.; Wang S.; Hamman B. D.; Rawlins D. B. Discovery of a type III inhibitor of LIM kinase 2 that binds in a DFG-out conformation. ACS Med. Chem. Lett. 2014, 10.1021/ml500242y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.