Abstract
Rhinoviruses (RV’s) are common human pathogens of the respiratory tract being the most frequent cause of mild diseases of the upper respiratory tract (common cold) but more importantly they are a major initiator of acute exacerbations of chronic airway diseases. Infections can be life threatening in the latter context however RV -induced common colds have an associated economic cost from loss of productivity due to absence from work or school. There are no appropriate antiviral therapies available and vaccine strategies have failed because of the large number of viral serotypes and the lack of cross-serotype protection generated. Here, approaches past and present for development of a vaccine to these widespread human pathogens are highlighted.
Keywords: rhinovirus, immunity, vaccine, human pathogens, mouse model, chronic airway diseases
Background
Rhinoviruses (RVs) are a species of human pathogens belonging to the genus enterovirus of the picornaviridae family of viruses [1]. RVs are very small viruses about 30nm in diameter that contain a positive sense ssRNA genome of approximately 7500bp that is surrounded by a protein capsid which is composed of 60 copies of four protein subunits that assemble an icosahedron. These structural proteins consist of the externally facing virus protein 1 (VP1), VP2 and VP3 and internal VP4 which lies at the interface of the capsid and RNA genome [2]. RVs infect and cause disease in all humans but most significantly in those individuals with underlying lung diseases such as asthma or chronic obstructive pulmonary disease, where they are a major precipitant of acute exacerbations [3]. The success of RV’s as human pathogens is due not only to their speed of infection and onward transmission but also to their ability to adapt and change, resulting in the existence of numerous antigenically distinct serotypes. The original definition and numbering of serotypes from 1 to 100 was based on antibody neutralisation properties with polyclonal antisera where little or no cross-serotype neutralization was observed [4]. Antibodies are directed against the outer surface of the RV capsid most commonly to exposed areas of VP1, VP2 and VP3 [5, 6]. Regions of the capsid sequences display a high degree of heterogeneity amongst serotypes where there are areas with less than 70% homology within the RV polyproteins (Figure 1) [7]. These result in variable surface-exposed immunodominant epitopes that can dictate serotypespecific immune responses. Based on RNA genome sequence analyses, RV’s have now been divided into three groups known as RV types A, B and C [8] and may be further classified by entry receptor usage. Approximately 90% of characterized RV strains (major group) use intercellular adhesion molecule-1 (ICAM-1) as receptors to enter host epithelial cells [9] whereas the minor group exploits members of the low-density lipoprotein (LDL) receptor family [10]. The entry receptor for group C RV’s has yet to be identified due to propagation difficulties in vitro [11] making it difficult to compare serological responses and to therefore pinpoint the exact number of RV serotypes. However, based upon the newer molecular methods to genetically assign RV groupings there is likely to be significantly more than the fully characterized 100 distinct serotypes [12]. When the numbers of distinct RV infections are characterized molecularly by sequencing the VP4/VP2 region, it has been estimated that 47% of infections are due to group A, 12% to group B and 39% to group C [13]. These numbers suggest that upwards of 160 RV serotypes exist and are in circulation. The fact that adults experience on average of 2-5 infections and children up to 10 infections per year [14], when coupled with the lack of cross protective immunity between serotypes [15], ensures that humans can expect a lifetime of RV infections. A broadly cross protective vaccine could alleviate many of these infections and the associated health and economic issues, particularly in those with underlying chronic airways diseases.
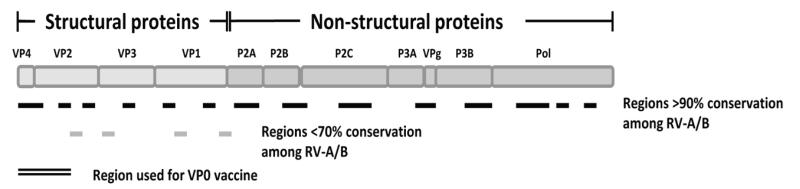
Figure 1.
Schematic diagram of RV polyprotein displaying individual proteins as boxes. The polyprotein is organised into the N terminal proximal structural proteins (capsid proteins VP4, VP2, VP3 and VP1) followed by the non-structural proteins (P2A, P2B, P2C, P3A, VPg, P3B, Pol) which are C terminal proximal. Regions with >90% conservation among the RV types A and B are denoted with a black line and regions displaying <70% conservation are marked with a grey line. The region at the N terminus that corresponds to the VP0 experimental vaccine is marked with a double line.
Early attempts at RV vaccines
During the late 1960s and early 1970s clinical trials were performed to investigate a common cold vaccine, largely through administration of a formalin inactivated single RV serotype (RV13) [16, 17, 18, 19, 20, 21]. This approach was found to provide only minimal protective effects and was abandoned in favour of testing of inactivated multivalent vaccines spanning 10 serotypes [22]. Although these vaccines attempted to address the issue of weak cross-serotype protection induced by monovalent vaccination, they also lost popularity when surprisingly they failed to induce significant cross protection amongst RV serotypes. Table 1 summarises these studies that have been performed in humans using inactivated RV preparations as vaccines. We now suspect that inactivation of RV for vaccine studies is unfavourable for the generation of significant cell mediated immune responses and that the antibody responses alone that are often generated in such situations are insufficient for broad protection [23]. Formalin treatment was the most common method for RV inactivation [16, 17, 18, 19, 20, 21] although alternative methods such as heat treatment (pasteurisation), low pH and UV treatment are also effective [24, 25, 26]. These methods, whilst largely safe for human application, are likely to destroy many epitopes required for optimal immune responses and therefore can impact negatively on vaccine efficacy by reducing preparation immunogenicity [27]. In addition, another potential reason as to why these prior studies displayed limited success is that there was no evidence indicating that an adjuvant was used to amplify immune responses. The use of adjuvants would most likely have improved vaccine efficacy significantly [28] however, at that time, Alum was the only approved adjuvant for use in humans whilst there are now several others available [29].
Table 1.
Summary of early clinical studies investigating efficacy of RV vaccines.
| Vaccine | Administration route | Findings | Reference |
|---|---|---|---|
| Formalin inactivated RV3, RV7, RV10, RV13, RV14, RV18, RV22, RV42, RV43, RV55 (decavalent) | Intramuscular | Minimal homologous and heterologous neutralizing antibody responses | Hamory 1975 [22] |
| Formalin inactivated RV13 | Subcutaneous | Homotypic neutralising antibody generated and reduced viral shedding upon homotypic challenge | Douglas 1972 [21] |
| Formalin inactivated RV13 | Intranasal | Resistance to homotypic challenge | Buscho 1972 [20] |
| Formalin inactivated RV13 | Intranasal | Protection to homotypic challenge | Perkins 1969 [19] |
| Formalin inactivated RV13 | Intranasal & intramuscular | Protection to homotypic challenge by intranasal immunization & correlated with level of nasal neutralising antibody | Perkins 1969 [18] |
| Formalin inactivated single strain | Intranasal | Protection to homotypic challenge but not heterotypic challenge | Mitchison 1965 [17] |
| Live & formalin inactivated single strain | Intranasal & intramuscular | Increased homotypic antibody responses with live intranasal and inactive intramuscular | Doggett 1963 [16] |
Following the human trials, experimental studies in immunised animals (rabbits and mice) began to determine some properties of antibody cross reactivity [30, 31, 32] briefly encouraging renewed hope for a RV vaccine as cross-serotype neutralising antibodies were convincingly demonstrated (Table 2). Despite these positive steps, RV vaccine research studies in the scientific literature then virtually disappeared for over 20 years before further studies in immunised animals with recombinant RV capsid protein subunits and synthetic peptides again proposed possibilities for cross-serotype protective antibodies generation. Here, short conserved regions at the N-terminus of the capsid protein VP4 were identified that elicit cross-serotype protective antibodies [33] and others found that the entire VP1 polypeptide had similar effects [34]. Despite these encouraging studies and the application of modern molecular analyses, the formal demonstration of protective vaccine responses to RV’s in in vivo settings remained elusive largely because of the absence of a small animal in vivo model of RV infections.
Table 2.
Summary of animal studies investigating RV antibodies after vaccination.
| Immunogen & Animal model | Administration route | Findings | Reference |
|---|---|---|---|
| Inactivated RV16 Cotton rat |
Intramuscular | Generation of cross-serotype neutralising antibody responses following intranasal challenge | Blanco 2014 [37] |
| Recombinant VP0 of RV16 Mouse |
Subcutaneous | Generation of cross-serotype neutralising antibody responses following intranasal challenge | Glanville 2013 [7] |
| Inactivated RV1B Mouse |
Subcutaneous | Generation of cross-serotype neutralising antibody responses following intranasal challenge | McLean 2012 [23] |
| Recombinant VP1 of RV89 & RV14 Rabbit & mouse |
Subcutaneous | Generation of cross-serotype neutralising antibody responses | Edlmayr 2011 [34] |
| VP4 peptides of RV14 Rabbit |
Subcutaneous | Generation of cross-serotype neutralising antibody responses | Katpally 2009 [33] |
| VP1 & VP3 peptides of RV14 Rabbit |
Subcutaneous | Generation of cross-serotype neutralising antibody responses | McCray 1987 [32] |
| Numerous RV serotypes individually Rabbit |
Intravenous | Extensive cross-serotype binding antibody responses | Cooney 1975 [30] |
Recent approaches using mouse models of human RV infection
The advent of a mouse model of human RV infection [26] has permitted new approaches for RV vaccine development where specific RV challenge following immunisation can be addressed. Previously, infection of mouse cells and indeed live mice with human RV’s was not thought possible due to significant sequence differences between the major group entry receptor human ICAM-1 and the mouse counterpart [35]. Furthermore, there was a lack of sustained intracellular viral replication in mouse cells despite minor group RV having the ability to enter via the mouse LDL receptor [36]. Mice transgenic for human ICAM-1, and improved methods for generating high titre RV inoculum, have now allowed the intranasal infection of mice with RV’s [26]. Here, RV was shown to replicate and cause acute lung inflammation as well as activating innate immune responses and initiating adaptive immune responses. Immunisation and challenge strategies have subsequently been investigated in this model system [7, 23], providing a basis for evaluating the immunological correlates of protection to RV’s in vivo. Hyper-immunisation of mice with inactivated RV1B, followed by homologous intranasal challenge, generated strong cross-serotype neutralising humoral immune responses which were directed at the capsid protein VP1 [23]. Although these antibody responses were neutralising in vitro, similar to prior experimentation in humans, very little protective effect was observed in vivo further confirming that the use of inactivated RV preparations as immunogens does not provide the appropriate immunological stimulation that can result in broad RV protection.
Thus an alternate approach was initiated that focussed on the induction of broadly reactive T cell immunity. Here, a conserved region (VP0) of the RV polyprotein amongst type A and type B strains was identified (Figure 1), the recombinant protein was produced in E. coli and used as an immunogen in mice [7]. In this study, recombinant VP0 derived from RV16 was immunogenic in vivo, inducing immunogen and RV-specific antibodies and cross-serotypic systemic cellular immune responses. Furthermore, the use of a Th1-promoting adjuvant in combination with VP0 induced cross-serotype cellular T lymphocytes producing the Th1 cytokine IFNγ and improved Th1-associated RV-specific antibody responses. It was also shown that immunised mice challenged with heterologous RV strains displayed enhanced cross-reactive cellular, increased memory CD4 T cell numbers and stronger humoral immune responses suggesting broad cross-serotype reactivity was obtained with this strategy. Most importantly, VP0 immunisation followed by live RV challenge improved the generation of neutralising antibodies to a variety of RV serotypes and also caused more rapid virus clearance in vivo. VP0 therefore represents a useful candidate for a subunit RV vaccine and may function by generating significant cross-reactive Th1 cells that upon heterologous RV challenge quickly stimulates additional protective immune responses [7]. Further experimentation in this model system of RV infection and translation to humans awaits.
Very recently it has been shown that cotton rats are permissive for RV16 infection and display characteristics similar to the mouse RV infection model [37]. Interestingly, in this model, prior immunisation with inactivated RV via the intramuscular route but not by the intranasal route produced significant neutralising antibody responses and reduced the viral load in the lungs upon homotypic challenge, confirming findings in the mouse model [7, 23]. In further experiments both prophylactic antibody administration and maternal immunity transfer to neonates were both protective although heterotypic responses were not evaluated [37]. The use of this model system in addition to the mouse model will complement human studies and hopefully aid in the identification and development of RV vaccines.
Public health challenges of RV vaccine delivery to humans
There is a large unmet medical need resulting from RV infections that would be corrected by a safe and effective vaccine. The major target population of an RV vaccine would be those suffering from chronic lung diseases such as asthma and chronic obstructive pulmonary disease where infections with RV are a major precipitant of life threatening acute exacerbations that can require hospitalisation [3]. What would be the risk that a RV vaccine might exacerbate airway inflammation in such chronic lung diseases? Since it is known that RV infection in asthma induces Th2 responses that are linked to lower airway disease [38] it is of concern that a RV vaccine might exacerbate this response, particularly following natural RV exposure. Thus, the favoured RV vaccine approach should promote Th1 responses which is hypothesised to reset the unbalanced immune responses observed in asthmatics. Such an approach as outlined above has been demonstrated already in mice [7]. Whether the vaccine induced enhancement of Th1 cell responses to RV will prove a safe strategy for preventing RV-induced disease awaits confirmation in a clinical trial.
A secondary population that would benefit from a RV vaccine are healthy individuals. Here a broadly protective RV vaccine could reduce the burden of the common cold. Clearly any population receiving a RV vaccine would require initial safety and efficacy testing in healthy individuals with subsequent careful monitoring of airways inflammation following both natural and experimental RV exposure. This would be necessary to eliminate the possibility of an undesirable disease augmentation occurring following challenge as had occurred previously when testing a formalin inactivated RSV vaccine in infants during the 1960s [39].
Conclusion
Attempts to produce a protective vaccine to RV’s have failed due to the large number of antigenically distinct serotypes and the lack of a suitable small animal model of infection to test candidates in. The recent discovery of a previously unrecognised clade of RV’s has complicated this further. Nevertheless, studies in immunised animals have demonstrated that significant cross-serotype protection is possible. With the advent of small animal immunisation and challenge models, suitable vaccine candidates can now be evaluated thoroughly before translation to humans. The quest for a RV vaccine now seems somewhat less forlorn than it did a decade ago.
Acknowledgements
Supported in part by Serendipity Award SA29/0513 from the Dunhill Medical Trust.
Abbreviations
- RV
rhinovirus
- VP
virus protein
- ICAM-1
intercellular adhesion molecule-1
- IFNγ
interferon gamma
- Th1
T cell helper type 1
- Th2
T cell helper type 2
Footnotes
Conflict of Interest The author is listed as an inventor on International Patent Application No. EP2014/052349 (Induction of Cross-Reactive Cellular Response Against Rhinovirus Antigens).
References
- [1].Waman VP, Kolekar PS, Kale MM, Kulkarni-Kale U. Population structure and evolution of Rhinoviruses. PLoS One. 2014;9:e88981. doi: 10.1371/journal.pone.0088981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rossmann MG, Arnold E, Erickson JW, Frankenberger EA, Griffith JP, et al. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985;317:145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- [3].Greenberg SB. Rhinovirus and coronavirus infections. Semin Respir Crit Care Med. 2007;28:182–192. doi: 10.1055/s-2007-976490. [DOI] [PubMed] [Google Scholar]
- [4].Conant RM, Hamparian VV. Rhinoviruses: basis for a numbering system. II. Serologic characterization of prototype strains. J Immunol. 1968;100:114–119. [PubMed] [Google Scholar]
- [5].Sherry B, Mosser AG, Colonno RJ, Rueckert RR. Use of monoclonal antibodies to identify four neutralization immunogens on a common cold picornavirus, human rhinovirus 14. J Virol. 1986;57:246–257. doi: 10.1128/jvi.57.1.246-257.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Appleyard G, Russell SM, Clarke BE, Speller SA, Trowbridge M, et al. Neutralization epitopes of human rhinovirus type 2. J Gen Virol. 1990;71:1275–1282. doi: 10.1099/0022-1317-71-6-1275. [DOI] [PubMed] [Google Scholar]
- [7].Glanville N, McLean GR, Guy B, Lecouturier V, Berry C, et al. Cross-serotype immunity induced by immunization with a conserved rhinovirus capsid protein. PLoS Pathog. 2013;9:e1003669. doi: 10.1371/journal.ppat.1003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Palmenberg AC, Spiro D, Kuzmickas R, Wang S, Djikeng A, et al. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science. 2009;324:55–59. doi: 10.1126/science.1165557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Greve JM, Davis G, Meyer AM, Forte CP, Yost SC, et al. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- [10].Hofer F, Gruenberger M, Kowalski H, Machat H, Huettinger M, et al. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc Natl Acad Sci USA. 1994;91:1839–1842. doi: 10.1073/pnas.91.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bochkov YA, Palmenberg AC, Lee WM, Rathe JA, Amineva SP, et al. Molecular modeling, organ culture and reverse genetics for a newly identified human rhinovirus C. Nat Med. 2011;17:627–632. doi: 10.1038/nm.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McIntyre CL, Knowles NJ, Simmonds P. Proposals for the classification of human rhinovirus species A, B and C into genotypically assigned types. J Gen Virol. 2013;94:1791–1806. doi: 10.1099/vir.0.053686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rahamat-Langendoen JC, Riezebos-Brilman A, Hak E, Scholvinck EH, Niesters HG. The significance of rhinovirus detection in hospitalized children: clinical, epidemiological and virological features. Clin Microbiol Infect. 2013;19:E435–442. doi: 10.1111/1469-0691.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Turner RB. Epidemiology, pathogenesis, and treatment of the common cold. Ann Allergy Asthma Immunol. 1997;78:531–539. doi: 10.1016/S1081-1206(10)63213-9. quiz 539–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Privolizzi R, Solari R, Johnston SL, McLean GR. The application of prophylactic antibodies for rhinovirus infections. Antivir Chem Chemother. 2014;23(5):173–177. doi: 10.3851/IMP2578. [DOI] [PubMed] [Google Scholar]
- [16].Doggett JE, Bynoe ML, Tyrrell DA. Some attempts to produce an experimental vaccine with rhinoviruses. Br Med J. 1963;1:34–36. doi: 10.1136/bmj.1.5322.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mitchison DA. Prevention of Colds by Vaccination against a Rhinovirus: A Report by the Scientific Committee on Common Cold Vaccines. Br Med J. 1965;1:1344–1349. [PMC free article] [PubMed] [Google Scholar]
- [18].Perkins JC, Tucker DN, Knopf HL, Wenzel RP, Kapikian AZ, et al. Comparison of protective effect of neutralizing antibody in serum and nasal secretions in experimental rhinovirus type 13 illness. Am J Epidemiol. 1969;90:519–526. doi: 10.1093/oxfordjournals.aje.a121098. [DOI] [PubMed] [Google Scholar]
- [19].Perkins JC, Tucker DN, Knope HL, Wenzel RP, Hornick RB, et al. Evidence for protective effect of an inactivated rhinovirus vaccine administered by the nasal route. Am J Epidemiol. 1969;90:319–326. doi: 10.1093/oxfordjournals.aje.a121076. [DOI] [PubMed] [Google Scholar]
- [20].Buscho RF, Perkins JC, Knopf HL, Kapikian AZ, Chanock RM. Further characterization of the local respiratory tract antibody response induced by intranasal instillation of inactivated rhinovirus 13 vaccine. J Immunol. 1972;108:169–177. [PubMed] [Google Scholar]
- [21].Douglas RG, Couch RB. Parenteral inactivated rhinovirus vaccine: minimal protective effect. Proc Soc Exp Biol Med. 1972;139:899–902. doi: 10.3181/00379727-139-36262. [DOI] [PubMed] [Google Scholar]
- [22].Hamory BH, Hamparian VV, Conant RM, Gwaltney JM. Human responses to two decavalent rhinovirus vaccines. J Infect Dis. 1975;132:623–629. doi: 10.1093/infdis/132.6.623. [DOI] [PubMed] [Google Scholar]
- [23].McLean GR, Walton RP, Shetty S, Paktiawal N, Kebadze T, et al. Rhinovirus infections and immunisation induce cross-serotype reactive antibodies to VP1. Antiviral Res. 2012;95:193–201. doi: 10.1016/j.antiviral.2012.06.006. [DOI] [PubMed] [Google Scholar]
- [24].Hughes JH, Mitchell M, Hamparian VV. Rhinoviruses: kinetics of ultraviolet inactivation and effects of UV and heat on immunogenicity. Arch Virol. 1979;61:313–319. doi: 10.1007/BF01315018. [DOI] [PubMed] [Google Scholar]
- [25].Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- [26].Bartlett NW, Walton RP, Edwards MR, Aniscenko J, Caramori G, et al. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med. 2008;14:199–204. doi: 10.1038/nm1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bachmann MF, Kundig TM, Kalberer CP, Hengartner H, Zinkernagel RM. Formalin inactivation of vesicular stomatitis virus impairs T-cell- but not T-help-independent B-cell responses. J Virol. 1993;67:3917–3922. doi: 10.1128/jvi.67.7.3917-3922.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- [29].Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11:865–872. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cooney MK, Wise JA, Kenny GE, Fox JP. Broad antigenic relationships among rhinovirus serotypes revealed by cross-immunization of rabbits with different serotypes. J Immunol. 1975;114:635–639. [PubMed] [Google Scholar]
- [31].Fox JP. Is a rhinovirus vaccine possible? Am J Epidemiol. 1976;103:345–354. doi: 10.1093/oxfordjournals.aje.a112233. [DOI] [PubMed] [Google Scholar]
- [32].McCray J, Werner G. Different rhinovirus serotypes neutralized by antipeptide antibodies. Nature. 1987;329:736–738. doi: 10.1038/329736a0. [DOI] [PubMed] [Google Scholar]
- [33].Katpally U, Fu TM, Freed DC, Casimiro DR, Smith TJ. Antibodies to the buried N terminus of rhinovirus VP4 exhibit cross-serotypic neutralization. J Virol. 2009;83:7040–7048. doi: 10.1128/JVI.00557-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Edlmayr J, Niespodziana K, Popow-Kraupp T, Krzyzanek V, Focke-Tejkl M, et al. Antibodies induced with recombinant VP1 from human rhinovirus exhibit cross-neutralisation. Eur Respir J. 2011;37:44–52. doi: 10.1183/09031936.00149109. [DOI] [PubMed] [Google Scholar]
- [35].Register RB, Uncapher CR, Naylor AM, Lineberger DW, Colonno RJ. Human-murine chimeras of ICAM-1 identify amino acid residues critical for rhinovirus and antibody binding. J Virol. 1991;65:6589–6596. doi: 10.1128/jvi.65.12.6589-6596.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yin FH, Lomax NB. Establishment of a mouse model for human rhinovirus infection. J Gen Virol. 1986;67:2335–2340. doi: 10.1099/0022-1317-67-11-2335. [DOI] [PubMed] [Google Scholar]
- [37].Blanco JCG, Core S, Pletneva LM, March TH, Boukhvalova MS, et al. Prophylactic antibody treatment and intramuscular immunization reduce infectious human rhinovirus 16 load in the lower respiratory tract of challenged cotton rats. Trials in Vaccinology. 2014;3:52–60. doi: 10.1016/j.trivac.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]