Abstract
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental pollutants, and have been reported to be a risk factor for human neural tube defects (NTDs). We investigated the relationship between PAH concentrations in maternal serum and NTD risk in offspring using a case-control study design, and explored the link between PAH concentrations to household energy usage characteristics and life styles. One hundred and seventeen women who had NTD-affected pregnancies (cases) and 121 women who delivered healthy infants (controls) were recruited in Northern China. Maternal blood samples were collected at pregnancy termination or at delivery. Twenty-seven PAHs were measured by gas chromatography–mass spectrometry. The concentrations of 13 individual PAHs detected were significantly higher in the cases than in the controls. Clear dose–response relationships between concentrations of most individual PAHs and the risk of total NTDs or subtypes were observed, even when potential covariates were adjusted for. High-molecular-weight PAHs (H-PAHs) showed higher risk than low-molecular-weight PAHs (L-PAHs). No associations between PAH concentrations and indoor life styles and energy usage characteristics were observed. It was concluded that maternal exposure to PAHs was associated with an increased risk of NTDs, and H-PAHs overall posed a higher risk for NTDs than L-PAHs.
Introduction
Neural tube defects (NTDs) are a group of common and serious congenital malformations that result from the failure of the neural tube to close by the 28th day postconception.1 Fetuses with anencephaly, a major anterior NTD subtype, are often stillborn or die shortly after birth, whereas fetuses with spina bifida, another major NTD subtype, are usually liveborn, but most often have significant life-long disabilities.1,2 Birth defects are the leading cause of infant mortality and physical disabilities, and NTDs are among the most common form of birth defects, with a prevalence of one in every 1000 established pregnancies worldwide.2 It has been estimated that more than 320 000 infants worldwide are affected by NTDs each year.3 Although folic acid supplementation has been proved to be effective in the prevention of NTDs by both observational studies and randomized trials,2 the underlying mechanism of NTD prevention with folic acid remains poorly understood.1 Meanwhile, it is also widely appreciated that maternal/embryonic exposure to environmental pollutants, especially polycyclic aromatic hydrocarbons (PAHs) during critical periods of gestation, are associated with increased risks for NTDs in offspring.4−6
PAHs are emitted from the incomplete combustion of fossil fuels or biomass and have potent adverse effects on human health. Some PAHs have been identified as being carcinogenic, mutagenic, and/or teratogenic.7 Animal studies have shown that benzo(a)pyrene-7,8-dihydrodiol-9,10-epoxide, an important metabolic derivative of benzo(a)pyrene, can cause a variety of malformations including: exencephaly (the mouse equivalent of human anencephaly), gastroschisis and phocomelia in mice.8 It was reported that Shanxi Province ranked among the highest PAH emission density in China.9 Epidemiological studies have suggested that maternal PAH exposure, determined by job title or information elicited by questionnaires is associated with an increased risk of NTDs.4,10 In a rural population in Shanxi Province in Northern China, maternal exposure to coal used for residential heating and cooking was linked to an increased NTD risk.10 Preliminary studies conducted by our group found higher concentrations of PAHs in the venous blood of 35 women who had pregnancies affected by NTDs, when compared to 18 women who delivered full term healthy infants.5 In addition, we found an association between higher concentrations of PAHs in placental tissue and an elevated risk for NTDs.6
Until now, published reports concerning a potential association between maternal PAH exposure, as indicated by internal exposure markers, and fetal NTD risk are scarce. Having hypothesized that maternal exposure to PAHs increases the risk of NTDs in offspring, we conducted a case-control study to examine the association between levels of PAHs in maternal serum and the risk of NTDs, and to identify factors regarding household energy usage and life styles that may be associated with PAH concentrations in maternal serum.
MATERIALS AND METHODs
Study Population
Detailed information about the study population has been described elsewhere.11 Briefly, subjects were recruited from Taigu, Pingding, Xiyang, Shouyang, and Zezhou counties, and Changzhi city in Shanxi Province in Northern China from November 2010 to March 2013. The counties in which the studies were conducted are considered to fall within the mean level of socioeconomic development in Shanxi Province. These areas were selected given the high NTD prevalence in this Province.6 When a woman with an NTD-affected pregnancy (including live birth, still birth, or pregnancy termination) was confirmed as a case, a woman who delivered a term healthy infant at the same birthing hospital was selected to serve as a control, matched to the case by the same county or city of residence, and last menstrual period. Information was collected using face-to-face interviews by trained local health workers prior to discharge (within the first week of delivery or pregnancy termination). The participation rate was approximately 80% for both case and control mothers. The questionnaire included information on maternal age (<25, 25–29, or >29 years old), occupation (farmer or nonfarmer), education (“primary or lower”, “junior high”, or “high school or above”), gravidity (1, 2 or >2 times), parity (1, 2 or >2 times); reproductive history (yes or no), periconceptional folate supplementation (yes or no), fever or flu during early pregnancy (yes or no), alcohol drinking (yes or no), active or secondhand smoking during the periconceptional period (yes or no), separate kitchen from living room/bedroom (yes or no), primary fuel used for cooking (soft coal, hard coal, biomass, or liquefied gas), and conception during the heating season (yes or no). If a conception occurred during the heating season, the following questions were asked: using a stove for heating (yes or no), stove in the living room/bedroom (yes or no), primary fuel used for heating (soft coal, hard coal, biomass, or liquefied gas), frequency of ventilation in the living room/bedroom (every day, 1–6 times per week, <1 time per week), and frequency of combustion exhausted gas in living room (every day, 1–6 times per week, <1 time per week). Fasting blood samples from 117 NTD case women (anencephaly, 44; spina bifida, 67; encephalocele, 6) and 121 control women were collected at delivery or termination of NTD-affected pregnancies with a 4 mL BD vacutainer (Becton, Dickinson and Company, USA). Maternal serum was separated within 1 h of collection, temporarily stored at −20 °C, and then transferred on dry ice to our laboratory for further processing, storage and analyses. The serum was stored in a 2 mL freezing tube from Greiner Bio-One GmbH Co., Germany, which was checked and determined to be free of any detectable PAH background. All the serum samples were kept at −80°C prior to extraction. The study protocol was approved by the institutional review board of Peking University, and signed consent was obtained from all the recruited subjects.
PAH Analysis
The extraction and cleanup procedures for PAH analysis have been described elsewhere12 and the detailed information about methods and reagents used in this study is provided in the Supporting Information. Twenty-seven parent PAHs from J&K Chemical, USA including acenaphthylene (ACY), acenaphthene (ACE), fluorene (FLE), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLU), retene (RET), pyrene (PYR), benzo(c)phenanthrene (BCP), cyclopenta(c,d)pyrene (CcdP), benz(a)anthracene (BAA), chrysene (CHR), benzo(b)fluoranthene (BBF), benzo(k)fluoranthene (BKF), benzo(e)pyrene (BEP), benzo(a)pyrene (BAP), perylene (PER), indeno(1,2,3-cd)pyrene (IcdP), dibenz(a,h)anthracene (DahA), benzo(g,h,i)perylene (BghiP), dibenzo(a,c)pyrene (dBacP), dibenzo(a,l)pyrene (dBalP), dibenzo(a,e)fluoranthene (dBaeF), coronene (COR), dibenzo(a,e)pyrene (dBaeP), dibenzo(a,i)pyrene (dBaiP), and dibenzo(a,h)pyrene (dBahP) were analyzed. The concentration of each PAH was determined using a gas chromatograph–mass spectrometer (Agilent 7890, USA) coupled with a mass spectrometry (Agilent 5975, USA) instrument equipped with a HP-5MS capillary column (30 m × 0.25 mm × 0.25 μm). Two procedure blanks and a reagent blank were included for each batch (18–20 samples) of serum samples. The PAH concentration of each sample was calculated by the measured PAH concentration subtracting the mean of their corresponding operation blanks. If the PAH concentrations of samples were lower than their operation blanks, they were set to zero. The recoveries of 2-fluorobiphenyl [surrogate standard for low-molecular-weight PAHs (L-PAHs) with 2–3 benzene rings (L-PAH) including ANY, ACE, FLE, PHE, ANT,FLU, and RET, and p-terphenyl D14 [surrogate standard for high-molecular-weight PAHs (H-PAHs) with 4–5 benzene rings including PYR, BAA, CHR, BBF, BKF, and BAP] were 65.1 ± 28.2% and 79.3 ± 18.7%, respectively. To evaluate the extraction efficiency of the PAHs, a serum sample was divided into six aliquots and 27 PAHs were spiked with three of them. Recoveries of the 27 PAH standards varied from 67.4% (dBaeF) to 106% (PHE) with mean and standard deviation being 84.9 ± 9.62%. The detailed recovery data are shown in Figure S1 (Supporting Information). The case-control status of the samples was masked to the person involved in chemical analysis.
Analysis of Triglycerides and Cholesterol
Triglycerides and cholesterol contents were measured by the oxidative method (INTEC (Xiamen) Technology Co., LTD, China) following the standard protocol. The optical density was quantified by an automatic biochemical analyzer (Olympus AU400, Japan).
Statistical Analyses
PAH concentration in serum was normalized with lipid content and reported as ng/g lipid. The total lipid content (TL, g/L) of serum was calculated from the concentrations of triglycerides (Tg, g/L) and total cholesterol (Tc, g/L) using the formula TL = 0.92 + 1.31 × (Tg + Tc).13 The mean and standard deviation of the lipid content of the 238 maternal samples was 7.33 ± 1.86 g/L. The low limits of detection (LOD) of all PAHs in serum ranged from 0.05 to 0.10 ng/mL and the PAH concentrations of the 238 serum samples in the final extracts were all within the calibration ranges. Samples with concentrations below the LOD were assigned to be zero.
Concentrations of PAHs in serum were not normally distributed, so the median with interquartile range (IQR) was used to describe their skewed distributions. Differences in concentrations of PAHs between cases and controls were evaluated using the Mann–Whitney U test. Maternal demographic information between the cases and the controls was compared using χ2 test, or Fisher’s exact test if cell expectation was less than five. PAH concentration quartiles of the controls were used as the cutoff values in dose–response analysis. The risk of NTDs associated with PAH concentrations was estimated by odds ratio (OR) with 95% confidence internal (CI).
The study was originally designed as a loosely matched case-control study. However, some blood samples were not available for evaluation because consent could not be obtained from some subjects, or collecting a blood sample from a control mother matched to a terminated case woman was often delayed because we had to wait until there was an appropriate term delivery for use as the control. To maximize the sample size, we had to separate the pairs and used an unconditional logistic model in odds ratio analyses. NTD status was taken as the dependent variable. Serum PAH concentration, dichotomized with the median of PAH in control group as the cutoff, was used as the independent variable. “Exposed” was defined as when the PAH concentration was above the median, and “Unexposed” as when the PAH concentration was below the median. Eleven factors were selected as potential covariates including maternal occupation, age, educational level, body mass index (BMI), previous birth defect history, gravidity, parity, periconceptional folic acid supplementation, active or secondhand smoking, alcohol drinking during the periconceptional period, and fever or flu during early pregnancy. Factors that are associated with both the risk of NTDs and levels of any individual PAHs in maternal serum were adjusted for in the unconditional Logistic model with “backward” method for further screening covariates. In dose–response or trend analyses, PAH concentration quartiles of the controls were used as the cutoff values. Statistical analyses were conducted using SPSS 16.0 (SPSS Inc.). A two-tail p value of <0.05 was taken as the statistical significance level.
Results
Characteristics
Detailed information on the demographic characteristics of this population was previously described11 and a brief summary description can be found in Table 1. The distribution of maternal age, BMI, education, parity, periconceptional folate supplementation, fever or flu status during early pregnancy, and active or passive smoking, was statistically different between the cases and controls. Generally, cases were older [26 (IQR: 22–30) years in cases vs 24 (IQR: 21–28) years in controls] and less educated, and had larger BMI [23.5 (IQR: 20.9–26.6) kg/m2 in cases vs 21.4 kg/m2 (IQR: 19.5–23.4) in controls], and higher parity. A lower proportion of cases (45%) reported periconceptional folate supplementation, compared to controls (58%). In addition, a higher proportion of cases reported exposure to passive tobacco smoke (67% in cases, 26% in controls), and had hyperthermia (fever or influenza) during early pregnancy (54% in cases, 24% in controls). Median concentrations of polycyclic aromatic hydrocarbons (PAHs) in maternal serum by population characteristics are compared in Table S1 in the Supporting Information. Seven of them were found to be associated with any of the individual PAH levels in serum, and selected as the potential covariates for developing logistic regression model including: maternal age, pregnancy BMI, occupation, parity, periconceptional folate supplementation, fever or flu during early pregnancy, and smoking or secondhand smoking.
Table 1. Characteristics of Women Who Had Pregnancies Affected by NTDs (Cases) and Women Who Delivered Healthy Infants (Controls) in Shanxi Province, China, 2010–2013.
| characteristics | cases (na = 117) | controls (n = 121) | pb |
|---|---|---|---|
| maternal age (y) | |||
| <25 | 45 (40)c | 69 (57) | 0.011 |
| 25–29 | 23 (29) | 31 (26) | |
| ≥30 | 35 (31) | 20 (17) | |
| maternal pregnancy BMI (kg/m2) | |||
| <18.5 | 12 (11) | 12 (10) | 0.032 |
| 18.5–24.9 | 60 (54) | 82 (70) | |
| ≥25 | 38 (35) | 23 (20) | |
| maternal education | |||
| primary or lower | 9 (8) | 2 (2) | <0.001 |
| junior high | 85 (73) | 67 (55) | |
| high school or above | 22 (19) | 52 (43) | |
| maternal occupation | |||
| farmer | 93 (80) | 84 (69) | 0.057 |
| nonfarmer | 23 (20) | 37 (31) | |
| previous birth defects history | |||
| yes | 6 (5) | 1 (1) | 0.066 |
| no | 108 (95) | 114 (99) | |
| gravidity | |||
| 1 | 52 (44) | 63 (54) | 0.191 |
| 2 | 65 (56) | 54 (46) | |
| parity | |||
| 1 | 98 (89) | 98 (99) | 0.003 |
| 2 | 12 (11) | 1 (1) | |
| periconceptional folate supplementation | |||
| yes | 52 (45) | 67 (58) | 0.041 |
| no | 64 (55) | 48 (42) | |
| fever or flu during early pregnancy | |||
| yes | 62 (54) | 28 (24) | <0.001 |
| no | 52 (46) | 88 (76) | |
| maternal smoking or secondhand smoking | |||
| yes | 74 (67) | 30 (26) | <0.001 |
| no | 37 (33) | 84 (74) | |
| alcohol drinking | |||
| yes | 16 (14) | 8 (7) | <0.001 |
| no | 101 (86) | 113 (93) | |
| separate kitchen from living room/bedroom | |||
| yes | 98 (84)c | 90 (74) | 0.051 |
| no | 18 (16) | 31 (26) | |
| primary fuel used for cooking | |||
| soft coal | 20 (18) | 19 (17) | 0.644 |
| hard coal | 43 (39) | 43 (38) | |
| firewood | 2 (2) | 0 (0) | |
| liquefied gas | 46 (41) | 51 (45) | |
| conception during the heating season | |||
| yes | 60 (52) | 74 (63) | 0.147 |
| no | 56 (48) | 44 (37) | |
| using a stove for heatingd | |||
| yes | 41 (69) | 36 (47) | 0.027 |
| no | 18 (31) | 40 (53) | |
| stove in the living room/bedroomd | |||
| yes | 28 (67) | 19 (53) | 0.456 |
| no | 14 (33) | 17 (47) | |
| primary fuel used for heatingd | |||
| soft coal | 21 (49) | 17 (46) | 0.994 |
| hard coal | 20 (47) | 18 (48) | |
| biomass | 1 (2) | 1 (3) | |
| liquefied gas | 1 (2) | 1 (3) | |
| frequency of ventilation in the living room/bedroomd | |||
| every day | 30 (75) | 28 (76) | 0.594 |
| 1–6 times per week | 8 (20) | 7 (19) | |
| <1 time per week | 2 (5) | 2 (5) | |
| frequency of combustion exhausted gas in living roomd | |||
| every day | 12 (31) | 6 (17) | 0.533 |
| 1–6 times per week | 4 (11) | 5 (15) | |
| <1 time per week | 22 (58) | 23 (68) | |
Number of subjects.
Pearson’s χ2 test, or Fisher’s exact test if cell expectation was less than 5.
Data are number (percentage). Total number may not be equal to the number of cases or controls due to missing or unknown data.
For women whose pregnancies occurred during the winter heating season.
Overall, there were no significant differences in life styles and energy usage characteristics of this population, with the exception of using a stove for heating (Table 1). In the case group, 69% used a stove for residential heating during winter, compared with 47% in control group. Coal was the primary energy source for heating. Some households, irrespective of case or control status, placed a stove in the living room (or bedroom). Coal and liquefied gas were the major fuel types for cooking, and firewood was less often used in this area. Most of the households kept living rooms ventilated every day during the heating season (75% in cases and 76% in control), and fewer proportions of them had indoor combustion exhausted gas in the living room every day (31% in cases and 17% in controls).
Serum PAH Concentrations and NTD Risks
Among the 27 measured PAHs, 13 had a detection rate of over 65% in all 238 serum samples, including seven L-PAHs (ANY, ACE, FLE, PHE, ANT, FLU, and RET) and six H-PAHs (PYR, BAA, CHR, BBF, BKF, and BAP). The median concentrations of the 13 PAHs among cases were approximately twice as high as the levels found in controls (Table 2). The sum of L-PAHs (ΣL-PAHs), H-PAHs (ΣH-PAHs), and all PAHs (ΣPAHs) in cases were well over twice as high as the concentrations determined in the controls, respectively. Differences in PAH concentrations were observed for all three NTD subtypes (Table 2). In the anencephaly subtype, the concentrations of the 13 individual PAHs, with the exceptions of BBF and BKF, were higher than control values, whereas all of the 13 individual PAHs of the spina bifida subtype group were higher than the controls. There were no differences for all PAHs between the anencephaly and spina bifida subtypes. For the encephalocele subtype, the limited sample size (six subjects) did not permit meaningful comparisons; however, the PAH concentrations in this subtype were similar to those in other NTD subtypes with only a few exceptions. Hence, we chose not to consider encephaloceles separately in the following analyses, including them in the total NTD calculations.
Table 2. Concentrations (ng/g Lipid) of Polycyclic Aromatic Hydrocarbons (PAHs) in Serum of Women Who Had Pregnancies Affected by Neural Tube Defects (NTDs), and Women Who Delivered Healthy Infants (controls) in Shanxi Province, China, 2010–2013.
| PAHsa |
total
NTDs (nb = 117) |
anencephaly (n = 44) |
spina
bifida (n = 67) |
encephalocele (n = 6) |
controls (n = 121) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| %c | median (IQR) | % | median (IQR) | % | median (IQR) | % | median (IQR) | % | median (IQR) | ||
| L-PAHs | ANY | 86 | 236 (43.6–479)** | 77 | 236 (17.5–482)* | 82 | 243 (48.4–466)** | 100 | 191 (138–318) | 78 | 93.8 (11.5–255) |
| ACE | 95 | 303 (162–606)*** | 86 | 312 (161–619)*** | 91 | 302 (162–571)*** | 100 | 355 (231–546)* | 84 | 99.1 (31.6–241) | |
| FLE | 97 | 814 (339–1922)*** | 91 | 878 (384–1879)** | 93 | 814 (339–1998)*** | 100 | 950 (419–1301) | 83 | 412 (110–969) | |
| PHE | 100 | 1820 (574–3454)*** | 95 | 1842 (564–3204)** | 96 | 1685 (532–3423)*** | 100 | 1843 (1274–3190)* | 91 | 796 (272–1420) | |
| ANT | 100 | 142 (93.2–314) *** | 95 | 143.9 (92.1–300)*** | 96 | 141 (88.9–312)*** | 100 | 191 (125–344)* | 90 | 54.4 (27.0–115) | |
| FLU | 95 | 345 (101–609)*** | 86 | 338.0 (79.4–512)** | 91 | 345 (138–656)*** | 100 | 422 (184–621)* | 74 | 134 (0–308) | |
| RET | 89 | 566 (154–1540)*** | 77 | 575.1 (77.8–2620)* | 90 | 565 (244–1434)*** | 83 | 259 (61.8–609) | 89 | 272 (93.0–503) | |
| H-PAHs | PYR | 99 | 441 (180–836)*** | 93 | 428.1 (171–815)** | 94 | 443 (179–1042)*** | 100 | 278 (225–523) | 93 | 186 (79.9–413) |
| BAA | 92 | 137 (61.0–272) *** | 84 | 135.7 (51.0–264)** | 90 | 139 (68.4–275)*** | 83 | 121 (51.8–289) | 83 | 56.9 (14.6–112) | |
| CHR | 91 | 188 (53.5–486)*** | 82 | 164.5 (42.4–674)** | 90 | 208 (82.4–401)*** | 83 | 120 (55.2–836) | 81 | 65.3 (10.8–148) | |
| BBF | 76 | 103 (0–216)*** | 61 | 84.77 (0–148) | 78 | 112 (42.9–232)*** | 83 | 107 (36.0–243) | 65 | 46.5 (0–98.6) | |
| BKF | 75 | 32.2 (0–114)** | 61 | 30.43 (0–106) | 76 | 40.8 (5.34–114)*** | 83 | 36.5 (6.00–146) | 64 | 11.3 (0–37.2) | |
| BAP | 89 | 54.7 (19.7–181)** | 82 | 56.61 (23.6–215)* | 88 | 65.7 (20.2–170)** | 67 | 12.3 (1.92–19.6) | 79 | 21.4 (3.06–96.3) | |
| ΣL-PAHs | 100 | 4712 (1930–9618)*** | 95 | 4743 (1877–9714)*** | 96 | 4789 (1740–9841)*** | 100 | 3686 (3180–6035)* | 98 | 2068 (1007–3818) | |
| ΣH-PAHs | 100 | 1164 (683–2142)*** | 95 | 1081 (662.6–1840)*** | 96 | 1240 (691.1–2185)*** | 100 | 1362 (674.1–1921) | 98 | 557 (300–944) | |
| ΣPAHs | 100 | 5839 (2661–13161)*** | 95 | 5752 (2762–14070)*** | 96 | 6302 (2523–12044)*** | 100 | 5466 (3813–7423)* | 98 | 2668 (1406–5065) | |
IQR, interquartile range; *p < 0.05, **p < 0.01, and ***p < 0.001, in comparison with the median of controls by Mann-Whiney U test.
The abbreviations of individual PAHs are acenaphthylene (ACY), acenaphthene (ACE), fluorene (FLE), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLU), retene (RET), pyrene (PYR), benz[a]anthracene (BAA), chrysene (CHR), benzo[b]fluoranthene (BBF), benzo[k]fluoranthene (BKF), benzo[a]pyrene (BAP). ΣL-PAHs is the sum of ANY, ACE, FLE, PHE, ANT, FLU and RET; ΣH-PAHs of PYR, BAA, CHR, BBF, BKF and BAP; ΣPAHs of all PAHs.
Number of subjects.
Detection rate above limit of detection.
Overall, all individual PAHs above the median concentration of the controls were associated with elevated risks of NTDs (Table 3). ΣH-PAHs conferred higher NTD risks than ΣL-PAHs, which was confirmed by taking ΣH-PAHs and ΣL-PAHs together in a binary logistic regression model [e.g., 4.14 (95%CI: 2.04–8.42) of ΣH-PAHs vs 1.22 (95%CI: 0.62–2.40) of ΣL-PAHs for total NTDs in Table S2 of the Supporting Information], whereas the highest OR for any individual PAHs was observed for ANT, a L-PAH, in either the unadjusted or adjusted model. The overall direction and magnitude of the ORs of all the individual PAHs for each subtype were similar. For the anencephaly subtype (Table 3), only three (ACE, ANT, and BAA) of the 13 PAHs were associated with an elevated risk in both unadjusted and adjusted models, with ΣH-PAHs having stronger associations than ΣL-PAHs. For the spina bifida subtype (Table 3), all PAHs except ANY, were associated with elevated risks for NTDs in the unadjusted model, whereas five of the H-PAHs and two L-PAHs were associated with elevated NTD risks in the adjusted model. Again, ΣH-PAHs had more significant OR values than ΣL-PAHs for the risk of the spina bifida subtype.
Table 3. Risks of Neural Tube Defects (NTDs) in Association with Levels of Polycyclic Aromatic Hydrocarbons (PAHs) above the Median Concentration in Maternal Serum of Controls in Shanxi Province, China, 2010–2013.
| PAHsa |
total
NTDs |
anencephaly |
spina
bifida |
|||||
|---|---|---|---|---|---|---|---|---|
| median (IQR)b | crude ORc (95% CI) | adjusted ORc,d (95% CI) | crude ORc (95% CI) | adjusted ORc,d (95% CI) | crude ORc (95% CI) | adjusted ORc,d (95% CI) | ||
| L-PAHs | ANY | 140 (19.8–368) | 1.82 (1.08–3.07)* | 1.98 (3.86–1.02)* | 1.17 (0.58–2.35) | 0.74 (1.93–5.01) | 1.60 (0.86–2.95) | 2.16 (0.95–4.90) |
| ACE | 194 (58.5–416) | 4.25 (2.37–7.62)*** | 4.50 (9.51–2.13)*** | 2.37 (1.12–5.04)* | 1.44 (4.38–13.28)** | 3.70 (1.83–7.47)*** | 3.84 (1.57–9.40)** | |
| FLE | 588 (204–1333) | 2.50 (1.46–4.29)** | 2.84 (5.62–1.44)** | 2.12 (1.01–4.45)* | 1.13 (3.14–8.74)* | 1.95 (1.04–3.65)* | 2.15 (0.97–4.79) | |
| PHE | 1051 (404–2396) | 2.40 (1.41–4.10)** | 2.99 (5.91–1.51)** | 1.37 (0.68–2.77) | 0.86 (2.25–5.87) | 1.89 (1.01–3.54)* | 2.01 (0.91–4.45) | |
| ANT | 95.4 (45.1–194) | 7.87 (4.00–15.5)*** | 8.53 (19.29–3.77)*** | 4.87 (2.01–11.8)*** | 1.96 (6.45–21.21)** | 7.89 (3.34–18.7)*** | 7.91 (2.83–22.11)*** | |
| FLU | 199 (42.8–462) | 2.70 (1.57–4.64)*** | 3.36 (6.71–1.68)*** | 1.67 (0.82–3.39) | 1.25 (3.48–9.64)* | 2.59 (1.36–4.95)** | 2.25 (1.00–5.05) | |
| RET | 378 (109–787) | 2.20 (1.30–3.73)** | 2.30 (4.53–1.16)* | 1.29 (0.64–2.59) | 0.57 (1.44–3.66) | 2.09 (1.11–3.94)* | 1.78 (0.79–4.04) | |
| H-PAHs | PYR | 289 (120–658) | 2.40 (1.41–4.10)** | 2.31 (4.60–1.16)* | 1.78 (0.87–3.65) | 1.09 (3.10–8.88)* | 2.16 (1.15–4.08)* | 2.11 (0.93–4.78) |
| BAA | 92.4 (30.1–190) | 3.23 (1.86–5.63)*** | 3.17 (6.46–1.56)** | 2.42 (1.16–5.08)* | 1.28 (3.60–10.11)* | 4.22 (2.09–8.53)*** | 4.41 (1.76–11.05)** | |
| CHR | 106 (24.3–285) | 2.82 (1.64–4.86)*** | 3.08 (6.26–1.51)** | 1.72 (0.84–3.53) | 1.07 (2.93–8.01)* | 2.62 (1.36–5.05)** | 2.84 (1.19–6.77)* | |
| BBF | 71.2 (0–143) | 2.11 (1.25–3.58)** | 2.40 (4.75–1.21)* | 1.72 (0.85–3.50) | 1.23 (3.44–9.62)* | 2.31 (1.23–4.35)** | 2.22 (0.98–5.00) | |
| BKF | 19.8 (0–69.0) | 1.97 (1.16–3.32)* | 2.56 (5.11–1.28)** | 1.67 (0.82–3.39) | 1.20 (3.36–9.39)* | 2.08 (1.11–3.90)* | 2.65 (1.15–6.09)* | |
| BAP | 36.4 (7.90–147) | 2.48 (1.45–4.24)*** | 2.46 (4.93–1.22)* | 3.15 (1.46–6.81)** | 0.68 (1.79–4.70) | 3.09 (1.60–5.95)*** | 2.83 (1.18–6.76)* | |
| ΣL-PAHs | 2851 (1307–6683) | 2.61 (1.52–4.49)*** | 2.91 (5.83–1.45)*** | 1.72 (0.84–3.53) | 0.95 (2.59–7.07) | 2.09 (1.11–3.94)* | 2.00 (0.89–4.49) | |
| ΣH-PAHs | 805 (420–1514) | 4.65 (2.57–8.40)*** | 5.89 (12.75–2.72)** | 2.76 (1.28–5.96)** | 1.48 (4.42–13.19)** | 4.69 (2.24–9.81)*** | 4.31 (1.71–10.88)** | |
| ΣPAHs | 3656 (1805–8294) | 2.95 (1.71–5.09)*** | 3.24 (6.80–1.55)** | 2.20 (1.05–4.60)* | 1.31 (3.76–10.77)* | 2.16 (1.15–4.08)* | 2.29 (1.01–5.17)* | |
OR, odds ratio; IQR, interquartile range; * p < 0.05, ** p < 0.01, and *** p < 0.001.
The abbreviations of individual PAHs are acenaphthylene (ACY), acenaphthene (ACE), fluorene (FLE), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLU), retene (RET), pyrene (PYR), benz[a]anthracene (BAA), chrysene (CHR), benzo[b]fluoranthene (BBF), benzo[k]fluoranthene (BKF), benzo[a]pyrene (BAP). ΣL-PAHs is the sum of ANY, ACE, FLE, PHE, ANT, FLU and RET; ΣH-PAHs of PYR, BAA, CHR, BBF, BKF and BAP; ΣPAHs of ΣL-PAHs and ΣH-PAHs.
Statistical results of all subjects.
Calculated by using binary logistic regression.
Adjust for maternal general characteristics and exposure, including BMI, parity, fever of flu during early pregnancy, and active or passive smoking and drinking.
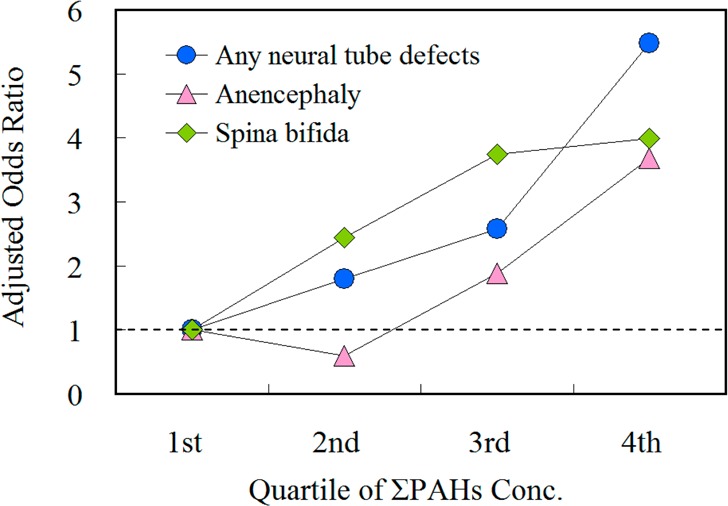
The association between higher concentrations of ΣL-PAHs, ΣH-PAHs, and ΣPAHs with the risk of NTDs indicated a clear dose–response relationship (Figure 1). When the lowest quartile was used as the referent group, increases in the risk of total NTDs of 1.66 (95% CI: 0.44–6.23), 3.96 (95% CI: 0.99–15.8), and 6.39 (95% CI: 2.07–19.8) were observed for women whose serum concentrations of ΣPAHs were in the second, third, and fourth quartiles, respectively. The detailed data about the risks by quartile of controls for total NTDs and its two subtypes can be found in Table S3 (Supporting Information).
Figure 1.
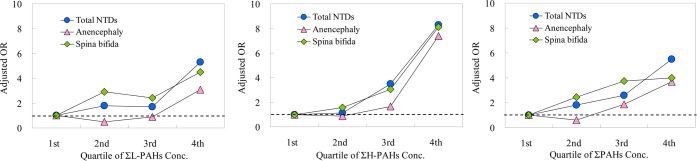
Quartiles of polycyclic aromatic hydrocarbons in maternal serum and risk of total neural tube defects (NTDs) in a population in Shanxi Province, China, 2010–2013. ΣL-PAHs (left panel) is the sum of acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, and retene; ΣH-PAHs (middle panel) of pyrene, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, and benzo[a]pyrene; ΣPAHs (right panel) of ΣL-PAHs and ΣH-PAHs.
Lifestyles and Energy Usage Characteristics and PAH Concentrations
Serum PAH concentrations in subgroups of different population life styles and energy usage characteristics are shown in Table S4 (Supporting Information). Overall, no significant differences were found between subgroups of primary fuel type for heating and cooking, isolation between living room (or bedroom), kitchen and stove location, heating by stove, ventilation in the living room, and seasonal time of conception.
Discussion
In this study, we examined the relationships between PAH concentrations in maternal serum and NTD risk in their offspring, as well as the association of maternal life style variables and energy usage characteristic with PAH concentrations in a population in Northern China. Overall, both individual and the total PAHs in maternal serum were associated with elevated risks for NTDs. No association between PAH concentrations in maternal serum and life styles and energy usage characteristics were found to be significant. Two previous epidemiological studies suggested that maternal exposure to PAHs or indoor coal combustion was associated with an elevated risk of NTDs in their offspring. One study conducted in the United States found that maternal occupational exposure to PAHs may be associated with increased risk of spina bifida in offspring of women who are either normal weight or underweight.4 Another study conducted in the same population as the present study found that indoor combustion, including cooking and heating during the periconceptional period, is associated with an elevated risk of NTDs.10 However, the exposure estimate based on questionnaire or job title may be subject to recall bias or inaccuracy. In addition, questionnaire or job title-based estimation is unable to include other sources of exposure, such as that which occurs via food preparation and consumption. The present study utilized actual PAHs determinations in maternal blood as markers of maternal internal PAH exposure from all sources, providing a much-needed quantification of exposure lacking in other earlier studies.
We found that PAHs are significantly associated with elevated NTD risks in unadjusted or adjusted models, with the exception of RET in the adjusted model. RET is usually used as a marker for softwood combustion,14 and less than 2% of the households of this population used firewood for either cooking or heating. Overall, our findings were consistent with the results of our previous pilot study in a smaller population in Shanxi Province, China.5 We also compared the PAH concentrations in placenta and found that the concentrations of the detected eight PAHs in NTD cases were significantly higher than in the controls from a previous study.6 These studies demonstrated that PAHs could be used as internal biomarker of exposure. Risks of spina bifida associated with maternal serum PAHs were overall higher than those of anencephaly in each quartile of PAH concentrations. This difference was also observed in our previous placenta study.6 Similarly, only an association between elevated risk of spina bifida in offspring and maternal occupational exposure to PAHs was observed a large population-based case-control study in the United States, while no association was observed for the anencephaly and encephalocele.4
PAHs are strong oxidative stressors to human health.15−18 Oxidative stress induced damage has been implicated as an underlying mechanism of NTD development related to maternal diabetes and high glucose, and a number of drugs that prenatal exposure leads to NTDs.19−22 Animal studies showed that benzo(a)pyrene-7,8-dihydrodiol-9,10-epoxide, a highly reactive intermediate of BAP, can induce exencephaly in mice.8 In this study, the ΣH-PAHs were shown to have higher OR values than ΣL-PAHs. It may be because five of the six detected H-PAHs are carcinogenic, and their higher potency may result in more oxidative stress damage than that observed in the L-PAHs,15 which in turn, produces a higher risk for NTDs in the exposed offspring.19 However, this remains only a hypothesis that needs to be mechanistically tested in future studies.
Coal was the major energy source for heating and cooking, whereas biomass was rarely used in this population. Coal combustion has been reported to be the major source of PAHs.23 A higher proportion of heating by using a stove was observed in NTD cases compared to controls. Our analysis revealed that coal combustion via stove use may be an important contributor to indoor PAH exposure in households of NTD cases, especially during the heating seasons. This is consistent with our previous study.10 However, we found no statistical differences in maternal serum PAH concentrations between women whose households were heated with a stove, and those households that do not use a stove. No association was observed between maternal PAH concentrations and life style or energy usage characteristics including primary fuel used for cooking or heating, separating kitchen from living room/bedroom, conception during the heating season, and ventilation frequency of the living room. Indoor burning of solid fuel increases indoor air PAH concentrations and population external inhalation exposure risk24,25 and internal PAH exposure markers should show an increase. This discrepancy may be caused by the contribution of PAHs from other pathways, like outdoor inhalation exposure and food ingestion. It has been reported that the main exposure to PAHs for general population is food in various countries, which is more than 90%.26,27 In future studies, information on daily activities and dietary characters of individual subjects, the corresponding PAH pollution in outdoor air, and food should also be determined.
This study has many strengths, including the largest number of subjects of its kind in investigating maternal PAH exposure and fetal NTD risk with the use of internal exposure biomarkers. The concentrations of 27 PAHs with 2–6 fused benzene rings were comprehensively checked in the maternal serum. Nonetheless, there are four limitations of this study that should be noted. First, blood samples were collected at delivery or termination of NTD-affected pregnancies from mothers and the measured PAH concentrations may not be fully representative for those in the first month postconception, which is the critical period of neural tube development.1 Further studies are needed to address the relevance of serum PAH concentrations at delivery to those during the periconceptional period. Second, covariates included in the analyses rely on self-reported information through questionnaire interview, which are subject to recall error. Prior to the onset of the study, we trained local health workers at hospitals and clinics to standardize the interview procedures. In addition, we structured the questionnaire such that it contained questions that are straightforward and unambiguous. We performed an independent survey on the accuracy and reproducibility of the case-control data for the major variables, and found the coincidence rate was over 90%. This could control the recall error to some extent. Third, the older mothers may have higher concentrations of PAHs in their serum resulting from bioaccumulation. Thus, residual confounding is possible. These limitations suggest the need for investigating the interaction between trace pollutants and risk of NTDs.
Conclusion
Our case-control study revealed that the maternal exposure to PAHs is associated with an increased risk of NTDs, and H-PAHs may render a higher risk than L-PAHs. This study adds to the existing evidence of PAHs’ adverse effect on human health, and highlights the need to reduce PAHs emission and maternal PAH exposure from before pregnancy to protect fetuses who are vulnerable to environmental pollutants. Further studies aiming to replicate the findings in other populations and to elucidate the underlying mechanism between PAH exposure and NTD development are needed.
Acknowledgments
This work is supported by grants from the National Natural Science Foundation of China (No. 31371523 and 41390240) and National Institutes of Health (ES021006). The funding agencies have no role in study design, implementation, data analysis, and interpretation.
Supporting Information Available
Solvents and reagents, extraction and cleanup for PAH analysis, median concentrations (ng/g lipid) of polycyclic aromatic hydrocarbons (PAHs) in maternal serum by population characteristics, risks of neural tube defects (NTDs) in association with levels of polycyclic aromatic hydrocarbons (PAHs) of high-molecular weight (H-PAHs) and low-molecular weight (L-PAHs) above the median concentration in maternal serum of controls, risks of fetal total neural tube defects (NTDs) in four quartile ranges of controls, median concentrations (ng/g lipid) of polycyclic aromatic hydrocarbons (PAHs) in the maternal serum in subgroups of life styles and energy usage characteristics in Shanxi Province, China, 2010–2013, and method recoveries of the twenty-seven spiked PAHs with serum. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
⊥ These authors contributed equally to this work.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Wallingford J. B.; Niswander L. A.; Shaw G. M.; Finnell R. H. The continuing challenge of understanding, preventing, and treating neural tube defects. Science 2013, 33961231222002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp A. J.; Stanier P.; Greene N. D. Neural tube defects: Recent advances, unsolved questions, and controversies. Lancet Neurol. 2013, 128799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March of Dimes Foundation. Global report on birth Defects: The hidden toll of dying and disabled children; March of Dimes Birth Defects Foundation: New York, 2006. [Google Scholar]
- Langlois P. H.; Hoyt A. T.; Lupo P. J.; Lawson C. C.; Waters M. A.; Desrosiers T. A.; Shaw G. M.; Romitti P. A.; Lammer E. J. National Birth Defects Prevention Study. Maternal occupational exposure to polycyclic aromatic hydrocarbons and risk of neural tube defect-affected pregnancies. Birth Defects Res., Part A 2012, 949693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naufal Z.; Zhiwen L.; Zhu L.; Zhou G. D.; McDonald T.; He L. Y.; Mitchell L.; Ren A.; Zhu H.; Finnell R.; Donnelly K. C. Biomarkers of exposure to combustion by-products in a human population in Shanxi, China. J. Exposure Sci. Environ. Epidemiol. 2010, 204310–319. [DOI] [PubMed] [Google Scholar]
- Ren A. G.; Qiu X. H.; Jin L.; Ma J.; Li Z. W.; Zhang L.; Zhu H. P.; Finnell R. H.; Zhu T. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proc. Natl. Acad. Sci. U. S. A. 2011, 1083112770–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashin Y. V.; Bakhitova L. M. Mutagenic and carcinogenic properties of polycyclic aromatic hydrocarbons. Environ. Health Perspect. 1979, 30, 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri O.; Ognio E.; Rossi O.; Astigiano S.; Rossi L. Embryotoxicity of benzo(a)pyrene and some of its synthetic derivatives in Swiss mice. Cancer Res. 1986, 46194–98. [PubMed] [Google Scholar]
- Zhang Y. X.; Tao S.; Cao J.; Coveney R. M. Emission of polycyclic aromatic hydrocarbons in china by county. Environ. Sci. Technol. 2007, 41, 683–687. [DOI] [PubMed] [Google Scholar]
- Li Z.; Zhang L.; Ye R.; Pei L.; Liu J.; Zheng X.; Ren A. Indoor air pollution from coal combustion and the risk of neural tube defects in a rural population in Shanxi Province, China. Am. J. Epidemiol. 2011, 1744451–458. [DOI] [PubMed] [Google Scholar]
- Wang B.; Yi D. Q.; Jin L.; Li Z. W.; Liu J. F.; Zhang Y. L.; Qiu X. H.; Liu W. X.; Tao S.; Ren A. G. Organochlorine pesticide levels in maternal serum and risk of neural tube defects in offspring in Shanxi Province, China: A Case-Control Study. Sci. Total Environ. 2014, 490, 1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.; Wang X.; Wang B.; Tao S.; Liu W.; Cao J.; Li B.; Lu X.; Wong M. H. Polycyclic aromatic hydrocarbon residues in human milk, placenta, and umbilical cord blood in Beijing, China. Environ. Sci. Technol. 2011, 452310235–10242. [DOI] [PubMed] [Google Scholar]
- Rylander L.; Nilsson-Ehle P.; Hagmar L. A simplified precise method for adjusting serum levels of persistent organohalogen pollutants to total serum lipids. Chemosphere 2006, 623333–336. [DOI] [PubMed] [Google Scholar]
- Shen G. F.; Tao S.; Wei S. Y.; Zhang Y. Y.; Wang R.; Wang B.; Li W.; Shen H. Z.; Huang Y.; Yang Y. F.; Wang W.; Wang X. L.; Simonich S. L. M. Retene emission from residential solid fuels in China and evaluation of retene as a unique marker for soft wood combustion. Environ. Sci. Technol. 2012, 4684666–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V. K.; Patel D. K.; Jyoti; Ram S.; Mathur N.; Siddiqui M. K. J. Blood levels of polycyclic aromatic hydrocarbons in children and their association with oxidative stress indices: An Indian perspective. Clin. Biochem. 2008, 413152–161. [DOI] [PubMed] [Google Scholar]
- Mori T.; Yoshinaga J.; Suzuki K.; Mizoi M.; Adachi S.; Tao H.; Nakazato T.; Li Y. S.; Kawai K.; Kasai H. Exposure to polycyclic aromatic hydrocarbons, arsenic and environmental tobacco smoke, nutrient intake, and oxidative stress in Japanese preschool children. Sci. Total Environ. 2011, 409152881–2887. [DOI] [PubMed] [Google Scholar]
- Yoon H. S.; Lee K. M.; Lee K. H.; Kim S.; Choi K.; Kang D. Polycyclic aromatic hydrocarbon (1-OHPG and 2-naphthol) and oxidative stress (malondialdehyde) biomarkers in urine among Korean adults and children. Int. J. Hydrogen Environ. Health 2012, 2154458–464. [DOI] [PubMed] [Google Scholar]
- Deng Q. F.; Dai X. Y.; Guo H.; Huang S. L.; Kuang D.; Feng J.; Wang T.; Zhang W. Z.; Huang K.; Hu D.; Deng H. X.; Zhang X. M.; Wu T. C. Polycyclic aromatic hydrocarbons-associated microRNAs and their interactions with the environment: Influences on oxidative DNA damage and lipid peroxidation in coke oven workers. Environ. Sci. Technol. 2014, 4874120–4128. [DOI] [PubMed] [Google Scholar]
- Chang T. I.; Horal M.; Jain S. K.; Wang F.; Patel R.; Loeken M. R. Oxidant regulation of gene expression and neural tube development: Insights gained from diabetic pregnancy on molecular causes of neural tube defects. Diabetologia 2003, 464538–545. [DOI] [PubMed] [Google Scholar]
- James S. J.; Cutler P.; Melnyk S.; Jernigan S.; Janak L.; Gaylor D. W.; Neubrander J. A. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr. 2004, 8061611–1617. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Mosley B. S.; Cleves M. A.; Melnyk S.; James S. J.; Hobbs C. A. Neural tube defects and maternal biomarkers of folate, homocysteine, and glutathione metabolism. Birth Defects Res., Part A 2006, 764230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I.; Gibert M. J.; Pintos C.; Noguera A.; Besalduch A.; Obrador A. Oxidative stress in mothers who have conceived fetus with neural tube defects: The role of aminothiols and selenium. Clin. Nutr. 2004, 234507–514. [DOI] [PubMed] [Google Scholar]
- Zhang Y. X.; Tao S.; Cao J.; Coveney R. M. Emission of polycyclic aromatic hydrocarbons in China by county. Environ. Sci. Technol. 2007, 413683–687. [DOI] [PubMed] [Google Scholar]
- Ding J. N.; Zhong J. J.; Yang Y. F.; Li B. G.; Shen G. F.; Su Y. H.; Wang C.; Li W.; Shen H. Z.; Wang B.; Wang R.; Huang Y.; Zhang Y. Y.; Cao H. Y.; Zhu Y.; Simonich S. L. M.; Tao S. Occurrence and exposure to polycyclic aromatic hydrocarbons and their derivatives in a rural Chinese home through biomass fuelled cooking. Environ. Pollut. 2012, 169, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G. F.; Tao S.; Wang W.; Yang Y. F.; Ding J. N.; Xue M. A.; Min Y. J.; Zhu C.; Shen H. Z.; Li W.; Wang B.; Wang R.; Wang W. T.; Wang X. L.; Russell A. G. Emission of oxygenated polycyclic aromatic hydrocarbons from indoor solid fuel combustion. Environ. Sci. Technol. 2011, 4583459–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alomirah H.; Al-Zenki S.; Al-Hooti S.; Zaghloul S.; Sawaya W.; Ahmed N.; Kannan K. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control 2011, 22122028–2035. [Google Scholar]
- Suzuki K.; Yoshinaga J. Inhalation and dietary exposure to polycyclic aromatic hydrocarbons and urinary 1-hydroxypyrene in non-smoking university students. Int. Arch. Occup. Environ. Health 2007, 811115–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


