Summary
Shunting vascular malformations of the brain and spinal cord are traditionally studied using digital subtraction angiography (DSA), the current gold standard imaging method routinely used because of its favourable combination in terms of spatial and temporal resolution. Because DSA is relatively expensive, time-consuming and carries a risk of silent embolic events and a small risk of transient or permanent neurologic deterioration, a non-invasive alternative angiographic method is of interest. New 320 row-detector CT scanners allow volumetric imaging of the whole brain with temporal resolution up to ≌ 3 Hz. Those characteristics make computed tomography angiography (CTA) an affordable imaging method to study the haemodynamics of the whole brain and can also be applied to the study of limited portions of the spinal cord. The aim of this paper is to make a brief summary of our experience in studying shunting vascular malformation of the brain and spinal cord using dynamic 4D-CTA, explaining the technical details of the studies performed at our institution, and the state-of-the-art major advantages and drawbacks of this new technique. We found that dynamic 4D-CTA is able to depict the main architectural characteristics of previously untreated vascular shunting malformations both in brain and spinal cord (i.e. their main arterial feeders and draining veins) allowing their correct diagnosis and exhaustive classification, limiting the use of DSA for therapeutic purposes.
Keywords: arteriovenous malformations, angiography, digital subtraction, central nervous system vascular malformations, radiation dose
Introduction
Shunting vascular malformations of the brain and spine are traditionally studied using digital subtraction angiography (DSA), the current gold standard imaging method routinely used in these pathologies because of its favourable combination in terms of spatial and temporal resolution.
Because DSA is relatively expensive, time-consuming and carries a risk of silent embolic events 1 and a small risk of transient or permanent neurologic deterioration 2-4, a non-invasive alternative angiographic method is of interest.
New 320 row-detector CT scanners allow volumetric imaging of the whole brain with temporal resolution up to ≌ 3 Hz. Those characteristics make computed tomography angiography (CTA) an affordable imaging method to study the haemodynamics of the whole brain and can also be applied to the study of limited portions of the spinal cord.
The aim of this paper is to make a brief summary of our experience in studying shunting vascular malformation of the brain and spine using dynamic 4D-CTA, explaining the technical details of the studies performed at our institution, and the state-of-the-art major advantages and drawbacks of this new technique.
Materials and Methods
Approval for this study was obtained from our institutional research ethics board in November 2012. Written informed consent was obtained from all patients.
In the period between December 2012 and December 2013 we performed a dynamic CTA study and a DSA study in 20 patients (18 men and 2 women - age range: 18-65, mean age: 39.3 years) with suspected arteriovenous malformations (AVM) of brain or spine. The mean delay between the two examinations was 2±1 days, with a maximum of four days. All patients underwent dynamic CTA for suspected and previously untreated AVM or dural arteriovenous fistulae (dAVF); none of the patients had already had a DSA study.
The hypothesis of such a type of vascular malformation was based on a previous MR without contrast. The suspicion of spinal cord vascular malformation was formulated after neurological examinations and MR study of the whole spine, showing tortuous hypertrophic veins in the posterior aspect of the spinal cord, associated with spinal cord swelling and worsening weakness to the inferior limbs without a recent history of major trauma.
This allowed us to perform a targeted dynamic CTA at the maximum available FOV (160 mm) in the area of the presumed arteriovenous shunt.
Exclusion criteria were known allergy for iodinated contrast agents, renal failure (indicated by a baseline serum creatinine level of >133 μmol/L) and lack of informed consent.
The 4D-CTA results were scored and classified according to the Spetzler-Martin classification (AVM) and Cognard classification (AVF) by two expert neuroradiologists (MG and AS) and one second year radiology resident (FD). Then, within a maximum of 72 hours from the 4D-CTA, DSA was performed in all patients, and the DSA results were analysed by the same groups.
4D-CTA (Brain)
All dynamic CTA scans were performed using an Aquilion ONE multidetector CT scanner (Toshiba Medical System), with 320×0.5 mm detector rows covering 160 mm of volume per each rotation. The examination was preceded by a bolus test acquisition performed just below the skull base to optimize the timing of dynamic CTA acquisition phase. Subsequently, intravenous infusion of 50mL of non-ionic iodinated contrast medium (Iomeron 400, Bracco) followed by 20 mL of saline at 5mL/s injection rate was performed during dynamic CTA acquisition sequences realized with a gantry rotation time of 0.35 s (≌ 3 Hz).
The dynamic acquisition sequence consisted of continuous "cine" CT acquisition with the following parameters: range: 160 mm, 80 KV, 120 mA, rot. time: 0.35 s, total scan duration: 15 seconds.
The raw data were reconstructed to obtain volume renderings or maximum intensity projections at the maximum available temporal resolution, generating time-resolved whole brain volumes using any part of the brain volume at any viewing angle, with temporal resolution of about three volumes per second.
4D-CTA (Spinal Cord)
The examination was preceded by a bolus test acquisition performed at the aortic level thought to correspond to the radiculo-medullary arteries feeding the shunt, to optimize the timing of the dynamic CTA acquisition phase. Subsequently, intravenous infusion of 90 mL of non-ionic iodinated contrast medium (Iomeron 400, Bracco) followed by 40 mL of saline at 6mL/s injection rate was performed during dynamic CTA acquisition sequences realized with a gantry rotation time of 0.35 s (≌ 3 Hz).
DSA (Brain)
DSA examinations were performed using standard biplane fluoroscopy equipment (Axiom Artis Zee, Siemens). All patients underwent bilateral injections of the ICA, external carotid artery and vertebral artery with additional selective injections of the occipital, ascending pharyngeal or maxillary arteries, if indicated.
DSA (Spinal Cord)
Patients with demonstrated previously untreated dAVF of the spinal cord underwent whole spine diagnostic DSA, with bilateral injections of the radiculo-medullary arteries to document any unknown arterial feeders that could not be investigated by the previously performed 4D-CTA.
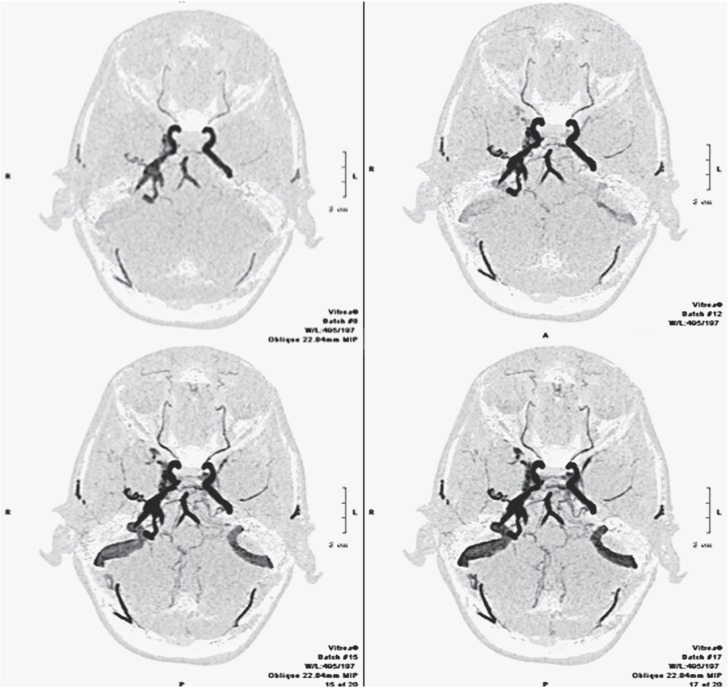
Figure 1.
Intracranial fistula. Dynamic CTA. Inverted MIP projection, axial plane. The images show different phases of progressive enhancement of the intracranial vessels, demonstrating an arteriovenous fistula between the middle meningeal artery on the right side and the ipsilateral petrous sinus.
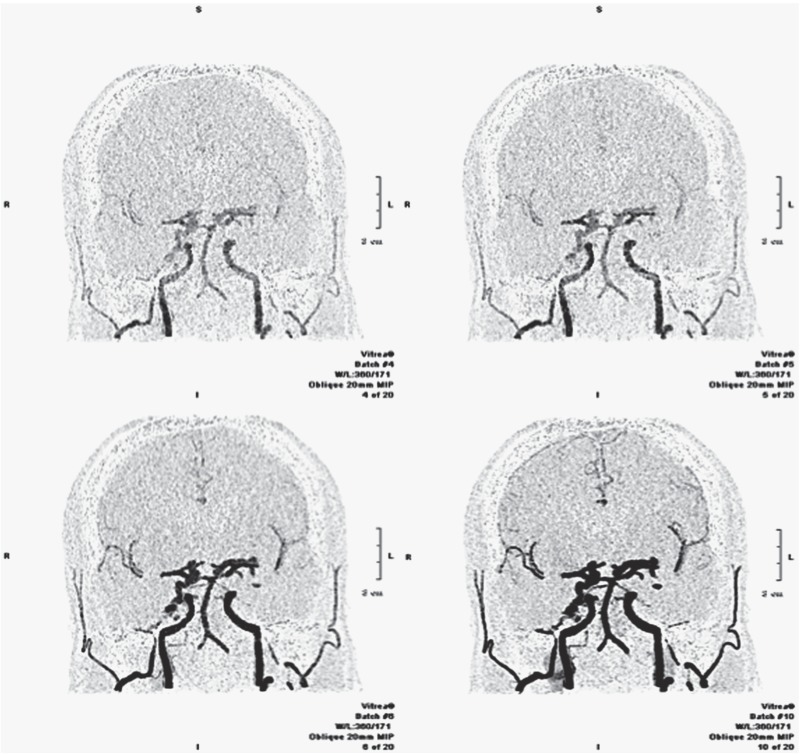
Figure 2.
Intracranial fistula. Dynamic CTA. Inverted MIP projection, coronal plane. The images show different phases of progressive enhancement of the intracranial vessels, demonstrating an arteriovenous fistula between the middle meningeal artery on the right side and the ipsilateral petrous sinus. Note the hypertrophy of the right middle meningeal artery in comparison with the contralateral vessel.
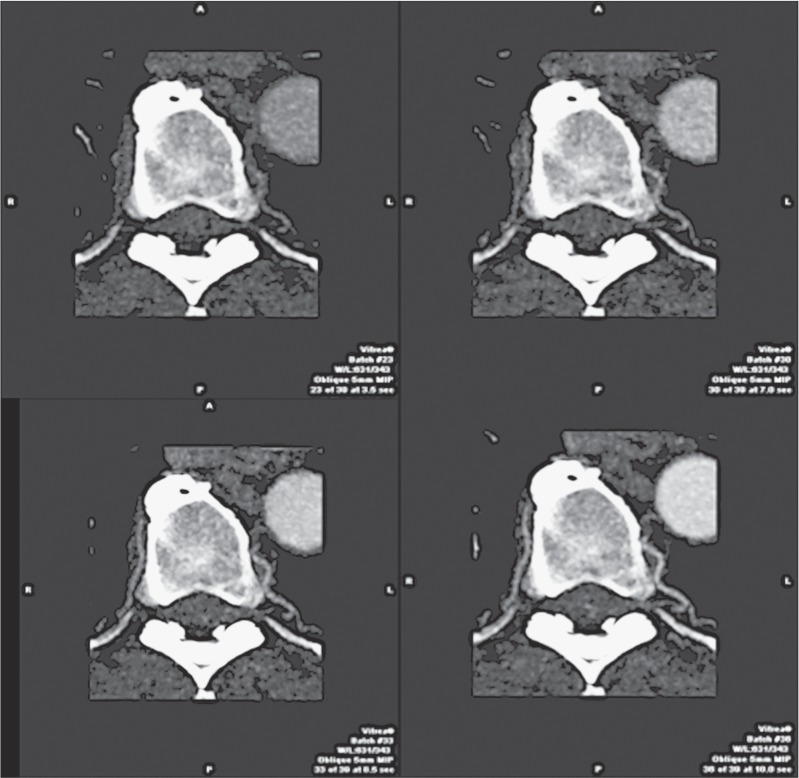
Figure 3.
Spinal cord fistula. Dynamic CTA. MIP projection, axial plane. The images show different phases of progressive enhancement of the left radiculo-medullary artery of D8-D9 level, establishing a dAVF. Note the hypertrophy of the artery and the presence of visible enhancement in the posterior aspect of the vertebral canal.
Figure 4.
Spinal cord fistula. Dynamic CTA. MIP projection, sagittal plane. Progressive appearance of tortuous vessels in the posterior aspect of the thoracic tract of the spinal cord due to the presence of a dAVF at D8-D9 level.
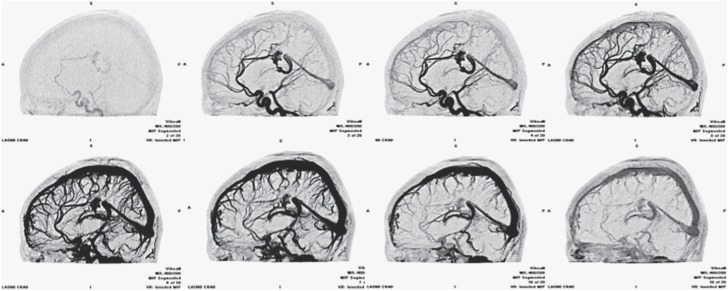
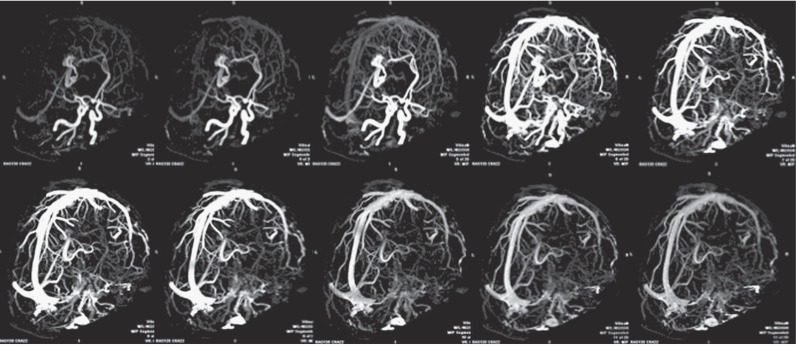
Figure 5.
AVM. Dynamic CTA. Inverted MIP projection, sagittal plane. Images showing a paracallosal AVM from early arterial to late venous phase. Its main arterial feeders (i.e. pericallosal arteries) and draining veins are easily recognizable (vein of Galen, straight sinus) as well as the nidus extension.
Figure 6.
AVM. Dynamic CTA. MIP projection, oblique sagittal plane. Same patient as Figure 5 seen from a different angle of view.
Figure 7.
AVM nidus. Dynamic CTA. Inverted MIP projection, oblique sagittal plane. Same patient as Figures 5 and 6. Images show dynamic contrast enhancement of the nidus, where a pseudo-aneurysm can be observed in the inferior aspect of the main draining vein.
Results
In all the subjects enrolled, the studies demonstrated an arteriovenous malformation. We found 12 brain AVMs (ten men, two women), two brain dAVF (two men), and six spinal cord dAVF (six men). Among the 12 brain AVMs, only one case was examined after acute haemorrhage (one day later), and in this patient the simultaneous presence of an unknown unruptured intracranial aneurysm, ipsilateral with the AVM, was discovered; 19/20 shunting vascular malformations were correctly detected using 4D-CTA.
In only one case was a dAVF of the lower thoracic spinal cord, the site of the shunt, not detected by 4D-CTA because of misinterpretation of the previous MR study, which caused wrong placement of the FOV during the planning phase of the 4D-CTA. In that case, whole spinal cord DSA successfully detected the feeding vessels and the site of the arteriovenous shunt. No complications were observed peri-procedurally.
All 4D-CTA studies allowed us to classify the vascular malformations observed, analysing the number of arterial feeders, the location of the draining veins, and any associated intranidal aneurysms of venous stenoses. The same elements were also evaluated after DSA with the same findings.
Discussion
Dynamic CTA is a novel technique to study cerebral, and - with some technical restrictions (due to the amplitude of the maximum available FOV) - spinal cord haemodynamics. This can be useful to detect and analyse the main features of previously unknown vascular shunting lesions both in brain and spinal cord. Currently, the technical characteristics of new multidetector CT scanners allow angiographic studies to be performed with sufficient temporal resolution (of about three frames/second) compared to traditional DSA studies or even if compared with recent dynamic MRA studies 5.
The main limitation of the technique is the implicit assumption that when performing CTA the reader is required to interpret the effects of simultaneous injection of all the vessels that constitute the malformation, doing without the diagnostic potential added by selective or super-selective injections of the feeding vessels. This can result in a "chaotic" vascular picture especially for lesions with a more complex architecture, made by several different arterial feeders and draining veins. On the other hand, the viewing angles of DSA are determined intra-procedurally: due to the implicit limitations of the technique, this means that several repeated injections are required to obtain views from different angles and this accounts for the higher radiation dose delivered to the patient throughout the procedure.
In relation to the total radiation burden delivered to the patient, it is widely accepted that a scan duration of 20 seconds is enough to obtain the information necessary to perform an exhaustive analysis and classification of these vascular lesions. With the above-cited technical parameters (range: 160 mm, 80 KV, 120 mA, rot. time: 0.35) for a "standard" dynamic CTA acquisition phase, the total radiation dose is approximately calculated to be 5.62 mSv; with the same scanner, when performing a study of intracranial arterial circulation (first scan without contrast, range:160 mm, 120 KV, 150 mA, rot. time: 0.75 s - scan with contrast, range 160 mm, 120 KV, 300 mA, rot. time: 0.35 s) the total radiation dose is expected to be 3.62 mSv.
It is more difficult to predict the radiation dose involved for DSA since it depends on many variables such as the fluoroscopy time needed (largely operator-dependent) to navigate into the feeding vessels, the number of injections performed, the equipment used, the execution or not of 3D DSA acquisition and so on.
In short, 4D-CTA is a non-invasive technique of angiographic imaging that can offer additional diagnostic information in comparison to traditional ("static" or non-dynamic) CTA or MRA.
Conclusions
Time-resolved CTA appears to be a valuable new diagnostic tool in the diagnostic work-up of brain and, with some limitations due to the too small FOV, spinal cord vascular shunting lesions. Although with some pitfalls, already highlighted also by other authors 6 when facing low-flow dAVFs, and some limitations mainly due to the simultaneous injection of all the feeding vessels and to inferior temporal resolution compared to traditional DSA, 4DCTA seems to be useful and convenient because of the lower costs, risks and time consumption.
Also according to the authors who first investigated this new technique 7,8, although the information obtained with dynamic CTA studies may not be complete in some cases, a dynamic CTA examination can yield all the most important information necessary to plan a subsequent phase of treatment, dispensing - perhaps in the majority of cases - with an invasive approach only for diagnostic purposes.
References
- 1.Bendszus M, Koltzenburg M, Burger R, et al. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet. 1999;354(9190):1594–1597. doi: 10.1016/S0140-6736(99)07083-X. doi: 10.1016/S0140-6736(99)07083-X. [DOI] [PubMed] [Google Scholar]
- 2.Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke. 1999;30(2):317–320. doi: 10.1161/01.str.30.2.317. doi: 10.1161/01.STR.30.2.317. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann TJ, Huston J, Mandrekar JN, et al. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology. 2007;243(3):812–819. doi: 10.1148/radiol.2433060536. doi: 10.1148/radiol.2433060536. [DOI] [PubMed] [Google Scholar]
- 4.Willinsky RA, Taylor SM, terBrugge K, et al. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227(2):522–528. doi: 10.1148/radiol.2272012071. doi: 10.1148/radiol.2272012071. [DOI] [PubMed] [Google Scholar]
- 5.Taschner CA, Gieseke J, Le Thuc V, et al. Intracranial arteriovenous malformation: time-resolved contrast-enhanced MR angiography with combination of parallel imaging, keyhole acquisition, and k-space sampling techniques at 1T. Radiology. 2008;246(3):871–879. doi: 10.1148/radiol.2463070293. doi: 10.1148/radiol.2463070293. [DOI] [PubMed] [Google Scholar]
- 6.Willems PW, Brouwer PA, Barfett JJ, et al. Detection and classification of cranial dural arteriovenous fistulas using 4D-CT angiography: initial experience. Am J Neuroradiol. 2011;32(1):49–53. doi: 10.3174/ajnr.A2248. doi: 10.3174/ajnr.A2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouwer PA, Bosman T, van Walderveen MA, et al. Dynamic 320-section CT angiography in cranial arteriovenous shunting lesions. Am J Neuroradiol. 2010;31(4):767–770. doi: 10.3174/ajnr.A1747. doi: 10.3174/ajnr.A1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willems PW, Taeshineetanakul P, Schenk B, et al. The use of 4D-CTA in the diagnostic work-up of brain arteriovenous malformations. Neuroradiology. 2012;54(2):123–131. doi: 10.1007/s00234-011-0864-0. doi: 10.1007/s00234-011-0864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]