Summary
The corpus callosal splenium is an uncommon location for Wallerian degeneration (WD), which may be mistaken for new pathology on magnetic resonance imaging (MRI). We describe the case of a 69-year-old woman with a left posterior cerebral artery infarct in whom progressive WD of the splenium of the corpus callosum seen on MRI was misinterpreted as new infarction or neoplasm. We address how magnetic resonance spectroscopy, perfusion MRI, diffusion tensor MRI, and serial imaging were utilized in establishing the correct diagnosis. Interestingly, the patient also presented with alexia without agraphia, which has never been reported in association with splenial WD. It is conceivable that WD affected critical splenial association fibers resulting in this uncommon dissociation syndrome.
Keywords: stroke, neuroradiology, MR imaging, magnetic resonance spectroscopy, Wallerian degeneration
Introduction
Wallerian degeneration (WD) is the secondary disintegration and demyelination of axons distal to the site of axonal or neuronal cell body injury occurring in both the central nervous system (CNS) and the peripheral nervous system (PNS). CNS WD is seen after ischemic stroke, intracerebral hemorrhage, multiple sclerosis and traumatic brain injury 1. WD after ischemic stroke is commonly seen after injury to the motor cortex or internal capsule 2. It has rarely been reported to occur in other white matter tracts 3.
WD is histopathologically visible within the first week after acute neurological injury 4 and may be detected by magnetic resonance imaging (MRI) 1.
We describe here the case of a patient exhibiting WD of the corpus callosal splenium who also suffered from alexia without agraphia. This patient's phenomenon was initially mischaracterized as a result of new infarction or neoplasm, but use of newer MR techniques established the true diagnosis and revealed the potential pathophysiology.
Case Report
A 69-year-old left-handed woman presented to her primary care physician with right vision loss. Head computed tomography (CT) revealed acute left occipital infarction. About a month later, she returned with complaints of worsening vision; however, her primary care physician detected no change in her existing severe right homonymous hemianopsia, intact left visual field and unchanged central visual acuities. Detailed history clarified that the patient could only read by using a magnifying glass to look at each individual letter. MRI revealed a now chronic left occipital infarct with a new signal abnormality on T2 and FLAIR imaging in the callosal splenium. MR angiogram showed a critical stenosis of the left posterior cerebral artery (PCA). A repeat MRI of the head obtained three months later showed increased T2 signal and mild expansion of the entire corpus callosal splenium extending into the right forceps major, in addition to the previously noted occipital infarction. There was no restriction of diffusion to indicate new infarction. Given the slightly expansile nature of the lesion, a glioma was also considered and dynamic contrast-enhanced perfusion imaging and MR spectroscopy was performed. There was no evidence of elevated relative cerebral blood volume to suggest a neoplasm. MR spectroscopy also revealed findings compatible with a degenerative process, demonstrating a slight decline in N-acetylaspartate, no significant elevation of choline and absence of a lactate peak. On DTI, there was loss of the expected anisotropy of the transverse splenial fibers.
Discussion
Although Wallerian degeneration (WD) of the pyramidal tracts after ischemic injury has been well-described 5,10,11,12, it is rarely seen in the corpus callosum. Alexia without agraphia is a rare disconnection syndrome that presents as difficulty reading words with preserved ability to write spontaneously 6,7. From a neuroanatomic standpoint, alexia without agraphia may be explained by involvement of the association fibers that connect the visual cortices with the left angular gyrus. In our patient, WD likely affected these association fibers. While most cases of this entity have been reported to occur after damage to the splenium by ischemia, trauma, surgery or neoplasms, its association with WD has not been described 13,14.
Clinically and radiologically, WD has a characteristic presentation unlike that of a new infarct. Degenerative changes occur within hours of infarct, and may be visible on diffusion-weighted sequences within days of infarct 10,11 (Figure 1A). Degeneration is visible weeks to months after the initial infarct, as T2 hyperintensity along the affected tract 11. WD follows four characteristic stages. Stage 1 occurs during the first four weeks after insult, and consists of physical degradation with minimal biochemical change in the myelin. This stage displays no signal abnormality on MRI. Stage 2 occurs from weeks four to 14 and involves myelin protein breakdown with myelin lipids remaining intact, resulting in hypointense signal on T2. In stage 3, myelin lipid breakdown and gliosis presents as T2 hyperintensity (Figure 1B). This is likely the stage in which the patient's complaints of “worsening vision” were recognized as alexia without agraphia. The final stage occurs years after the initial insult and presents as atrophy 2.
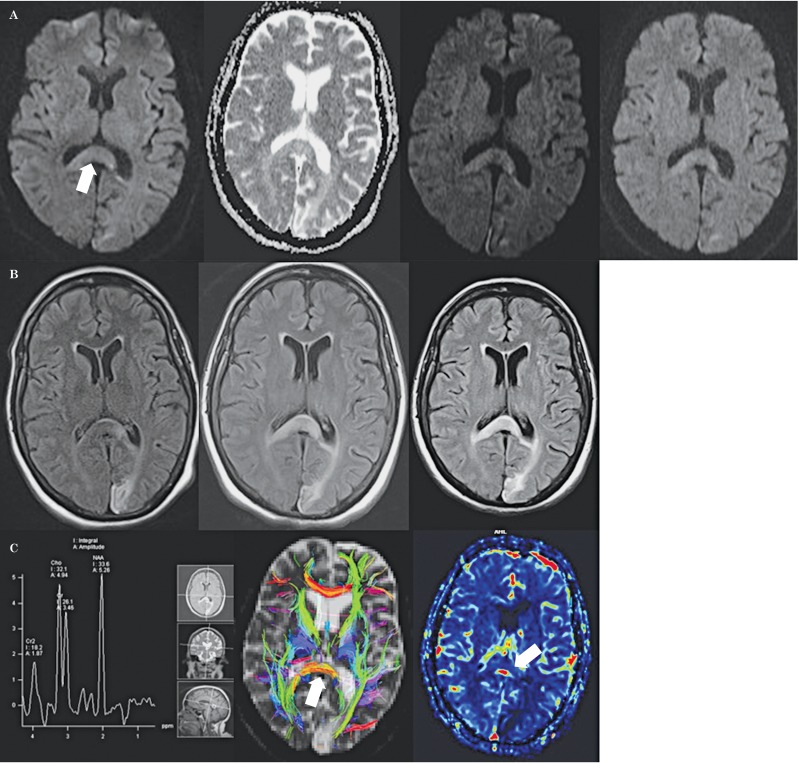
Figure 1.
A) Axial diffusion-weighted images obtained 1, 3, and 6 months after left occipital infarction show progressively increasing signal and swelling in the corpus callosal splenium (arrows). Note the lack of corresponding ADC abnormality. Similar findings are evident on the corresponding FLAIR images (B). C) On the MR spectroscopy image (left) note the relative preservation of metabolic spectra. On the DTI tractography image (middle) note the lack of orderly arrangement of preserved splenial fibers compared to the callosal genu. The dynamic contrast-enhanced perfusion MR image (left) shows decreased CBV.
Abnormal splenial MR signal intensity may be seen with tumors, multiple sclerosis, infection, hypoglycemia, trauma, electrolyte abnormalities and seizures 8,9. Our patient's new MRI findings did not conform to a specific arterial territory, nor did they demonstrate restriction of diffusion as one would expect for a new acute infarct, further suggesting a new stroke as an unlikely cause of pathology. Importantly, a six-month follow-up MRI did not reveal volume loss in this region, a finding that would be unusual for an evolving infarct. The lack of any perfusion or spectral abnormality also helped exclude neoplasm. On perfusion MRI, a neoplasm would be expected to demonstrate increased cerebral blood volume (CBV). CBV was decreased in the splenium in our patient (Figure 1C). Likewise, on MR spectroscopy (Figure 1C), markedly low n-acetylaspartate and elevated choline and creatine peaks, findings encountered with high-grade gliomas, were conspicuously absent in our patient. On DTI (Figure 1C), despite the loss of the normal orderly arrangement of callosal fibers (anisotropy), the fibers themselves were preserved, unlike the case with infarction. Thus, a combination of clinical and imaging findings enabled a diagnosis of WD.
WD of the callosal splenium is an uncommon event that must not be mistaken for new pathology. It may have prognostic significance in that it may indicate the existence of alexia without agraphia, warranting appropriate rehabilitatory efforts.
References
- 1.Sawlani V, Gupta R, Singh M, et al. MRI demonstration of Wallerian degeneration in various intracranial lesions and its clinical implications. J Neurol Sci. 1997;146(2):103–108. doi: 10.1016/s0022-510x(96)00299-7. doi: 10.1016/S0022-510X(96)00299-7. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn MJ, Mikulis DJ, Ayoub DM, et al. Wallerian degeneration after cerebral infarction: evaluation with sequential MR imaging. Radiology. 1989;172(1):179–182. doi: 10.1148/radiology.172.1.2740501. doi: 10.1148/radiology.172.1.2740501. [DOI] [PubMed] [Google Scholar]
- 3.Meguro K, Constans JM, Courtheroux P, et al. Atrophy of the corpus callosum correlates with white matter lesions in patients with cerebral ischaemia. Neuroradiology. 2000;42(6):413–419. doi: 10.1007/s002340000302. doi: 10.1007/s002340000302. [DOI] [PubMed] [Google Scholar]
- 4.Venkatasubramanian C, Kleinman J, Fischbein N, et al. Natural history and prognostic value of corticospinal tract Wallerian degeneration in intracerebral hemorrhage. J Am Heart Assoc. 2013;2(4):e000090. doi: 10.1161/JAHA.113.000090. doi: 10.1161/JAHA.113.000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pujol J, Martì-Vilalta JL, Junqué C, et al. Wallerian degeneration of the pyramidal tract in capsular infarction studied by magnetic resonance imaging. Stroke. 1990;21(3):404–409. doi: 10.1161/01.str.21.3.404. doi: 10.1161/01.STR.21.3.404. [DOI] [PubMed] [Google Scholar]
- 6.Bub DN, Arguin M, Lecours AR. Jules D‚jerine and his interpretation of pure alexia. Brain Lang. 1993;45(4):531–559. doi: 10.1006/brln.1993.1059. doi: 10.1006/brln.1993.1059. [DOI] [PubMed] [Google Scholar]
- 7.Martin A. Shades of Déjerine--forging a causal link between the visual word form area and reading. Neuron. 2006;50(2):173–175. doi: 10.1016/j.neuron.2006.04.004. doi: 10.1016/j.neuron.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Uchino A, Takase Y, Nomiyama K, et al. Acquired lesions of the corpus callosum: MR imaging. Eur Radiol. 2006;16(4):905–914. doi: 10.1007/s00330-005-0037-9. doi: 10.1007/s00330-005-0037-9. [DOI] [PubMed] [Google Scholar]
- 9.Doherty MJ, Jayadev S, Watson NF, et al. Clinical implications of splenium magnetic resonance imaging signal changes. Arch Neurol. 2005;62(3):433–437. doi: 10.1001/archneur.62.3.433. doi: 10.1001/archneur.62.3.433. [DOI] [PubMed] [Google Scholar]
- 10.DeVetten G, Coutts SB, Hill MD, et al. Acute corticospinal tract wallerian degeneration is associated with stroke outcome. Stroke. 2010;41(4):751–756. doi: 10.1161/STROKEAHA.109.573287. doi: 10.1161/STROKEAHA.109.573287. [DOI] [PubMed] [Google Scholar]
- 11.Thomalla G, Glauche V, Koch MA, et al. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage. 2004;22(4):1767–1774. doi: 10.1016/j.neuroimage.2004.03.041. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 12.Yu C, Zhu C, Zhang Y. A longitudinal diffusion tensor imaging study on Wallerian degeneration of corticospinal tract after motor pathway stroke. Neuroimage. 2009;47(2):451–458. doi: 10.1016/j.neuroimage.2009.04.066. doi: 10.1016/j.neuroimage.2009.04.066. [DOI] [PubMed] [Google Scholar]
- 13.Tamhankar MA, Coslett HB, Fisher MJ, et al. Alexia without agraphia following biopsy of a left thalamic tumor. Pediatr Neurol. 2004;30(2):140–142. doi: 10.1016/S0887-8994(03)00417-X. doi: 10.1016/S0887-8994(03)00417-X. [DOI] [PubMed] [Google Scholar]
- 14.Quint DJ, Gilmore JL. Alexia without agraphia. Neuroradiology. 1992;34(3):210–214. doi: 10.1007/BF00596338. doi: 10.1007/BF00596338. [DOI] [PubMed] [Google Scholar]