Summary
Leptomeningeal metastasis (LM) is a rare but increasingly common condition in which malignant cells migrate to the meninges. The gold standard for diagnosing LM is detection of cancer cells in the cerebrospinal fluid (CSF). Contrast enhanced-magnetic resonance imaging (CE-MRI) is also used to diagnose LM. We describe a case of LM in which CE-MRI of the neuroaxis was initially negative for meningeal enhancement but F-18 fluorodeoxyglucose positron-emission tomography/computed tomography (F-18 FDG PET/CT) revealed hypermetabolism within the lumbar spinal canal. Positive F-18 FDG PET findings have rarely been reported in LM and, to our knowledge, have never been reported in the context of initially negative CE-MRI scanning of the neuroaxis. F-18 FDG PET/CT may represent an alternative modality for diagnosing LM in patients who are unable to undergo CE-MRI and/or LP or in patients for whom initial CE-MRI and/or LP are negative for LM.
Keywords: leptomeningeal metastasis, PET imaging, MR imaging
Introduction
The incidence of leptomeningeal metastasis (LM) is rising secondary to improvements in systemic cancer treatment and prolonged survival 1. Despite the increased incidence and improved neuroimaging modalities for its detection, the diagnosis of LM remains clinically challenging. The gold standard for diagnosis is detection of cancer cells in the cerebrospinal fluid (CSF) 2. A single lumbar puncture (LP) is insensitive for the confirmation of LM 3. While contrast enhanced-magnetic resonance imaging (CE-MRI) can detect meningeal involvement suggestive of LM prior to LP and in the presence of negative cytology, the sensitivity of MRI is modest 4,5.
We describe a case of LM in which CE-MRI of the neuroaxis was initially negative for meningeal enhancement but F-18 fluorodeoxyglucose positron-emission tomography/computed tomography (F-18 FDG PET/CT) revealed hypermetabolism within the lumbar spinal canal.
Case Report
A 27-year-old woman with a medical history of triple-negative stage III breast cancer one year removed by bilateral mastectomy, chemotherapy, and radiation presented with intractable lower back pain of three weeks duration. The pain was gradually progressive and located in the lower lumbar region with radiation to the posterior aspect of her legs bilaterally. The pain was associated with bilateral paresthesias in her feet and mild subjective lower extremity weakness. The patient denied any difficulty with bowel or bladder function.
The patient had been previously admitted two days prior to this presentation with similar symptoms. During her previous admission, an MRI of her lumbar spine did not reveal any pathological findings (Figure 1). She was discharged with significant improvement in her symptoms on narcotic pain medication. She returned to the hospital with increased low back pain that was now associated with headache, nausea, and vomiting.
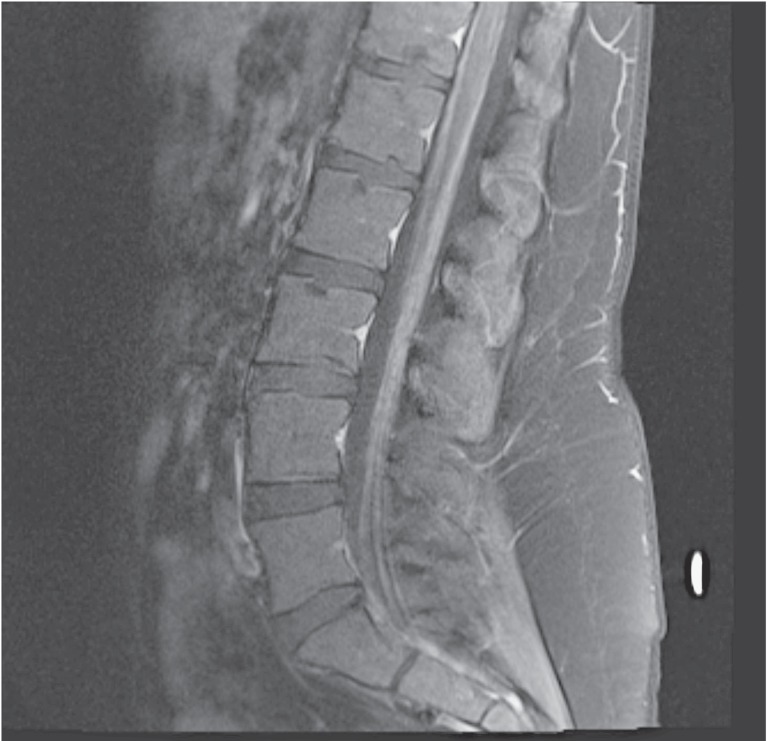
Figure 1.
Sagittal contrast-enhanced T1-weighted image of the lumbar spine showed no cord compression, no clumping of nerve roots, and no abnormal meningeal enhancement.
Non-contrast CT scan of the patient's head revealed an isodense mass producing mass effect on the fourth ventricle with surrounding edema extending into the cerebellar hemispheres. CE-MRI further delineated the lesion as a 2.5 × 3.0 × 1.7 cm dural-based, extra-axial, enhancing soft tissue mass arising from the right tentorium cerebelli with vasogenic edema and mild mass effect upon the right cerebellar hemisphere and fourth ventricle (Figure 2A,B). The MRI findings were consistent with a meningioma; however, a dural metastasis could not be excluded due to the patient's history of malignancy.
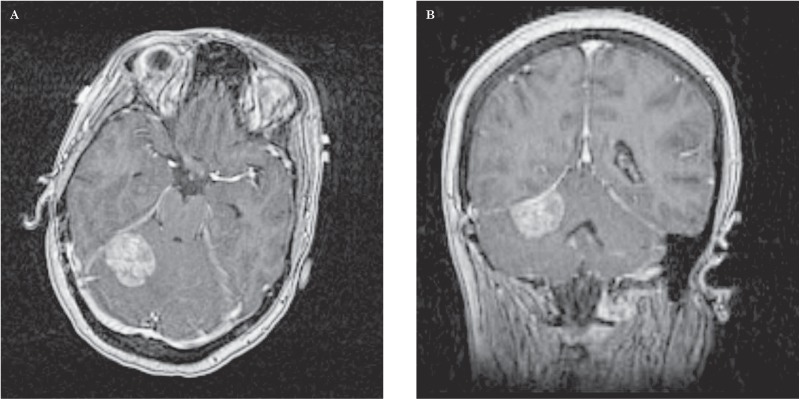
Figure 2.
Axial (A) and coronal (B) contrast-enhanced T1-weighted MRI of the brain revealed a 2.5 × 3.0 × 1.7 cm dural-based, extra-axial, enhancing soft tissue mass arising from the right tentorium cerebelli with vasogenic edema and mild mass effect upon the right cerebellar hemisphere and fourth ventricle. The lesion was favored to represent a meningioma. Given the patient's history of cancer, however, a solitary broad-based dural metastasis could not be excluded.
Dexamethasone treatment was initiated to reduce the patient's intracranial pressure. Magnetic resonance angiogram (MRA) was performed to evaluate the tumor's vasculature. MRA showed no significant intracranial arterial blood supply to the right infratentorial, dural-based mass, a finding non-discriminatory for distinguishing meningioma from dural metastasis 6.
On hospital day 3, the patient developed significant bilateral lower extremity weakness graded as 3/5. She was unable to walk at this time. MRI of thoracic and cervical spine revealed no pathological explanation for the patient's symptoms. Electromyography was performed to evaluate the patient's weakness but no abnormal electrodiagnostic findings were detected.
On hospital day 4, the patient developed double vision and tongue deviation on protrusion. Patient underwent an F-18 FDG PET/CT scan. Dedicated brain PET/CT showed the right infratentorial mass to be intensely hypermetabolic suggesting malignancy, either high-grade meningioma or metastasis (Figure 3A). Whole body PET/CT demonstrated a vertical linear pattern of intense hypermetabolic activity surrounding the lumbar spinal cord, concerning for LM (Figure 3B). A second lumbar CE-MRI was performed at this time which revealed interval progression of diffuse sugarcoating enhancement of the terminal cord and nerve roots as well as nerve root impingement consistent with metastatic disease (Figure 3C). The patient underwent lumbar puncture (LP) that was significant for elevated protein level of 218 mg/dl, decreased glucose level of 4 mg/dl, and increased white blood cell count of 175. CSF cytology showed adenocarcinoma, confirming a diagnosis of LM secondary to the patient's breast cancer. The patient was treated with local radiation to her lumbar spine and initiated on intrathecal methotrexate. Despite aggressive therapy, the patient did not regain lower-extremity strength and was eventually discharged to a hospice.
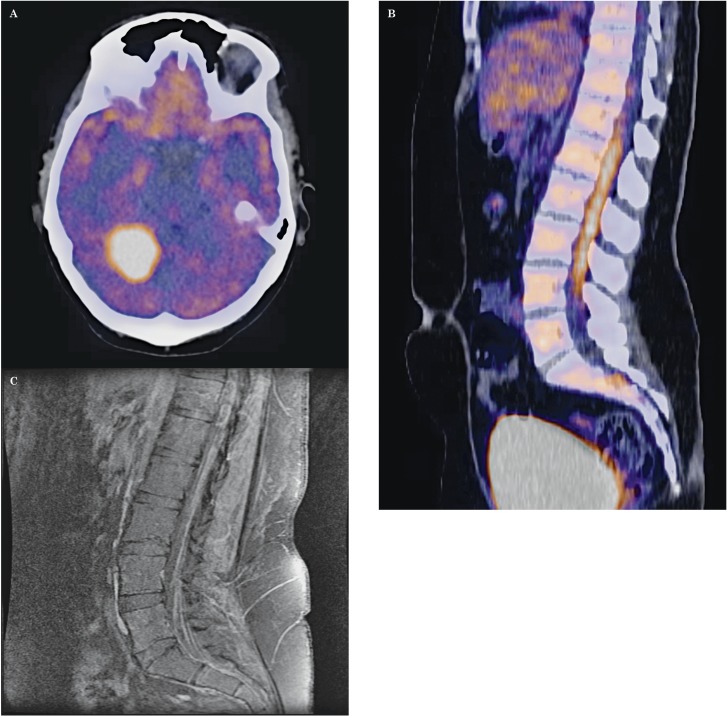
Figure 3.
A) Axial PET/CT of the brain revealed a large hypermetabolic lesion adjacent to the right cerebellum, corresponding to the tentorium cerebelli-based lesion seen on the prior MRI study. Intense tracer uptake it suggests malignancy, either malignant meningioma or metastasis. B) Sagittal PET/CT of the whole body showed a vertical linear pattern of intense hypermetabolic activity surrounding the lumbar spinal cord, concerning for drop metastases. C) Repeat sagittal contrast-enhanced T1-weighted image of the lumbar spine showed interval development of spinal cord sugarcoating and multilevel nerve impingement consistent with LM.
Discussion
The early diagnosis of LM can be difficult. The gold standard for diagnosis is the detection of malignant cells in the CSF 2. A single LP yields positive cytology in only about 50% of patients with LM. When three high-volume LPs are obtained, the sensitivity improves to 90% 3. CE-MRI of the brain and/or spine should be performed when the clinical suspicion for LM is high because positive findings allow clinicians to diagnose LM even with negative CSF findings or without performing LP altogether 7-9. A majority of patients with LM secondary to breast cancer will have co-existing parenchymal brain metastasis, as in our patient 10. The presence of metastasis causing mass effect could increase the risk of herniation secondary to LP. In this patient, the mild mass effect of the intracranial metastasis and the theoretical increased risk of herniation resulted in delay in performance of LP and ultimately delayed the final diagnosis and treatment.
CE-MRI can detect meningeal involvement suggestive of LM prior to LP and with negative cytology on LP, however the overall sensitivity of CE-MRI even in the presence of positive CSF cytology is only 65-71% 4,5. Because CE-MRI meningeal enhancement can be caused by a number of conditions, the specificity of MRI for diagnosis of LM is heavily dependent upon pre-test probability but can be as high as 77% 8.
In this case, initial CE-MRI of the entire neuroaxis showed no obvious meningeal enhancement to suggest LM. Ultimately, F-18 FDG-PET/CT provided the first imaging finding suggestive of LM. Repeat CE-MRI of the lumbar spine showed interval development of spinal cord sugarcoating and multilevel nerve impingement consistent with the patient's interval development of lower extremity weakness. The literature has multiple reports in which the diagnosis of LM was suggested in both MRI and PET scanning. PET imaging utilizing the radioisotopes F-18 FDG 11-15 as well as C-11 methionine 16 have shown intense radiotracer uptake suggestive of LM. In these cases, corollary CE-MRI was also suggestive of LM. To our knowledge, this is the first reported case in which F-18 FDG PET/CT suggested LM in the context of initially normal CE-MRI findings. F-18 FDG PET/CT may represent an alternative modality for diagnosing LM in patients who are unable to undergo CE-MRI and LP or in patients for whom initial CE-MRI and LP are negative for LM.
References
- 1.Clarke JL, Perez HR, Jacks LM, et al. Leptomeningeal metastases in the MRI era. Neurology. 2010;74(18):1449–1454. doi: 10.1212/WNL.0b013e3181dc1a69. doi: 10.1212/WNL.0b013e3181dc1a69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass JP, Melamed M, Chernik NL, et al. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology. 1979;29(10):1369–1375. doi: 10.1212/wnl.29.10.1369. doi: 10.1212/WNL.29.10.1369. [DOI] [PubMed] [Google Scholar]
- 3.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49(4):759–772. doi: 10.1002/1097-0142(19820215)49:4<759::aid-cncr2820490427>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Singh SK, Leeds NE, Ginsberg LE. MR Imaging of leptomeningeal metastases: comparison of three sequences. Am J Neuroradiol. 2002;23(5):817–821. [PMC free article] [PubMed] [Google Scholar]
- 5.Sze G, Soletsky S, Bronen R, et al. MR imaging of the cranial meninges with emphasis on contrast enhancement and meningeal carcinomatosis. Am J Neuroradiol. 1989;10(5):965–975. [PMC free article] [PubMed] [Google Scholar]
- 6.Bendszus M, Warmuth-Metz M, Burger R, et al. Diagnosing dural metastases: the value of 1H magnetic resonance spectroscopy. Neuroradiology. 2001;43(4):285–289. doi: 10.1007/s002340000419. doi: 10.1007/s002340000419. [DOI] [PubMed] [Google Scholar]
- 7.Freilich RJ, Krol G, Deangelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol. 1995;38(1):51–57. doi: 10.1002/ana.410380111. doi: 10.1002/ana.410380111. [DOI] [PubMed] [Google Scholar]
- 8.Straathof CS, de Bruin HG, Dippel DW, et al. The diagnostic accuracy of magnetic resonance imaging and cerebrospinal fluid cytology in leptomeningeal metastasis. J Neurol. 1999;246(9):810–814. doi: 10.1007/s004150050459. doi: 10.1007/s004150050459. [DOI] [PubMed] [Google Scholar]
- 9.Maroldi R, Ambrosi C, Farina D. Metastatic disease of the brain: extra-axial metastases (skull, dura, leptomeningeal) and tumour spread. Eur Radiol. 2005;15(3):617–626. doi: 10.1007/s00330-004-2617-5. doi: 10.1007/s00330-004-2617-5. [DOI] [PubMed] [Google Scholar]
- 10.Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. doi: 10.1002/cncr.11436. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 11.Komori T, Delbeke D. Leptomeningeal carcinomatosis and intramedullary spinal cord metastases from lung cancer: detection with FDG positron emission tomography. Clin Nucl Med. 2001;26(11):905–907. doi: 10.1097/00003072-200111000-00001. doi: 10.1097/00003072-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Rangarajan V, Purandare N, Luthra K, et al. 18F-FDG uptakes in leptomeningeal metastases from carcinoma of the breast on a positron emission tomography/computerized tomography study. Indian J Cancer. 2007;44(3):115. doi: 10.4103/0019-509x.38944. doi: 10.4103/0019-509X.38944. [DOI] [PubMed] [Google Scholar]
- 13.Renard D, Dufour S, Collombier L, et al. Cerebral FDG-PET hypermetabolism in carcinomatous meningitis. Eur Neurol. 2011;66(6):332–333. doi: 10.1159/000334096. doi: 10.1159/000334096. [DOI] [PubMed] [Google Scholar]
- 14.Rohren EM, Provenzale JM, Barboriak DP, et al. Screening for cerebral metastases with FDG PET in patients undergoing whole-body staging of non-central nervous system malignancy. Radiology. 2003;226(1):181–187. doi: 10.1148/radiol.2261010920. doi: 10.1148/radiol.2261010920. [DOI] [PubMed] [Google Scholar]
- 15.Shinohara M, Kosaka S, Okamura T, et al. FDG-PET in meningeal lymphomatosis. J Neurol Neurosurg Psychiatry. 2007;78(9):974. doi: 10.1136/jnnp.2007.116129. doi: 10.1136/jnnp.2007.116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padma MV, Jacobs M, Kraus G, et al. 11C-methionine PET imaging of leptomeningeal metastases from primary breast cancer - a case report. J Neurooncol. 2001;55(1):39–44. doi: 10.1023/a:1012973504866. doi: 10.1023/A:1012973504866. [DOI] [PubMed] [Google Scholar]