Abstract
Introduction: The aim of this study is the evaluation of the effect of Antimicrobial Photodynamic Therapy with Radachlorin on Staphylococcus aureus and Escherichia coli. New windows are open in the antimicrobial field so-call Photodynamic therapy that incorporates a nonpoisonous photosensitizer (PS) with innocuous special wavelength photons to excite the PS.
Methods: Two strains of bacteria used in this study were Methicillin resistant Staphylococcus aureus (ATCC 33591; PTCC 1764) and Escherichia coli (ATCC 25922; PTCC1399). Concentrations of 0.2 ml of Radachlorin® were applied on 0.2 ml of bacterial suspensions and placed in a 48-well microtiter plate. The following groups were used: (I) L− PS− (no laser, no photosensitizer), (II) L−PS+ (treated only with PS), (III) L+ PS− (treated only with laser) and (IV) L+ PS+ (treated with laser and PS: photodynamic therapy group). Aliquots of bacterial suspensions were sensitized with Radachlorin® for 15 minutes in the dark at room temperature and then bacterial suspensions in group III and IV were irradiated with 210 mW (power density) and 12 J/cm2 (energy density) on continuous mode.
Results: This study showed that photodynamic therapy reduces 0.14 log 10 in E.Coli (group IV) and there were significant differences for group IV (P<0.01). Photodynamic therapy in S.Aureus showed 6.28 log 10 colony count reduction (group IV) and there were highly significant differences in Photodynamic therapy group (P<0.0001).
Conclusion: Radachlorin® have bactericidal effect on S.aureus (6.28 log 10) and bacteriostaticeffect on E.coli (0.14 log 10).
Keywords: photodynamic therapy, staphylococcus aureus, escherichia coli
Introduction
Presently, two of the overt multidrug-resistant pathogens causing worldwide worry are Methicillin-resistant Staphylococcus aureus (MRSA) and “extended-spectrum β-lactamase (ESBL) “producing Escherichia coli 1,2. Some studies showed significant increasing trends of S.Aureus and E.Coli in urinary tract infections (UTI), respiratory tract infections (RTI), and surgical site infections (SSI)3,4. Many efforts have been done to overcome the pathogens such as producing new antibiotics but the microorganisms are wily and by different methods annihilate the antibiotics5. New windows are open in antimicrobial field so-call Photodynamic therapy that incorporates a nonpoisonous photosensitizer (PS) with innocuous special wavelength photons to excite the PS to its reactive triplet state, which will then produce reactive oxygen species, such as superoxide and singlet oxygen that are poisonous to cells and kill them 6,7. In many research, chlorin e6 as a photosensitiser has been used generally 8- 12. Radachlorin® which is a chlorophyll derivative, including sodium chlorine е6, chlorine р6, purpurine 5, that have been successfully used in tumors diagnosis and tumors treatment 13. There have been only few studies on the antimicrobial effects of Radachlorin®, although there have been several studies on chlorin e6, which is a major component of Radachlorin® 8- 12. Fekrazad et al reported that the combination of Radachlorin® and laser was more effective on Streptococcus mutans than Radachlorin® or laser alone (p < 0.05)14. Vahabi et al reported an in vitro study that toluidine blue O (TBO) mediated photodynamic therapy seems to be more efficient than Radachlorin® in reducing the viability of Streptococcus mutans15. We can’t find any reaseach on Staphylococcus aureus and Escherichia coli with Radachlorin®. The main purpose of this study was to explore the antimicrobial photodynamic therapy effect of Radachlorin® on Persian Type Culture Collection(PTCC) S. aureus and E. coli.
Methods
Bacteria
Two strains of bacteria used in this study were Methicillin resistant Staphylococcus aureus; Persian Type Culture Collection (PTCC 1764) and Escherichia coli (PTCC 1399). These bacteria were maintained by weekly subculture on nutrient agar (Merck). These bacteria were grown in brain-heart infusion broth in an orbital shaker at 37°C for 24 h. An aliquot of this suspension was then added to nutrient broth and grown to mid-log phase (OD600 = 0.6, 108 cells/mL).
Photosensitizers and laser sources
Radachlorin® gel (0.1%, 25 g) was obtained from RADA-FARMA Ltd, Russia and stored at 0–8 °C in the dark. The laser source used was a diode laser (Milon-LAHTA, Russia) with a fiber optic diameter of 800 micrometer, a maximum output of 2.5 W and a predominant wavelength of 662 nm.
Photodynamic therapy
Preparation of suspension of microbial cells was performed, preparation of liquid media (brain-heart infusion broth, BHI, for bacteria) and autoclave. Preparation of solid media was performed by addition of 1.5% microbiological agar to above broth and poured into 10 × 10 cm square petri dishes. Concentration of 0.2 ml of Radachlorin® was applied on 0.2 ml of the bacterial suspensions and placed in a 48-well microtiter plate. The following groups were used: (I) L− PS− (no laser, no photosensitizer), (II) L−PS+ (treated only with PS), (III) L+ PS− (treated only with laser) and (IV) L+ PS+ (treated with laser and PS: photodynamic therapy group). Aliquots of bacterial suspensions were sensitized with Radachlorin® for 15 minutes in the dark at room temperature and then bacterial suspensions in group III and IV were irradiated with continuous mode, 23 second, 213 mW (power density) and 12 J/cm2 (energy density). The focal point of laser was matched by one of 48-well microtiter plate and the fiber optic of the laser was at 1mm above the microtiter plate. The plates were incubated at 37°C overnight. The laboratory technician was blinded to the study and the numbers of colonies was counted to determine the survival fractions.
Statistical analysis
Values were expressed as log 10 means ± standard deviation. Comparisons between means of groups were used as well as the univariate analysis of variance and Post P<0.05 was considered statistically significant.
Results
This study showed that photodynamic therapy reduces 0.14 log 10 in E.Coli (group IV) and there were significant differences for group IV (P<0.01) (Table 1) but no differences in other groups and conrol group (group I) were obtained.
Table 1. Mean value log 10 E.coli colony count for Radachlorin®, (I) L− PS− (no laser, no photosensitizer), (II) L−PS+ (treated only with PS), (III) L+ PS−(treated only with laser) and (IV) L+ PS+ (treated with laser and PS: photodynamic therapy group) .
| Group | Mean | Std. Deviation | N |
| (I) | 7.1530 | .06542 | 20 |
| (II) | 7.0930 | .06602 | 20 |
| (III) | 7.0445 | .07244 | 20 |
| (IV) | 7.0105 | .15388 | 20 |
Profile pilot diagram of E.Coli show colony count mean values in each group (Figure 1).
Figure 1 .
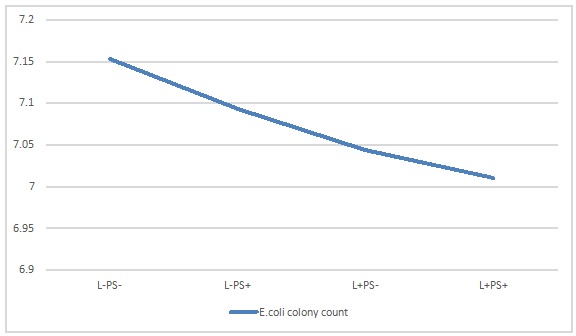
Mean value log 10 E.coli colony count , (I) L− PS− (no laser, no photosensitizer), (II) L−PS+ (treated only with PS), (III) L+ PS−(treated only with laser) and (IV) L+ PS+ (treated with laser and PS: photodynamic therapy group)
Photodynamic therapy in S.Aureus showed 6.28 log 10 colony count reduction (group IV) and there were highly significant differences in Photodynamic therapy group (P<0.0001) and other groups (Table 2).
Table 2. Mean value log 10 S.Aureus colony count for Radachlorin®, (I) L− PS− (no laser, no photosensitizer), (II) L−PS+ (treated only with PS), (III) L+ PS−(treated only with laser) and (IV) L+ PS+ (treated with laser and PS: photodynamic therapy group) .
| Group | Mean | Std. Deviation | N |
| (I) | 7.1285 | .08881 | 20 |
| (II) | 7.0780 | .23768 | 20 |
| (III) | 6.8610 | .06340 | 20 |
| (IV) | 0.84 | 2.84518 | 20 |
Other groups of S.Aureus (group II and III) showed no significant differences in comparison to the control group. Profile pilot diagram of S.Aureus shows colony count mean values in each group (Figure 2).
Figure 2 .
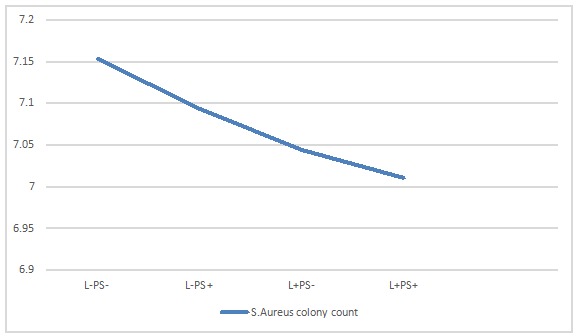
Mean value log 10 S.Aureus colony count , (I) L− PS− (no laser, no photosensitizer), (II) L−PS+ (treated only with PS), (III) L+ PS−(treated only with laser) and (IV) L+ PS+ (treated with laser and PS: photodynamic therapy group)
For analysis of difference between E.Coli and S.Aureus in response to photodynamic therapy Independent t-test was performed. This test showed that colony count of S.Aureus was significantly reduced compared to E.Coli (between group II and group IV) (P<0.0001).
Discussion
The ability of Radachlorin® to act as a photosensitizer after irradiation with laser photons has been demonstrated in a few studies but several studies were done on chlorin e6, which is a major component of Radachlorin® 8- 12. In the present study, we examined the antimicrobial effect of Radachlorin® mediated PDT against S.aureus and E.coli. Statistical analysis showed that Radachlorin® mediated PDT is very effective in inhibiting the growth of S. aureus. Bactericidal activity of an antimicrobial agents means >3 log10 reduction of bacterial counts and Bacteristatic activity of one antimicrobial agents means <3 log10 reduction of bacterial counts 16. According to this study Radachlorin® has Bactericidal effect on S.aureus (6.28 log 10) and Bacteristatic effect on E.coli (0.14 log 10). Several studies showed that Gram negative bacteria are largely resistant to antimicrobial photodynamic therapy due to their special cell wall structure 17,18. Park et al reported pure chlorin e6 mediated PDT also nearly inhibited the colony formation of S. aureus and P.aeruginosa, and partially inhibited that of E. coli and S .Typhimurium 19. Fomichev et al. reported the effectiveness of E.coli photoinactivation in the presence of chlorines was 100-200 times lower as compared with that of B. subtilis20. Hope et al. reported that The SnCe6/Phi11 conjugate achieved a statistically significant reduction in the number of viable bacteria of both Staphylococcus aureus NCTC 8325-4 and EMRSA-16 strains by 2.31 log (10) and 2.63 log(10), respectively. The conjugate could not however instigate lethal photosensitization of Escherichia coli 21.
The advantages of antimicrobial photodynamic therapy over usual antimicrobial agents are non-invasive nature, good selectivity, no resistance to drugs, rapid killing of target microorganisms in a few minutes depending on the energy densities delivered and antimicrobial effects of PS may be confined to the site of the lesion 22.
Although Radachlorin® mediated antimicrobial photodynamic therapy is very effective in inhibiting Gram-positive bacteria such as S.aureus, it is further necessary to design suitable strategies enhancing the permeability of the outer wall for PS in Gram negative bacteria such as E. coli.
Conclusion
Radachlorin® have Bactericidal effect on S.aureus (6.28 log 10) and Bacteristatic effect on E.coli (0.14 log 10).
Acknowledgments
Best gratitude for good consultation of Dr N.Kashef.
Disclosure Statement:
Support was provided by the AJA University of Medical Sciences (grant 689103).
Please cite this article as follows:
Fekrazad R, Zare H, Mohammadi Sepahvand S, Morsali P. The Effect of Antimicrobial Photodynamic Therapy with Radachlorin on Staphylococcus Aureus and Escherichia Coli: An in Vitro Study. J Lasers Med Sci 2014;5(2):82-5
References
- 1.Bootsma MC, Diekmann O, Bonten MJ. Controlling methicillin-resistant Staphylococcus aureus: quantifying the effects of interventions and rapid diagnostic testing. Proc Natl Acad Sci. 2006;103:5620–5. doi: 10.1073/pnas.0510077103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giamarellou H. Multidrug resistance in Gram-negative bacteria that produce extended spectrum beta-lactamases (ESBLs) Clin Microbiol Infect. 2005;11 Suppl 4:1–16. doi: 10.1111/j.1469-0691.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 3.Kang J, Sickbert-Bennett EE, Brown VM, Weber DJ, Rutala WA. Relative frequency of health care-associated pathogens by infection site at a university hospital from 1980 to 2008. Am J Infect Control. 2012;40:416–20. doi: 10.1016/j.ajic.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Underwood J, Klein JL, Newsholme W. Escherichia coli bacteraemia: how preventable is it? J Hosp Infect. 2011;79:364, 5. doi: 10.1016/j.jhin.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Liu ZZ, Xiong YL, Fan XJ, Gao YY, Yu RJ. The correlation between expression level of femB and resistance phenotype of methicillin-resistant Staphylococcus aureus (MRSA) Sichuan Da Xue Xue Bao Yi Xue Ban. 2011;42:661–5. [PubMed] [Google Scholar]
- 6.Ana P Castano, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one photosensitizers, photochemistry and cellular localization. Photodiagn Photodyn Ther. 2004;1:279–93. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tegos GP, Masago K, Aziz F, Higginbotham A, Stermitz FR, Hamblin MR. Inhibitors of bacterial multidrug efflux pumps potentiate antimicrobial photoinactivation. Antimicrob Agents Chemother. 2008;52:3202–9. doi: 10.1128/AAC.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drulis-Kawa Z, Bednarkiewicz A, Bugla-Ploskonska G, Strek W, Doroszkiewicz W. The susceptibility of anaerobic bacteria isolated from periodontal diseases to photodynamic inactivation with Fotolon (chlorin e6) Pol J Microbiol. 2005;54:305–10. [PubMed] [Google Scholar]
- 9.Gad F, Zahra T, Hasan T, Hamblin MR. Effects of growth phase and extracellular slime on photodynamic inactivation of gram-positive pathogenic bacteria. Antimicrob Agents Chemother. 2004;48:2173–8. doi: 10.1128/AAC.48.6.2173-2178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamblin MR, O'Donnell DA, Murthy N, Rajagopalan K, Michaud N, Sherwood ME. et al. Polycationic photosensitizer conjugates: effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J Antimicrob Chemother. 2002;49:941–51. doi: 10.1093/jac/dkf053. [DOI] [PubMed] [Google Scholar]
- 11.Jeong H, Huh M, Lee SJ, Koo H, Kwon IC, Jeong SY. et al. Photosensitizer-conjugated human serum albumin nanoparticles for effective photodynamic therapy. Theranostics. 2011;1:230–9. doi: 10.7150/thno/v01p0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang HM, Hamblin MR, Yow CM. A comparative in vitro photoinactivation study of clinical isolates of multidrug-resistant pathogens. J Infect Chemother. 2007;13:87–91. doi: 10.1007/s10156-006-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Privalov VA, Seliverstov OV, Revel'-Muroz Z, Lappa AV, Demidov AK, Faĭzrakhmanov AB. Percutaneous laser-induced thermotherapy of nodular goiter. Khirurgiia (Mosk) 2001;1:10–3. [PubMed] [Google Scholar]
- 14.Fekrazad R, Bargrizan M, Sajadi S. Evaluation of the effect of photoactivated disinfection with Radachlorin (R) against Streptococcus mutans (an in vitro study) Photodiagn Photodyn Ther. 2011;8:249–53. doi: 10.1016/j.pdpdt.2011.03.337. [DOI] [PubMed] [Google Scholar]
- 15.Vahabi S, Fekrazad R, Ayremlou S, Taheri S, Zangeneh N. The effect of antimicrobial photodynamic therapy with radachlorin and toluidine blue on streptococcus mutans: an in vitro study. J Dent Tehran. 2011;8:48–54. [PMC free article] [PubMed] [Google Scholar]
- 16.Stegemann MR, Sherington J, Blanchflower S. Pharmacokinetics and pharmacodynamics of cefovecin in dogs. J Vet Pharmacol Ther. 2006;29:501–11. doi: 10.1111/j.1365-2885.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 17.Malik Z, Hanania J, Nitzan Y. Bactericidal effects of photoactivated porphyrins--an alternative approach to antimicrobial drugs. J Photochem Photobiol. 1990;5:281–93. doi: 10.1016/1011-1344(90)85044-w. [DOI] [PubMed] [Google Scholar]
- 18.Bertoloni G, Salvato B, Dall'Acqua M, Vazzoler M, Jori G. Hematoporphyrin-sensitized photoinactivation of Streptococcus faecalis. Photochem Photobiol. 1984;39:811–6. doi: 10.1111/j.1751-1097.1984.tb08864.x. [DOI] [PubMed] [Google Scholar]
- 19.Park JH, Moon YH, Bang IS, Kim YC, Kim SA, Ahn SG. et al. Antimicrobial effect of photodynamic therapy using a highly pure chlorin e6. Lasers Med Sci. 2010;25:705–10. doi: 10.1007/s10103-010-0781-1. [DOI] [PubMed] [Google Scholar]
- 20.Fomichev AI, Zorin VP, Zorina TE, Cherenkevich SN. Photodamage of gram-positive and gram-negative bacterial cells in the presence of chlorin e6 derivatives. Mikrobiologiia. 1991;60:507–11. [PubMed] [Google Scholar]
- 21.Hope CK, Packer S, Wilson M, Nair SP. The inability of a bacteriophage to infect Staphylococcus aureus does not prevent it from specifically delivering a photosensitizer to the bacterium enabling its lethal photosensitization. J Antimicrob Chemother. 2009;64:59–61. doi: 10.1093/jac/dkp157. [DOI] [PubMed] [Google Scholar]
- 22.Luan XL, Qin YL, Bi LJ, Hu CY, Zhang ZG, Lin J. et al. Histological evaluation of the safety of toluidine blue-mediated photosensitization to periodontal tissues in mice. Lasers Med Sci. 2009;24:162–6. doi: 10.1007/s10103-007-0513-3. [DOI] [PubMed] [Google Scholar]


