Abstract
Introduction: An attack of acute myocardial infarction (MI) poses the threat of great damage to cardiac tissue. Operative therapeutic modalities such as coronary artery bypass grafting (CABG) may enhance myocardial perfusion in high-grade coronary vasculature occlusions. It has been shown previously that Low-Level Laser Therapy (LLLT) significantly reduces infarct size following induction of myocardial infarction in rats and dogs. The aim of this study was to investigate the effects of LLLT on cardiac tissue healing markers following grafting operations for coronary vessel occlusion.
Methods: Thirty-two cases having each two or three coronary vessel occlusions (2VD/3VD) underwent low-level laser therapy post-CABG, and 28 patients who did not undergo laser therapy were studied as a control group. Diode laser (810 nm, 500 mW) was used as LLLT protocol for 3 successive days post-CABG. Repeated measurements of blood cell count (CBC) and cardiac damage markers (CPK, CPK-MB, LDH) attained before CABG and during the 5 days of LLLT post-operatively, taken at one and 12 hours after daily laser irradiation.
Results: In a comparison of the mean levels of the control and laser group, the variables were statistically different on 5th day after intervention for WBC, Neutrophil and Lymphocyte counts and WBC and lymphocyte changes. A statistically significant difference was seen in changes of CPK, CPK-mb and LDH over time P<0.001.
Conclusion: It is concluded that low-level laser irradiation after CABG surgery could decrease cardiac cellular damage and help accelerate the repair of cardiac tissue post-operatively. This may lower post-operative disability as well as bed rest period in these patients.
Keywords: low level laser therapy, heart disease, coronary artery bypass
Introduction
Coronary artery bypass grafting (CABG), which has become the standard operative treatment of ischemic heart disease was first established by Carrel1 . Since the first successful open-heart surgery carried out by John Lewis in humans in 19532 , many techniques have been used to improve surgery prognosis and reduce its side effects.
Laser biostimulation or Low Level Laser Therapy (LLLT) has been applied in medicine for more than 30 years. It may directly affect the intracellular mechanisms and activation of heat-independent tissue repair processes without causing any tissue damage3. Studies have shown that laser therapy may be effective in a variety of complications, including lymphoedema and muscular trauma, and this therapeutic modality is now approved by the Food and Drug Administration for the treatment of carpal tunnel syndrome4 .
Laser light can increase mitochondrial respiration and ATP synthesis5 , decrease inflammatory response after injury, elevate neoformation of blood vessels in the injured area, and accelerate wound healing and skeletal muscle regeneration after injury6. The stimulatory effects of LLLT on wound healing cells such as fibroblasts have been shown in vitro and in vivo7-10.
Although in vivo studies have mainly focused on accessible tissues like skin, the ability of infrared wavelengths to penetrate deeper tissues11,12 have been reported. Tran cranial laser irradiation can stimulate angiogenesis, transforming growth factor signaling pathways and protein synthesis in the brain13-15. Similarities between cardiac and cerebral ischemia, further the possibility of transdermal cardiac treatment. Studies have shown that laser treatment has been able to reduce lesion size in rats’ hearts following myocardial infarction16-18. A further study has demonstrated enhancement of protein synthesis in skeletal muscle myoblasts following laser therapy19,20.
Although there are limited studies with respect to the effects of LLLT on cardiac cells and it is controversial and has no established mechanism of action, nevertheless, as a noninvasive, nonpharmacologic cardiac treatment, it would appear worthy of investigation. We therefore decided to investigate the effects of LLLT on cardiac tissue healing markers following CABG.
Methods
Sixty-two patients having each two or three coronary vessel occlusions (2VD/3VD) and who were candidates for CABG in the Heart Surgery Department of Milad Hospital were selected. Patients with pregnancy, epilepsy, photosensitivity or pacemakers were excluded, because of laser contraindications21. Diabetic patients (because of the negative effect of diabetes on wound healing) were also excluded. After completing informed consent forms, the patients were randomly divided into case and control groups. Demographic differences between groups such as COPD and smoking history were the same. The patients’ conditions, including surgery team, method of surgery and nursing services were identical. Blood cell count (CBC) and cardiac damage markers (CPK, CPK-MB, and LDH) were measured before CABG as routine. Thirty-two cases (selected randomly) underwent low-level laser therapy post CABG and 28 patients were allocated to the control group and received standard treatment without laser. We used a 810 nm, 500 mW gallium aluminum arsenide (GaAlAs) diode laser with 1cm2 surface area (from RJ, physiolaser-olympic, Germany), 6 J/cm2, continuous mode, for 3 successive days post-CABG (immediately after surgery and subsequent 2 days). The laser probe was placed in direct contact with the skin. To avoid contamination, the laser probe was placed in a very thin transparent sterile plastic cover. The laser was irradiated over the surgery incision (mid sternum) every 1 cm and over the pericardium (left parasternal 2nd, 3rdintercostal spaces and apex). Blood cell count (CBC) and cardiac damage markers (CPK, CPK-MB, LDH) were measured before CABG, at one and 12 hours after daily laser irradiation and finally 5 days post-operatively (before patients were discharged from hospital). In the control group (28 patients with two or three coronary vessel occlusions) the same blood sampling was carried out (which was routine sampling for all operative patients in the heart surgery department). All values are expressed as means ±SD. SPSS (version 17.0, Chicago, USA) was used for the analyses. Data were analyzed by t test to compare the quantitative variables between control and laser group. Repeated measures ANOVA, followed by Bonferroni test was used for pair comparisons. Differences were considered statistically significant at P<0.05.
Results
Sixty patients, 32 in the laser group and 28 in the control group, of whom 46 (76.7%) were male and 14 (23.3%) were female, with equal distribution of sex in the two groups, were recruited for this study. Mean (SD) for age was 57.51(13.82) years. In comparing the mean of the control and laser group, the variables were statistically different before intervention only for lymphocyte count, and were statistically different at 5th day after intervention for WBC, Neutrophil and Lymphocyte counts as well as WBC and lymphocyte changes (after minus before). Mean and SD for comparison of the two groups for cardiac enzymes and blood cells counts are exhibited in Table 1 .
Table 1 . Mean SD and P value of variables.
| Variables | Group |
Pre intervention Mean (SD)v |
P value |
After intervention (5th day after intervention) Mean(SD) |
P value |
| CPK | laser | 156.53(116.21) | 0.98 | 524.87(326.6) | .85 |
| control | 157.39(198.79) | 503.4(445.36) | |||
| CPK-mb | laser | 25.75(19.71) | 0.39 | 21(7.9) | .35 |
| control | 21.98(12.41) | 23.07(8.24) | |||
| LDH | laser | 475.21(139.01) | 0.55 | 622.64(185.5) | .7 |
| control | 496.1(109.64) | 600.57(169.8) | |||
| WBC | laser | 8.43(4.28) | 0.351 | 9.94(3.27) | 0.003 |
| control | 7.55(2.54) | 12.77(3.64) | |||
| WBC changes* | laser | 1.9(3.06) | <0.001 | ||
| control | 5.21(3.39) | ||||
| Neutrophil | laser | 83.73(87.62) | 0.231 | 70.62(9.41) | 0.001 |
| control | 63.42(13.99) | 78.35(7.23) | |||
| Lymphocyte | laser | 11.5(7.91) | 0.001 | 22.93(8.09) | 0.01 |
| control | 30.92(12.98) | 17.53(7.51) | |||
| Lymphocyte changes | laser | 12.67(8.47) | <0.001 | ||
| control | -13.39(11.83) |
*changes = after-before
In addition, Table 2 presents the results of repeated measures analysis of variance for changes of cardiac enzymes in multiple measurements and compares these changes in the two groups. A statistically significant difference was seen in changes of CPK, CPK-MB and LDH over time P<0.001.
Table 2. Results of repeated measures ANOVA for cardiac enzymes .
| Variable | Changes over time | Comparison between groups |
| CPK | F(5,160)=16.78 P<0.001 |
F(1,32)=0.31 P=0.58 |
| CPK-MB | F(5,205)=15.87 P<0.001 |
F(1,41)=1.72 P=0.19 |
| LDH | F(5,75)=5.78 P<0.001 |
F(1,15)=0.22 P=0.65 |
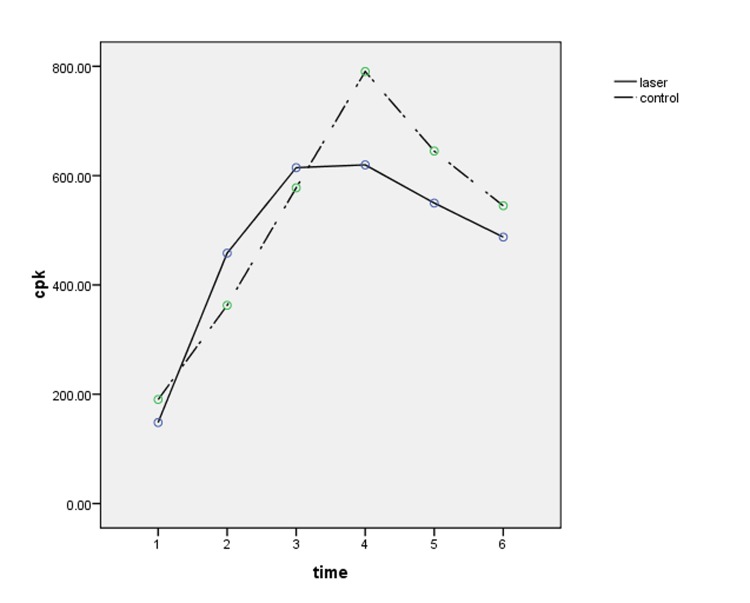
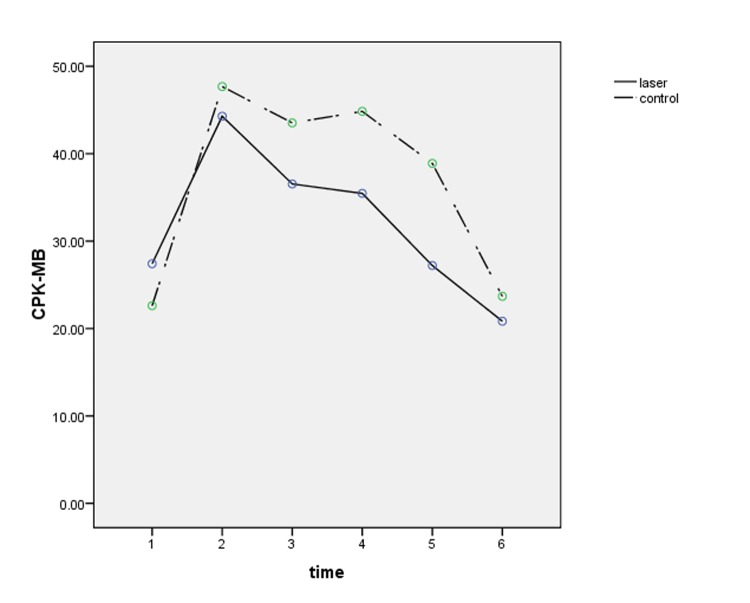
Figures number 1 to 3 present changes of cardiac enzymes.
Figure 1 .
CPK changes
Figure 3 .
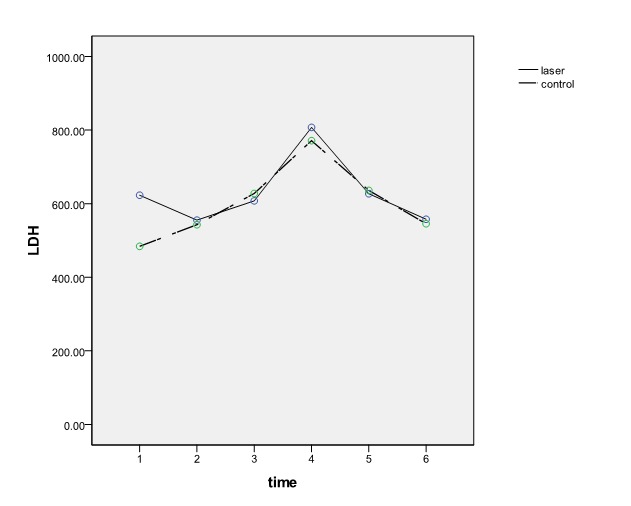
LDH changes
Figure 2 .
CPK-MB changes
Discussion
The results of this study demonstrate an increase of WBC count in both groups. But in the control group, this was significantly higher than in the laser group. Lymphocyte changes were not in the same direction in the two groups. Lymphocyte counts decreased in the control group, but increased in the laser group, although significant difference of lymphocyte count before intervention is not a confounder for significant difference of this variable after intervention.
According to the results of Table 2 , changes of cardiac enzymes in the laser group were significant over time, but comparison between groups was not significant. This non-significant difference between the groups may be the result of low sample size. We propose a similar study with a larger sample size to evaluate the effects of this type of laser in cardiac enzymes after cardiac surgery.
There are numerous reports of the use of LLLT in various medical fields, although controversial issues are published. The main interest of scientific research is its effect on intracellular processes, their excitation pathways, and the possibility of laser tissue interactions22-24.
Studies mostly performed on animal models and in vitro, show anti-inflammatory effects, microcirculation improvement, cardioprotective effects with respect to reducing infarct area after artificially induced myocardial infarction (MI) and significant influence on postinfarction myocardial remodeling17,18. These mechanisms may have an important impact on post operation status and prognosis in patients following CABG.
Whittaker et al occluded left coronary arteries in rats and evaluated the effect of LLLT in ventricular remodeling post acute myocardial infarction. This study demonstrated that laser irradiation attenuated infarct-associated remodeling. The effect of lasers on intramyocardial blood vessels in the infarcted area was also studied. In the laser treated group, lumen area was significantly larger than in the control group, which suggests better blood supply in this group25.Oron et al indicated that LLLT causes a noticeable reduction in scar formation after induction of MI in dogs. By electron microscopic examination in ischemic zone, 4 hours after Left Anterior Descending (LAD) occlusion, they noted a 60% reduction in the number of damaged mitochondria and therefore a much higher level of ATP in the laser treated group, compared with the non-laser group.
They concluded that the rate of degeneration in the injured laser-irradiated cells is probably much slower, because of an increase in ATP production in this group. The decrease in the number of injured cardiomyocytes may also significantly reduce the inflammatory response after LAD occlusion in the myocardium, as Bibikova et al demonstrated in their study on skeletal muscles after injury26. They additionally reported a significantly lower mortality rate after LAD occlusion in the laser treated dogs. Release of troponin T from the myocardium into blood was also significantly reduced. They reported a correlation between troponin-T release and infarct size in the laser group. Under ischemic conditions, cells generate and accumulate reactive oxygen species, which take part in the degenerative process. This study demonstrated that catalase, as a major enzyme in the antioxidative process of cells, was significantly elevated in the serum of the laser treated dogs 24 hours after LAD occlusion, compared with the non-laser group, which may show a possible link between laser and heat-shock proteins and their cardioprotective effect in ischemic conditions 18,27,28. Zhu et al demonstrated that argon dye laser (660-nm wavelength) can improve the functional recovery of cold-stored, isolated, rat cardiomyocytes29.
In a study by Ad et al, Low energy level irradiation (LLLI) of the infarcted area in the myocardium of experimentally induced MI rats significantly reduced the loss of myocardial tissue when compared to non irradiated rats. They concluded that this phenomenon may have an important beneficial effect on patients following MI or ischemic heart disease16. The early studies on LLLT performed by Kipshidze et al in patients suffering from MI and treated with intravascular irradiation, resulted in a reduction of ischaemic area and incidence of cardiac arrhythmia, and decreased levels of myocardial necrosis markers30,31.
In a further study they used intravenous laser to prevent post endovascular restenosis.
This method was highly effective in preventing neointima formation32-34. They suggested that laser may accelerate reendothelialization and increase VEGF production. External irradiation of patients with ischaemic heart disease was also effective, resulting in reduced angina complaints, inhibited lipid peroxidation processes35, improvement in blood rheology, and an increase in the stability of erythrocytes. Rechciński et al evaluated the effect of transdermal LLLT on tissue repair processes in patients with advanced coronary artery disease. They reported improvement of functional capacity and less frequent angina symptoms during exercise tests with no significant change in left ventricular function.
The LLLT effects may be either local or systemic, thus not only the sites of the direct influence of laser radiation are involved36.
Although our study indicated that laser therapy may accelerate wound healing in cardiomyocyte tissue following CABG, additional clinical studies and follow up are required to evaluate the long term effects, in particular its regulatory effect on gene translation and protein synthesis37.
Conclusion
We have shown that transdermal LLLT effectively reduces cardiac damage markers if initiated a few hours following CABG. Overall, our study indicates that LLLT is a promising candidate for development prognosis following CABG.
Small sample size was one of the limitations of our study. It would have been more effective if we could have compared basic clinical characteristics including different patient status (stable angina, unstable angina, Acute MI (AMI) and old MI (OMI)), heart function, risk factors of coronary artery disease, coronary artery disease history, severity of vessels (total occlusion or stenosis) between the LLLT group and control group, as these clinical characteristics may affect outcomes, but this was not possible as it would have resulted in very small subgroups.
Further studies are recommended to investigate the effect of LLLT on cardiac function and its rehabilitation post surgery.
Acknowledgment
We would like to acknowledge the support of Bahsaz Laser Instruments Co. Ltd, Department of Heart Surgery at Milad hospital, L. Habibi, S. Talebi and Y. Jahangiri Noudeh.
Funding: There has been no funding to declare.
Competing interests: None declared.
Please cite this article as follows:
Kazemi Khoo N, Babazadeh K, Lajevardi M, Hashem Dabaghian F, Mostafavi E. Application of Low-Level Laser Therapy Following Coronary Artery Bypass Grafting (CABG) Surgery. J Lasers Med Sci 2014;5(2):86-91
References
- 1.Carrel A. Carrel AVIIIOn the Experimental Surgery of the Thoracic Aorta and Heart. Ann Surg. 1910;52:83. doi: 10.1097/00000658-191007000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis F, Taufic M. Closure of atrial septal defects with the aid of hypothermia; experimental accomplishments and the report of one successful case. Surgery. 1953;33:52. [PubMed] [Google Scholar]
- 3.Zyci ski P, Krzemi ska-Paku a M, Peszy ski-Drews C, Kierus A, Trzos E, Rechci ski T. et al. Laser biostimulation in end-stage multivessel coronary artery disease- a preliminary observational study. Kardiol Pol. 2007;65(1):13–21. [PubMed] [Google Scholar]
- 4.Backman C, Fridénl J, Widmark A. Blood flow in chronic Achilles tendinosis: Radioactive microsphere study in rabbits. Acta Orthopaedica. 1991;62:386–7. doi: 10.3109/17453679108994477. [DOI] [PubMed] [Google Scholar]
- 5.Yu W, Naim JO, McGowan M, Ippolito K, Lanzafame RJ. Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem Photobiol. 1997;66:866–71. doi: 10.1111/j.1751-1097.1997.tb03239.x. [DOI] [PubMed] [Google Scholar]
- 6.Bibikova A, Belkin V, Oron U. Enhancement of angiogenesis in regenerating gastrocnemius muscle of the toad (Bufo viridis) by low-energy laser irradiation. Anat Embryol. 1994;190:597–602. doi: 10.1007/BF00190110. [DOI] [PubMed] [Google Scholar]
- 7.Webb C, Dyson M, Lewis W. Stimulatory effect of 660 nm low level laser energy on hypertrophic scar derived fibroblasts: possible mechanisms for increase in cell counts. Lasers Surg Med. 1998;22:294–301. doi: 10.1002/(sici)1096-9101(1998)22:5<294::aid-lsm6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Grossman N, Schneid N, Reuveni H, Halevy S, Lubart R. 780 nm low power diode laser irradiation stimulates proliferation of keratinocyte cultures: involvement of reactive oxygen species. Lasers Surg Med. 1998;22:212–8. doi: 10.1002/(sici)1096-9101(1998)22:4<212::aid-lsm5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 9.Calatrava IR, Valenzuela JMS, Gomez-Villamandos R, Redondo JI, Gomez-Villamandos JC, Jurado IA. Histological and clinical responses of articular cartilage to low-level laser therapy: Experimental study. Laser Med Sci. 1997;12:117–21. [Google Scholar]
- 10.Reddy GK, Stehno Bittel L, Enwemeka CS. Laser photostimulation of collagen production in healing rabbit Achilles tendons. Lasers Surg Med. 1998;22:281–7. doi: 10.1002/(sici)1096-9101(1998)22:5<281::aid-lsm4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 11.Welch A, Torres JH, Cheong WF. Laser physics and laser-tissue interaction. Tex Heart Inst J. 1989;16:141. [PMC free article] [PubMed] [Google Scholar]
- 12.Vargas G, Chan EK, Barton JK, Rylander HG 3rd, Welch AJ. Use of an agent to reduce scattering in skin. Lasers Surg Med. 1999;24:133–41. doi: 10.1002/(sici)1096-9101(1999)24:2<133::aid-lsm9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Lapchak PA, Wei J, Zivin JA. Transcranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbits. Stroke. 2004;35:1985. doi: 10.1161/01.STR.0000131808.69640.b7. [DOI] [PubMed] [Google Scholar]
- 14.Maulik N. Redox regulation of vascular angiogenesis. Antioxid Redox Signal. 2002;4:783–4. doi: 10.1089/152308602760598927. [DOI] [PubMed] [Google Scholar]
- 15.Leung MCP, Lo SCL, Siu FKW, So KF. Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up regulates the expression of transforming growth factor beta 1. Lasers Surg Med. 2002;31:283–8. doi: 10.1002/lsm.10096. [DOI] [PubMed] [Google Scholar]
- 16.Ad N, Oron U. Impact of low level laser irradiation on infarct size in the rat following myocardial infarction. Int J Cardiol. 2001;80:109–16. doi: 10.1016/s0167-5273(01)00503-4. [DOI] [PubMed] [Google Scholar]
- 17.Yaakobi T, Shoshany Y, Levkovitz S, Rubin O, Ben Haim SA, Oron U. Long-term effect of low energy laser irradiation on infarction and reperfusion injury in the rat heart. J Appl Physiol. 2001;90:2411. doi: 10.1152/jappl.2001.90.6.2411. [DOI] [PubMed] [Google Scholar]
- 18.Oron U, Yaakobi T, Oron A, Mordechovitz D, Shofti R, Hayam G. et al. Low-energy laser irradiation reduces formation of scar tissue after myocardial infarction in rats and dogs. Circulation. 2001;103:296–301. doi: 10.1161/01.cir.103.2.296. [DOI] [PubMed] [Google Scholar]
- 19.Shefer G, Barash I, Oron U, Halevy O. Low-energy laser irradiation enhances de novo protein synthesis via its effects on translation-regulatory proteins in skeletal muscle myoblasts. Biochim Biophys Acta. 2003;1593:131–9. doi: 10.1016/s0167-4889(02)00350-6. [DOI] [PubMed] [Google Scholar]
- 20.Cambier DC, Vanderstraeten GG, Mussen MJ, van der Spank JT. Low-power laser and healing of burns: a preliminary assay. Plast Reconstr Surg. 1996;97:555. doi: 10.1097/00006534-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins D, Houreld N, Abrahamse H. Low level laser therapy (LLLT) as an effective therapeutic modality for delayed wound healing. ANYAS. 2005;1056:486–93. doi: 10.1196/annals.1352.040. [DOI] [PubMed] [Google Scholar]
- 22.Derkacz A. Derkacz ALaser therapy application in invasive cardiologyCurrent state and future trends] PrzeglaĚ § d lekarski. 2004;61:35. [PubMed] [Google Scholar]
- 23.Karu T. Low-power laser therapy. Biomedical photonics handbook. 2003;3:1–25. [Google Scholar]
- 24.Karu T. Cellular mechanism of low power laser therapy: new questions. Lasers Med Dent. :79–100. [Google Scholar]
- 25.Whittaker P, Patterson MJ. Ventricular remodeling after acute myocardial infarction: Effect of low intensity laser irradiation. Lasers Surg Med. 2000;27:29–38. doi: 10.1002/1096-9101(2000)27:1<29::aid-lsm4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Bibikova A, Oron U. Promotion of muscle regeneration in the toad (Bufo viridis) gastrocnemius muscle by low energy laser irradiation. Anat Rec. 1993;235:374–80. doi: 10.1002/ar.1092350306. [DOI] [PubMed] [Google Scholar]
- 27.Currie RW, Karmazyn M, Kloc M, Mailer K. Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res. 1988;63:543–9. doi: 10.1161/01.res.63.3.543. [DOI] [PubMed] [Google Scholar]
- 28.Yue TL, Ma XL, Wang X, Romanic AM, Liu GL, Louden C. et al. Possible involvement of stress-activated protein kinase signaling pathway and Fas receptor expression in prevention of ischemia/reperfusion-induced cardiomyocyte apoptosis by carvedilol. Circ Res. 1998;82:166. doi: 10.1161/01.res.82.2.166. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Q, Yu W, Yang X, Hicks GL, Lanzafame RJ, Wang T. Photo irradiation improved functional preservation of the isolated rat heart. Lasers Surg Med. 1997;20:332–9. doi: 10.1002/(sici)1096-9101(1997)20:3<332::aid-lsm12>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 30.Kipshidze N, Chapidze G, Bokhua M, Marsagishvili LA, Dolidze NK, Eristavi DG. Intravascular laser therapy of acute myocardial infarction. Angiology. 1990;41:801–8. [PubMed] [Google Scholar]
- 31.Kipshidze N, Chapidze G, Marsagishvili L, Bokhua MR, Salukhvadze NS, Dolidze NK. Effect of intravascular irradiation of blood by helium-neon laser on the markers of the area of ischemic myocardial damage in patients with acute myocardial infarction. Kardiologiia. 1990;30:44. [PubMed] [Google Scholar]
- 32.Kipshidze N, Sahota H, Wolinsky H, Komorowski R, Boer- boom LE, Keane SD. Photoremodeling of the atherosclerotic arterial wall inhibits myointimal hyperplasia following balloon angioplasty. Circulation. 1994;90:332. [Google Scholar]
- 33.Kipshidze N, Nikolaychik V, Keelan MH, Shankar LR, Khanna A, Kornowski R. et al. Low power helium: Neon laser irradiation enhances production of vascular endothelial growth factor and promotes growth of endothelial cells in vitro. Lasers Surg Med. 2001;28:355–64. doi: 10.1002/lsm.1062. [DOI] [PubMed] [Google Scholar]
- 34.De Scheerder IK, Wang K, Kaul U, Singh B, Sahota H, Keelan MH. et al. Intravascular low power laser irradiation after coronary stenting: Long term follow up. Lasers Surg Med. 2001;28:212–5. doi: 10.1002/lsm.1040. [DOI] [PubMed] [Google Scholar]
- 35. Vasil'ev A, Strel'tsova N, Senatorov IN. Efficacy of laser therapy in patients with ischemic heart disease]. Voprosy kurortologii, fizioterapii, i lechebnoÄ fizicheskoÄ kultury; 10. [PubMed]
- 36.Zyciński P, Krzemińska-Pakuła M, Peszyński-Drews C, Kierus A, Trzos E, Rechciński T. et al. Laser biostimulation in end-stage multivessel coronary artery disease--a preliminary observational study. Kardiol Pol. 2007;65:13. [PubMed] [Google Scholar]
- 37. Karu T. The science of low-power laser therapy: Gordon and Breach Science Publishers. 1998.