Abstract
Introduction: Intestinal ischemia and reperfusion (i-I/R) is an insult associated with acute respiratory distress syndrome (ARDS). Herein we evaluate the dose-response effect of low-level laser therapy (LLLT) on lung inflammation induced by i-I/R.
Methods: Mice were subjected to mesenteric artery occlusion (45 min) and killed after clamp release and intestinal reperfusion (2h). Increasing doses (1, 3, 5 and 7,5 J/cm2) of laser irradiation (660 nm) was carried out on the mice skin over the upper bronchus for 5 min after initiating reperfusion. Neutrophils activation was determined by myeloperoxidase (MPO) activity. The mRNA expression and protein concentration of inflammatory mediators IL-1β, IL-6, TNF and IL-10 in lung were measured by RT-PCR and ELISA, respectively.
Results: With exception of 1J/cm2, LLLT reduced MPO activity as well as IL-1β levels in the lungs from inflamed mice. LLLT was also markedly effective in reducing both IL-6 and TNF expression and levels in the lungs from mice submitted to i-I/R in all laser doses studied. Otherwise, LLLT significantly increased the protein levels of IL-10 in inflamed mice by i-I/R; however only in the dose of 1J/cm2.
Conclusion: We conclude that the LLLT is able to control the neutrophils activation and proinflammatorycytokines release into the lungs in a model of i-I/R in mice.
Keywords: respiratory distress syndrome, Acute, inflammatory mediators, laser therapy, doseresponse, mice
Introduction
Intestinal ischemia/reperfusion (i-I/R) is associated with induction of systemic inflammatory response, beyond to present a high prevalence of pulmonary effects, a fact that may indicate a causal link between mediators released during systemic inflammation and the pulmonary dysfunction in acute respiratory distress syndrome (ARDS) 1. It was shown that neutrophilendothelial cell adhesion with consequent neutrophil accumulation and increasing in vascular permeability might be a rate?limiting step in the pathogenesis of lung injury induced by intestinal ischemia/reperfusion (i-I/R) 2,3.
There is a marked intestinal and lung inflammation characterized by increased vascular permeability, neutrophil influx into the tissues with exacerbated pro-inflammatory cytokines production. These inflammatory events are accompanied by a high lethality, which is remarkably associated with the elevated concentration of TNF and IL- 1β 4,5. Several studies have shown the ability of IL-1β for inducing the production of TNF beside cooperates with TNF effects during acute and chronic inflammation 6,7. Moreover, some authors showed that IL-1β has a pivotal role in the inflammatory lesions following i-I/R8,9. Souza et al. showed that neutralization and/or antagonism of IL-1β were associated with a marked prevention of the reperfusion injury as well as induction of IL-10 anti-inflammatory cytokine10. Some authors have demonstrated that IL-10 attenuates the pro-inflammatory cytokine production and tissue injury following ischemia and reperfusion injury6.
Low-level laser therapy (LLLT) has been used for the treatment of several inflammatory pathologies 11,12 as well as in experimental models of acute and chronic inflammation 13,14. Some reports have referred that laser therapy can interfere positively in order to relieve the clinical signals and the late and early symptoms of lung inflammation 15,16. Some authors are focused in which cellular signalling is responsible for the anti-inflammatory effects of LLLT in lung and airways disorders. Mafra de Lima et al. showed that laser irradiation reduces both the cholinergic hyper-reactivity and β2-adrenoceptor hypo-responsiveness induced by TNF17. In another study, we showed that LLLT acts as cAMP-elevating agent similarly to PDE inhibitor (rolipram) in a model of ARDS in rats 18.
Regarding the i-I/R model of ARDS, we have found a dual effect of LLLT on the acute lung inflammation with marked drop of IL-1β level at the same time of increasing in the IL-10 concentration19. Furthermore, we also demonstrated that LLLT restores the oxidative stress balance in acute lung injury induced by gut ischemia and reperfusion 18
Since that several studies demonstrate that LLLT presents beneficial effects in clinical trials for treatment of allergic lung disease as well as in experimental model of acute lung inflammation, and considering the lack of studies investigating the effects of different doses of laser in lung diseases, the present study was designed to investigate the effects of 1, 3, 5 and 7,5 J/cm2 of 660nm laser on the lung inflammatory response in a model of ARDS induced by intestinal ischemia and reperfusion in mice.
Methods
Animals
C57/Bl6 mice (n = 28 animals) were randomly allocated into 4 groups. All animal care was in accordance with the guidelines of the Nove de Julho University for animal care. The experiments were carried out on female mouse weighting 20-22 g each, maintained under standard conditions of temperature (22-250C), relative humidity (40-60%) and light/dark cycle with access to food and water ad libitum. The animals were provided by the Central Animal House of the Nove de Julho University. All mice were placed in a common box and divided randomly into 4 groups of seven animals (n=7) each.
Intestinal Ischemia/Reperfusion (I-I/R) Mouse Model
Mice were pre-anaesthetized with acepromazine (0.1 mg.kg-1) and anesthetized with chloridrate of zolazepam (0.1 mg.kg-1) + Tiletamine Chloridrate (0.1 mg.kg-1). Laparotomy was done under anaesthesia and then the mice were submitted to occlusion of superior mesenteric artery with a microsurgical clip (Vascu-statt no 1001-531; Scalan International, St. Paul, MN, USA) during 45 min, as described by Cavriani et al.3 After the occlusion period, the clip was removed and the intestinal perfusion was re-established. The animals were sacrificed under deep anaesthesia 2 hours after reperfusion by exsanguination via abdominal aorta.
Laser Irradiation
A 660 nm laser diode (MM Optics, CW diode laser, São Carlos, SP) with an output power of 30 mW and 0.08 cm2 of spot size was employed. The optical power was calibrated utilizing a Newport Multifunction Optical Meter model 1835C. The stability of laser during the laser irradiation was measured collecting light with a partially reflecting surface (4 %).The dose of laser irradiation was (1, 3, 5, 7.5 J/cm2), applied punctually at half an hour after the beginning of reperfusion. All animals received laser punctually on the skin in direction of the bronchus.
Lung Myeloperoxidase (MPO) Activity
MPO was measured as an index of the neutrophils’ presence and activation. Lung tissue samples were obtained from mice euthanized 2 hours after intestinal reperfusion. The lungs were perfused using phosphate-buffered saline (PBS) containing 5 IU/mL-heparin, pH 7.0. . Briefly, to normalize the pulmonary MPO activity among the groups, the whole lungs was homogenized with 3 mL/g PBS containing 0.5 % of hexadecyltrimethylammonium bromide and 5 mM EDTA, pH 6.0. The homogenized samples were sonicated (Vibra Cell, Sonics Materials, Newtown, CT) for 1 min and were then centrifuged at 37,000 g for 15 min. Samples of lung homogenates (20µL) were incubated for 15 min with H2O2 and ortodianisidine; the reaction was stopped by the addition of 1% NaNO3. Absorbance was determined at 460 nm using a microplate reader (Bio-Tek Instruments, Synergy™ H4, Winooski, VT).
Lung Tissue Sampling And Processing Mediators
After performing the bronchoalveolar lavage (BAL), the thoracic cavity was exposed and, the heart and lung were removed in bloc. The two major lung lobes were dissected, and the pulmonary vasculature of the lobes was perfused with ice-cold sterile phosphate buffer solution (PBS), using a peristaltic pump (Sellex, USA), aiming to remove the blood poll of cells. Then, lobes were cut into 5-mm pieces using a tissue chopper, flash frozen in liquid nitrogen and stored at -80oC for cytokines (IL-1β, IL-6, IL-10 and TNF) analysis through Enzyme Linked Immuno Sorbent Assay (ELISA), by using commercial ELISA kits from BD Biosciences, according to the manufacturer’ recommendations.. The detection limit of these assays was found to be in the range of 1-5 pg.ml-1. For lung tissue, cytokine levels were further corrected for protein content using the assay of Lowry. The protein data in lung tissue were expressed as pg.mg-1.
Messenger RNA (mRNA) Expression of Pro-Inflammatory and Anti-Inflammatory Mediators
At the final of reperfusion, the thoracic cavity of mice was exposed and, the heart and lung were removed in bloc. The pulmonary artery was cannulated and then the pulmonary vasculature was perfused with ice-cold sterile phosphate buffer solution (PBS) using a peristaltic pump (Thermo Fisher Scientific, Suwannee, GA) to remove the intravascular blood. Lung fragments were cut into 5-mm pieces using a tissue chopper, flash frozen in liquid nitrogen and stored at -80oC for Real Time-PCR (RT-PCR) analysis. For that, lung total RNA was isolated using TRIzol reagent (GibcoBRL, Gaithersburg, MD), according to the manufacturer´s protocol. RNA was subjected to DNase I digestion, followed by reverse transcription to cDNA. PCR was performed in a 7000 Sequence Detection System (ABI Prism, Applied Biosystems, Foster City, CA) using the SYBRGreencore reaction kit (Applied Biosystems). Primers used for IL-1β, IL-6, TNF and IL-10 mRNA quantification were IL-1β 195-305 (GenBankTMaccession number X66539) forward primer 5’-CTGGTTGGGAACAAGAAGGA-3’ and reverse primer 5’-CAAAAACCTCCCTCACTCCA-3’ (GenBankTMaccession number D00475), IL-6 532–610 (GenBankTM accession number E02522) forward primer 5'- TCCTACCCCAACTTCCAATGCTC-3' and reverse primer 5'- TTGGATGGTCTTGGTCCTTAGCC -3' (GenBankTM accession number M26745), TNF forward 5’-AAATGGGCTCCCTCTATCAGTTC-3’ and reverse primer 5’-TCTGCTTGGTGGTTTGCTACGAC-3’ (GenBankTM accession number D00475), IL-10 forward primer 5'- TGACAATAACTGCACCCACTT-3' and reverse primer 5' TCATTCATGGCCTTGTAGACA (GenBankTM accession number NW036214) and GAPDH primer 3474-3570 (GenBankTM accession number J00691) 5’-AAGTCCCTCACCCCTCCCAAAAG-3’ and primer reverse 5’- TCTGCTTGGTGGTTTGCTACGAC -3’ (GenBankTM accession number V01217)’ were used as control. Quantitative values for IL-1β, IL-6, TNF, IL-10 and GAPDH mRNA transcription were obtained from the threshold cycle number, where the increase in the signal growth of PCR products begins to be detected. Melting curves were generated at the end of every run to ensure product uniformity. The relative target gene expression level was normalized on the basis of GAPDH expression as endogenous RNA control. ΔCt values of the samples were determined by subtracting the average Ct value of IL-1β, IL-6, TNF and IL-10 mRNA from the average Ct value of the internal control GAPDH. Since it is uncommon to use ΔCt as a relative data due to the logarithmic characteristic, the 2-ΔCt parameter was used to express the relative expression data. Results are expressed as a ratio relative to the sum of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcript level as internal control.
Statistical Analysis
Statistical differences were evaluated by analysis of variance (ANOVA) and Tukey-Kramer Multiple Comparisons Test to determine differences between groups. The results were considered significant when p< 0.05.
Results
LLLT on Myeloperoxidase Activity
The Figure 1 illustrates neutrophils activation measured through MPO activity in the lungs for all mice of all experimental groups. It shows that i-I/R induces a marked increase in MPO content in lung homogenates in comparison with mice from basal group. Except for the dose of 1J/cm2, the rise in lung MPO activity was significantly attenuated after laser therapy at doses of 3, 5 and 7.5 J/cm2. Of note, laser irradiation on basal group did not affect MPO activity when compared with basal group non-irradiated.
Figure 1 .
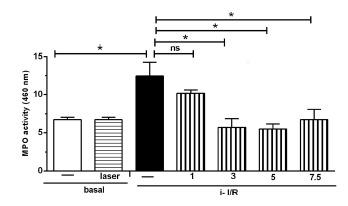
Effect of LLLT on MPO Content in Lung Tissue after i-I/R. Mice were treated with laser 1 hour after the beginning of i-I/R. MPO activity was measured in lung homogenates 2 hours after i-I/R to identify the neutrophils recruitment. Data expressed as mean ± SEM of 7 animals. The significant results when p < 0.05.
LLLT on Pro- and Anti- Inflammatory Mediators
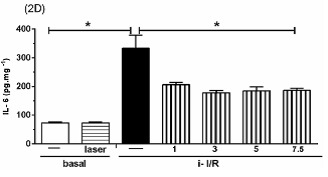
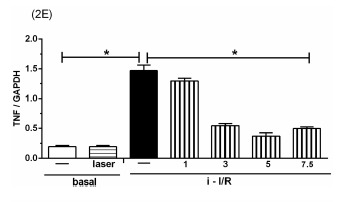
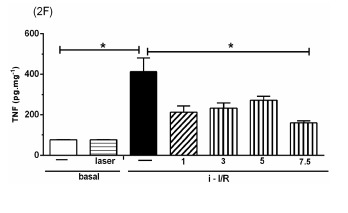
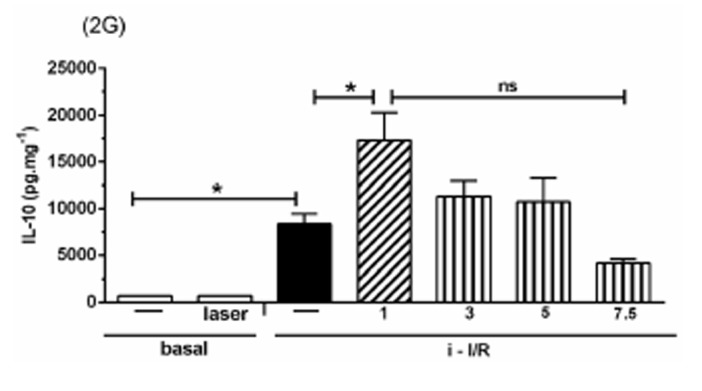
The Figure 2 shows the gene activation (2A, 2C, 2E) and protein concentration (2B, 2D, 2F, 2G) of pro-inflammatory mediators IL-1β, IL-6 and TNF as well as the anti-inflammatory mediator IL-10 in lung homogenate by assessing mRNA expression and ELISA technique, respectively. As shown in Figure 2 there was a marked increase in mRNA expression as well as in protein level of all inflammatory mediators studied in response to i-I/R in comparison with basal group. Otherwise, the LLLT reduced both the mRNA expression and the protein concentration of pro-inflammatory mediators in doses of 3, 5 and 7.5 J/cm2 evaluated 2 h after initial of reperfusion. Except for the dose of 1 J/cm2, the LLLT was not effective in reducing the IL-1β protein level in lung from mice submitted to i-I/R. On the contrary, the dose of 1 J/cm2 provoked a profound reduction in mRNA expression for IL-1β. The laser effect on the IL-1β mRNA expression in lung from inflamed mice presented a dose-dependent response. Regarding to anti-inflammatory protein IL-10, the Figures 2G illustrate that the i-I/R provoked a significant increase in IL-10 protein level in lung of mice from i-I/R group when compared with basal group. The Figure 2G represent also a LLLT dual effect on IL-10 since that the dose of 1 J/cm2 caused a significant rise of IL-10 higher than levels found in mice subjected to i-I/R; Otherwise, 7.5 J/cm2 reduced the IL-10 concentration when compared with mice from i-I/R group. It is observed that laser irradiation on basal group did not affect mRNA expression neither the protein concentration of pro- and anti- inflammatory mediators when compared with basal group.
Figure 2.
Effect of LLLT on pro-inflammatory and anti-inflammatory mediator expression and protein in mice lung. Mice were treated with laser 30 minutes after initial of reperfusion. A representative RT-PCR analysis showing IL-1β, IL-6, TNF and IL-10 together with housekeeping expression GAPDH were used as internal control. The content of IL-1β, IL-6, TNF and IL-10 protein in lung was measured by ELISA technique. Inflammatory mediators mRNA expression and protein were measured 2 hours post i-I/R. Data were expressed as mean±SEM of seven animals. The results were considered significant when P<0.05.
(2A) .
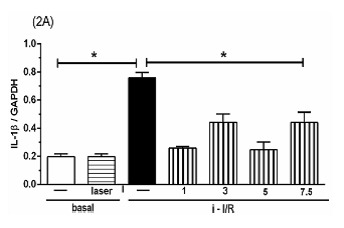
(2B) .
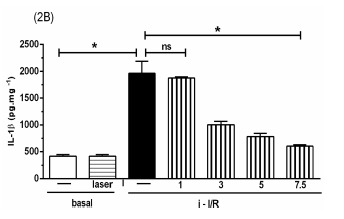
(2C) .
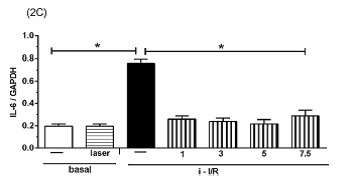
(2D) .
(2E) .
(2F) .
(2G) .
Discussion
The present study reports by the first time the effects of different doses of LLLT on pulmonary inflammation in an ARDS model induced by intestinal I/R in mice. Once again, we showed the anti-inflammatory effects of laser applied only for few minutes in a non-invasive manner modulating neutrophils activation and the pro- and anti- inflammatory cytokines expression in the lungs.
Some authors have reported the laser therapy presents beneficial effects for the treatment of asthmatic patients 22, pleurisy 23, chronic bronchitis 24, tuberculosis 25. Some authors have evidenced that LLLT acts on the bronchial hyperreactivity and on the lung inflammation by a cellular mechanism that involved the reduction of cellular migration as well as the release of inflammatory mediators through activation of transcription factors, as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), for instance 26,17,27,19,18
In the present study, our results showed that after i-I/R there was a significant augment of neutrophils influx into the lung tissue since the MPO activity was increased, and that LLLT significantly reduced the MPO activity. These results evidenced the participation of neutrophils and their role in the development of i-I/R, which is in agreement with Souza et al.28. According to Soares et al, Toll-like receptors (TLRs), notably TLR 2/4, play a critical role for induction of neutrophils recruitment into the lungs as observed by increased lung MPO activity21. However, in the present study the expression of TLR 2/4 was not investigated and will be the goal for further studies.
In our study we used a 660 nm laser at doses of 1, 3, 5 and 7.5 J/cm2. The goal was to observe which dose would have a better response against inflammation, as demonstrated through the evaluation of the influx of neutrophils, cytokines levels and mRNA expression. We observed that in general, 660 nm LLLT presented beneficial effects with most doses, but especially at doses (3, 5, and 7.5 J/cm2), although in some situations the lowest and the highest dose were not effective to inhibit the inflammatory mediators. On the contrary, we observe that only the dose of 1J/cm2 was effective to increased IL-10 levels and mRNA expression in i-I/R group.
A depiction from Arndt Schulz model illustrates a possible dose “sweet spot” at the target tissue. This law suggests that insufficient power density or too short time exposition will result in no effect on the pathological process and, that too much power density and / or long time exposition may have inhibitory effects, requiring studies aiming to obtain the best time and dose for the different organs, tissues and pathologies.
In experimental model of oral mucositis in hamsters treated using 660-nm laser at two different irradiances (55 mW/cm2 during 16 seconds per point or 155 mW/cm2 during 6 seconds per point) 29, the authors reported reduced severity of clinical mucositis and lower expression of COX-2 for 55 mW/cm2 group, while for155 mW/cm2 group, no beneficial effects was observed. In another condition, other authors compared the effects of delivering 5 J/cm2 of 670-nm laser at different power densities on wound tensile strength in a rat model. They found that 670 nm laser achieved a significant effect using 4mW/cm2 applied for 20 min but that this effect was lost if the same 5 J/cm2 fluence was delivered at 15 mW/cm2 for 5 min 30.
Based on i-I/R-acute lung inflammation, we have previously demonstrated that LLLT reduces the protein concentration of TNF in lung homogenates. This indicates that LLLT is truly efficient in reducing this powerful pro-inflammatory cytokine 19. In the present study we observed that TNF at mRNA expression and / or protein levels was increased after i-I/R and that LLLT was effective in reducing its expression and levels. Following prolonged (120-min) ischemia of the vascular territory irrigated by the superior mesenteric artery, there is a marked pro-inflammatory cytokine production. These inflammatory events are accompanied by a high degree of lethality, which is remarkably associated with the serum concentration of TNF 5.
Regarding the effects of LLLT on IL-1β induced by i-I/R, in the present study we evidenced that LLLT reduced the protein concentration of IL-1β in lung homogenates in doses of 3, 5 and 7.5 J/cm2 when compared with i-I/R group. Was also observed that the most effective dose reducing IL-1β was 7.5 J/cm2. Related to expression of IL-1β mRNA, we observed that LLLT reduced IL-1β mRNA in all doses studies. In fact, some authors have demonstrated that IL-1β plays a central role in the cascade of events leading to TNF production and TNF mediated injury-I/R. To this end, two strategies were used to block the action of IL-1β, neutralization of the protein using anti-IL-1 antiserum and administration of IL-1ra, a natural antagonist of IL-1 5. The results have shown that both strategies were associated with an overall enhancement of tissue injury, pro-inflammatory cytokine expression, and lethality, suggesting a pivotal role for IL-1β in models of i-I/R. Indeed, IL-1β has been implicated in the expression of cell adhesion molecules and neutrophil influx following ischemia and reperfusion injury 6,7, reinforcing its role in inflammatory processes
Concerning the effects of IL-6 in models of i-I/R, some authors have shown that the levels of IL-6 are extremely elevated following i-I/R injury, pointing out the value of IL-6 on the pathophysiology of i-I/R-induced ARDS 28. The profile of circulating levels of IL-6 has been considered as a marker of severity of gastrointestinal inflammatory trauma 31. In addition, serum levels of IL-6 and IL-10 were reported as biomarkers of mortality of patients upon pneumonia after hospital discharge 32. IL-6 is one of the most important mediators of fever and in ARDS high plasma and BALF levels are predictive of poor outcomes of the disease. Though IL-6 can activates both pro and anti-inflammatory pathways, while in early phase of ARDS IL-6 is correlated with a pro-inflammatory profile with increased levels seen in response to LPS and i-I/R 33. Herein, we showed that the treatment with laser irradiation was effective to reduce the IL-6 protein concentration as well as the mRNA expression in lung homogenates in all doses studied (1, 3, 5 and 7.5 J/cm2), while the most effective dose was 3 and 5 J/cm2.
IL-10 is an anti-inflammatory cytokine 34,35that inhibits the release of pro-inflammatory cytokines (IL-6, IL-1β and TNF) from monocytes/macrophages, thus preventing subsequent tissue damage 20,36. These findings highlighted the potential importance of the imbalance between pro-inflammatory and anti-inflammatory cytokines in acute lung inflammation, which is corroborated by the ratio of IL-10 and TNF in the lung. Our results showed that LLLT significantly induced an increased in the expression and in the levels of IL-10 in animals subjected to i-I/R, reinforcing its anti-inflammatory role. These results are in agreement with those reported by Souza and Teixeira that evidenced that LLLT increases the levels of IL-10 in animals submitted to severe i-I/R37. Other authors reported also that LLLT acts as anti-inflammatory mediator by reducing the classical features of tendinitis by increasing the IL-10 concentration in inflamed tissue14. These results are interesting due to the fact that, as previously reported herein, the presence of TNF in conditions of inflammation leads to an increase of IL-10. Moreover, most of the studies using animal models recognize that the rise of TNF is ordinarily accompanied by increasing of IL-10 38. Our results corroborates with these authors, since it was observed that TNF as well as IL-10 increased 4 h after reperfusion. This concomitant increase in TNF and IL-10 is accredited to be a counterbalancing effect of the system aiming to inhibit the deleterious effect of TNF. Therefore, our results support the beneficial effect of LLLT in i-I/R injury accounting the participation of IL-10, at least as part of the mechanisms involved.
Finally, our results evidenced that LLLT control the acute lung inflammation by reducing the neutrophils activation as well as the generation of pro-inflammatory mediators. Moreover we showed that LLLT has protective effect on lung inflammation via increase of IL-10. Therefore, the LLLT attenuates the i-I/R-induced acute lung inflammation by modulating the release of pro- and anti-inflammatory cytokines. In addition, the present study demonstrated for the first time that several LLLT doses may be effective to reduce i-I/R-induced acute lung inflammation.
Please cite this article as follows:
Mafra de Lima F, Aimbire F, Miranda H, Vieira R, Ligeiro de Oliveira AP, Albertini R. Low-Level Laser Therapy Attenuates the Myeloperoxidase Activity and Inflammatory Mediator Generation in Lung Inflammation Induced By Gut Ischemia and Reperfusion: A Dose-Response Study. J Lasers Med Sci 2014;5(2):63-70
References
- 1.Van Soeren MH, Diehl-Jones WL, Maykut RJ, Haddara WM. Pathophysiology and implications for treatment of acute respiratory distress syndrome. AACN Clin Issues. 2000;11:179–97. doi: 10.1097/00044067-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Kuzu MA, Köksoy C, Kuzu I, Gürhan I, Ergün H, Demirpence E. Role of integrins and intracellular adhesion molecule-1 in lung injury after intestinal ischemia reperfusion. Am J Surg. 2002;183(1):70–4. doi: 10.1016/s0002-9610(01)00846-7. [DOI] [PubMed] [Google Scholar]
- 3.Cavriani G, Oliveira-Filho RM, Trezena AG, da Silva ZL, Domingos HV, de Arruda MJ. et al. Lung microvascular permeability and neutrophil recruitment are differently regulated by nitric oxide in a rat model of intestinal ischemia-reperfusion. Eur J Pharmacol. 2004;494:241–9. doi: 10.1016/j.ejphar.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 4.Carden DL, Granger DN. Pathophysiology of ischemia reperfusion injury. J Pathol. 2000;190:255. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Souza DG, Mendonça VA, de A Castro MS, Poole S, Teixeira MM. Role of tachykinin NK receptors on the local and remote injuries following ischemia and reperfusion of the superior mesenteric artery in the rat. Br J Pharmacol. 2002;135:303. doi: 10.1038/sj.bjp.0704464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto S, Tanabe M, Wakabayashi G, Shimazu M, Matsumoto K, Kitajima M. The role of tumor necrosis factor alpha and interleukin-1 beta in ischemia reperfusion injury of the rat small intestine. J Surg Res. 2001;99:134. doi: 10.1006/jsre.2001.6106. [DOI] [PubMed] [Google Scholar]
- 7.Feldmann M, Brennan FM, Foxwell BM, Maini RN. The role of TNF-alpha and IL-1 in rheumatoid arthritis. Curr Dir Autoimmun. 2001;3:188. doi: 10.1159/000060522. [DOI] [PubMed] [Google Scholar]
- 8.Koga S, Ogawa S, Kuwabara K, Brett J, Leavy JA, Ryan J. et al. Synthesis and release of interleukin 1 by reoxygenated human mononuclear phagocytes. J Clin Invest. 1992;90:1007. doi: 10.1172/JCI115913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, Kaneda Y. et al. Overexpression of interleukin-1 receptor antagonist provides cardioprotection against ischemia reperfusion injury associated with reduction in apoptosis. Circulation. 2001;104:I308–I3. doi: 10.1161/hc37t1.094871. [DOI] [PubMed] [Google Scholar]
- 10.Souza DG, Guabiraba R, Pinho V, Bristow A, Poole S, Teixeira MM. IL-1 driven endogenous IL-10 production protects against the systemic and local acute inflammatory response following intestinal reperfusion injury. J Immunol. 2003;170:4759–66. doi: 10.4049/jimmunol.170.9.4759. [DOI] [PubMed] [Google Scholar]
- 11.Bjordal JM, Johnson MI, Iversen V, Aimbire F, Lopes-Martins RA. Low level laser therapy in acute pain: a systemic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed Laser Surg. 2006;24:2,158–68. doi: 10.1089/pho.2006.24.158. [DOI] [PubMed] [Google Scholar]
- 12.Chow RT, Johnson MI, Lopes Martins RA, Bjordal JM. Efficacy of low level laser therapy in the management of neck pain: a systematic review and meta analysis of randomised placebo or active treatment controlled trials. Lancet. 2009;374(9705):1897–1898. doi: 10.1016/S0140-6736(09)61522-1. [DOI] [PubMed] [Google Scholar]
- 13.Albertini R, Aimbire FS, Correa FI, Ribeiro W, Cogo JC, Antunes E. et al. Effects of different protocol doses of low power gallium-aluminum arsenate (Ga-Al-As) laser radiation (650nm) on carrageenan induced rat paw oedema. J Photochem Photobiol B. 2006;74:2–3,101. doi: 10.1016/j.jphotobiol.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Xavier M, David DR, de Souza RA, Arrieiro AN, Miranda H, Santana ET. et al. Anti inflammatory effect of low level light emitting diode therapy on Achilles tendinitis in rats. Lasers Surg Med. 2010;42(6):553–8. doi: 10.1002/lsm.20896. [DOI] [PubMed] [Google Scholar]
- 15.Landyshev IuS, Avdeeva NV, Goborov ND, Krasavina NP, Tikhonova GA, Tkacheva SI. Efficacy of low intensity laser irradiation and sodium nedocromil in the complex treatment of patients with bronchial asthma. Ter Arkh. 2002;74(3):25–8. [PubMed] [Google Scholar]
- 16.Nikitin AV, Esaulenko IE, Shatalova OL. Effectiveness of laser puncture in elderly patients with bronchial asthma. Vopr Kurortol Fizioter Lech Fiz Kult. 2008;6:38–9. Russian. [PubMed] [Google Scholar]
- 17.de Lima FM, Costa MS, Albertini R, Silva Jr JA R, Aimbire F. Low level laser therapy (LLLT): Attenuation of cholinergic hyperreactivity, beta adrenergic hyporesponsiveness and TNF alpha mRNA expression in rat bronchi segments in Ecoli Lipopolysaccharide- induced airway inflammation by a NF-KB dependent mechanism. Lasers Surg Med. 2009;41:68–74. doi: 10.1002/lsm.20735. [DOI] [PubMed] [Google Scholar]
- 18.de Lima FM, Vitoretti L, Coelho F, Albertini R, Breithaupt-Faloppa AC, de Lima WT. et al. Suppressive effect of low level laser therapy on tracheal hyperresponsiveness and lung inflammation in rat subjected to intestinal ischemia and reperfusion. Lasers Med Sci. 2013;28:2,551–64. doi: 10.1007/s10103-012-1088-1. [DOI] [PubMed] [Google Scholar]
- 19.de Lima FM, Villaverde AB, Albertini R, Corrêa JC, Carvalho RL, Munin E. et al. Dual effect of low level laser therapy (LLLT) on the acute lung inflammation induced by intestinal ischemia and reperfusion: Action on anti and pro inflammatory cytokines. Lasers Surg Med. 2011;43:410–20. doi: 10.1002/lsm.21053. [DOI] [PubMed] [Google Scholar]
- 20.Seitz M, Loetscher P, Dewald B, Towbin H, Gallati H, Baggiolini M. Interleukin-10 differentially regulates cytokines inhibitor and chemokines release from blood mononuclear cells and fibroblasts. Eur J Immunol. 1995;25(4):1129–32. doi: 10.1002/eji.1830250443. [DOI] [PubMed] [Google Scholar]
- 21.Soares AL, Coelho FR, Guabiraba R, Kamal M, Vargaftig BB, Li L. et al. Tumor necrosis factor is not associated with intestinal ischemia reperfusion induced lung inflammation. Shock. 2010;34(3):306–13. doi: 10.1097/SHK.0b013e3181cdc585. [DOI] [PubMed] [Google Scholar]
- 22.Mikhaĭlov VA, Aleksandrova OIu, Gol'dina EM. The immunomodulating action of low-energy laser radiation in the treatment of bronchial asthma. Vopr Kuortol Fizioter Lech Fiz Kult. 1998;4:23–5. Russian. [PubMed] [Google Scholar]
- 23.Milojevic M, Kuruc V. Laser biostimulation in the treatment of pleurisy. Med Pregl. 2003;56(11-12):516–20. [PubMed] [Google Scholar]
- 24.Kashanskaia EP, Fedorov AA. Low intensity laser radiation in the combined treatment of patients with chronic obstructive bronchitis. Vopr Kurortol Fiz Kult. 2009;2:19–22. Russian. [PubMed] [Google Scholar]
- 25.Nikolaeva OD. Use of electropuncture diagnostics and laseropuncture in patients with pulmonary tuberculosis. Lik Sprava. 2006;1-2:31–4. Russian. [PubMed] [Google Scholar]
- 26.Aimbire F, Ligeiro de Oliveira AP, Albertini R, JC Corrêa, CB Ladeira de Campos, JP Lyon. et al. Low level laser therapy (LLLT) decreases pulmonary microvascular leakage, neutrophil influx and IL-1β levels in airway and lung from rat subjected to LPS-induced inflammation. Inflammation. 2008;31(3):189–97. doi: 10.1007/s10753-008-9064-4. [DOI] [PubMed] [Google Scholar]
- 27.Mafra de Lima F, Villaverde AB, Salgado MA, Castro-Faria-Neto HC, Munin E, Albertini R. et al. Low intensity laser therapy (LILT) in vivo acts on the neutrophils recruitment and chemokines/ cytokines levels in a model of acute pulmonary inflammation induced by aerosol of lipopolysaccharide from Escherichia coli in rat. J Photochem Photobiol B. 2010;101(3):271–78. doi: 10.1016/j.jphotobiol.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Souza DG, Cassali GD, Poole S, Teixeira MM. Effects of inhibition of PDE4 and TNF alpha on local and remote injuries following ischemia and reperfusion injury. Br J Pharmacol. 2001;134:985. doi: 10.1038/sj.bjp.0704336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes NN, Plapler H, Chavantes MC, Lalla RV, Yoshimura EM, Alves MT. Cyclooxygenase -2 and vascular endothelial growth factor expression in 5-fluorouracil induced oral mucositis in hamsters: Evaluation of two low intensity laser protocols. Support Care Cancer. 2009;17(11):1409, 15. doi: 10.1007/s00520-009-0603-9. [DOI] [PubMed] [Google Scholar]
- 30.Gál P, Mokrý M, Vidinský B, Kilík R, Depta F, Harakalová M. et al. Effect of equal daily doses achieved by different power densities of low level laser therapy at 635 nm on open skin wound healing in normal and corticosteroid treated rats. Lasers Med Sci. 2009;24(4):539–47. doi: 10.1007/s10103-008-0604-9. [DOI] [PubMed] [Google Scholar]
- 31.Farmer DG, McDiarmid SV, Kuniyoshi J, Robert ME, Shaked A, Busuttil RW. Intragraft expression of messenger RNA for Interleukin 6 and tumor necrosis factor alpha is a predictor of rat small intestine transplant rejection. J Surg Res. 1994;57(1):138–42. doi: 10.1006/jsre.1994.1121. [DOI] [PubMed] [Google Scholar]
- 32.Menéndez R, Sahuquillo-Arce JM, Reyes S, Martínez R, Polverino E, Cillóniz C. et al. Cytokine activation patterns and biomarkers are influenced by microorganisms in community acquired pneumonia. Chest. 2012;141(6):1537–45. doi: 10.1378/chest.11-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Grady NP1, Preas HL, Pugin J, Fiuza C, Tropea M, Reda D. et al. Local inflammatory response following bronchial endotoxin instillation in humans. Am J Respir Crit Care Med. 2001;163(7):1591–8. doi: 10.1164/ajrccm.163.7.2009111. [DOI] [PubMed] [Google Scholar]
- 34.Kasama T, Strieter RM, Lukacs NW, Burdick MD, Kunkel SL. Regulation of neutrophil derived chemokine expression by IL-10. J Immunol. 1994;152(7):3559–69. [PubMed] [Google Scholar]
- 35.Pajkrt D, Van der Poll T, Levi M, Cutler DL, Affrime MB, van den Ende A. et al. Interleukin-10 inhibits activation of coagulation and fibrinolysis during human endotoxemia. Blood. 1997;89(8):2701–5. [PubMed] [Google Scholar]
- 36.Lo CJ, Fu M, Cryer HG. Interleukin 10 inhibits alveolar macrophage production of inflammatory mediators involved in adult respiratory distress syndrome. J Surg Res. 1998;79(2):179–84. doi: 10.1006/jsre.1998.5418. [DOI] [PubMed] [Google Scholar]
- 37.Souza DG, Teixeira MM. The balance between the production of tumor necrosis fator alpha and interleukin 10 determines tissue injury and lethality during reperfusion injury. Mem Inst Oswaldo Cruz. 2005;1:59–66. doi: 10.1590/s0074-02762005000900011. [DOI] [PubMed] [Google Scholar]
- 38.Inoue G. Effect of interleukin 10 (IL-10) on experimental LPS induced acute lung injury. J Infect Chemother. 2000;6(1):51–60. doi: 10.1007/s101560050050. [DOI] [PubMed] [Google Scholar]


